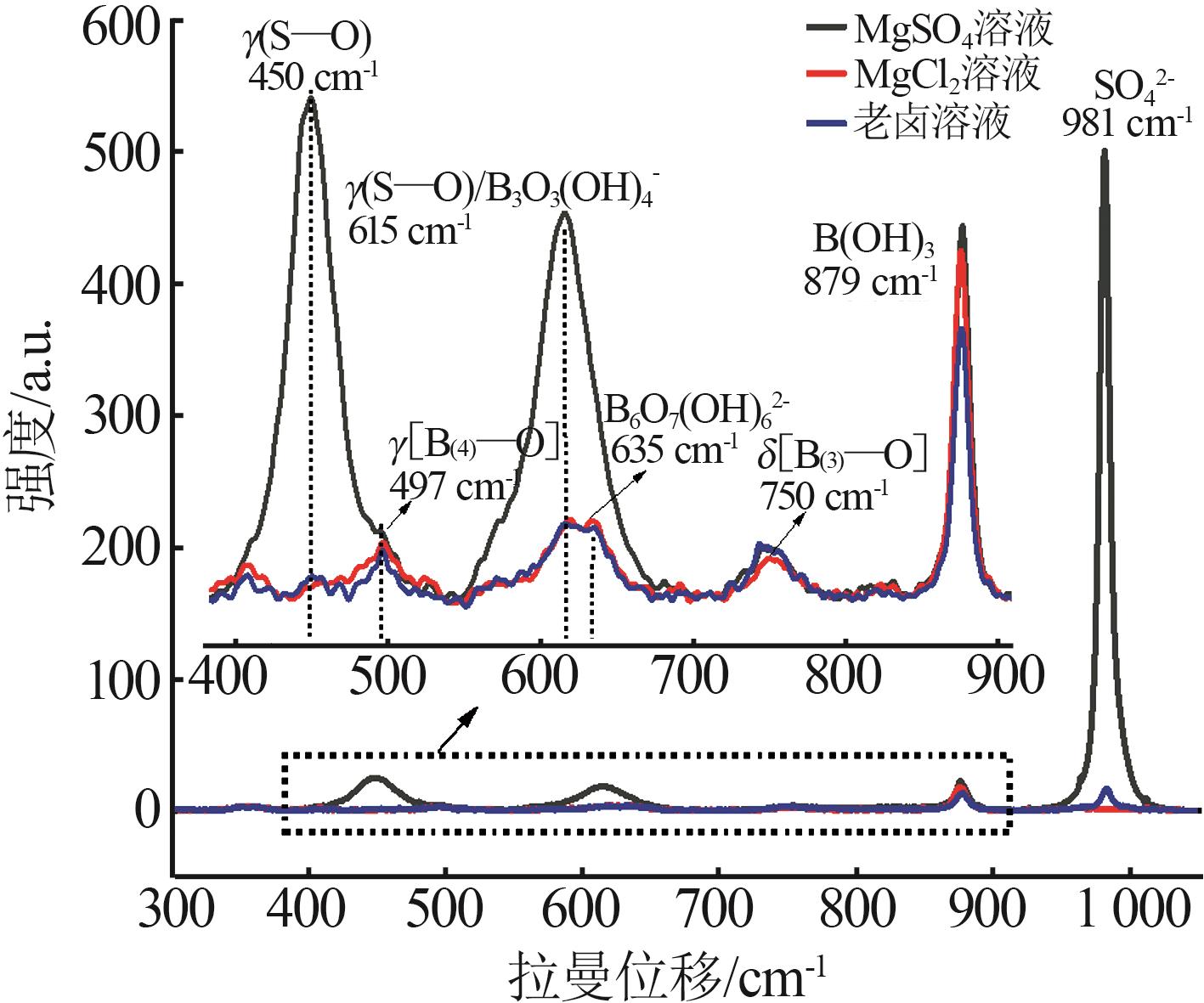
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 64-74.doi: 10.19964/j.issn.1006-4990.2024-0683
• Research & Development • Previous Articles Next Articles
Study on crystallization process and kinetic of macallisterite in concentrated MgCl2-xNa2O·yB2O3-H2O solution
HU Zhenrong1,2,3( ), ZHANG Manman2,3, SHI Chenglong1(
), ZHANG Manman2,3, SHI Chenglong1( ), PENG Jiaoyu2,3(
), PENG Jiaoyu2,3( ), ZHOU Yannan2,3, DONG Yaping2,3, LI Wu2,4
), ZHOU Yannan2,3, DONG Yaping2,3, LI Wu2,4
- 1. School of Chemistry and Materials Science,Qinghai Minzu University,Xining 810007,China
2. Key Laboratory of Green and High-end Utilization of Salt Lake Resources,Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
3. Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Xining 810008,China
4. Key Laboratory of Salt Lake Resources Chemistry of Qinghai Province,Xining 810008,China
-
Received:2024-12-17Online:2025-10-10Published:2025-07-17 -
Contact:SHI Chenglong, PENG Jiaoyu E-mail:huzr2023@163.com;qhmuscl@126.com;pengjy@isl.ac.cn
CLC Number:
Cite this article
HU Zhenrong, ZHANG Manman, SHI Chenglong, PENG Jiaoyu, ZHOU Yannan, DONG Yaping, LI Wu. Study on crystallization process and kinetic of macallisterite in concentrated MgCl2-xNa2O·yB2O3-H2O solution[J]. Inorganic Chemicals Industry, 2025, 57(10): 64-74.
share this article
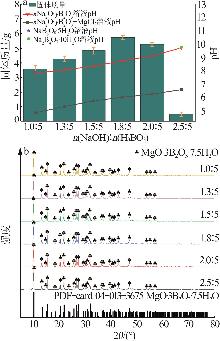
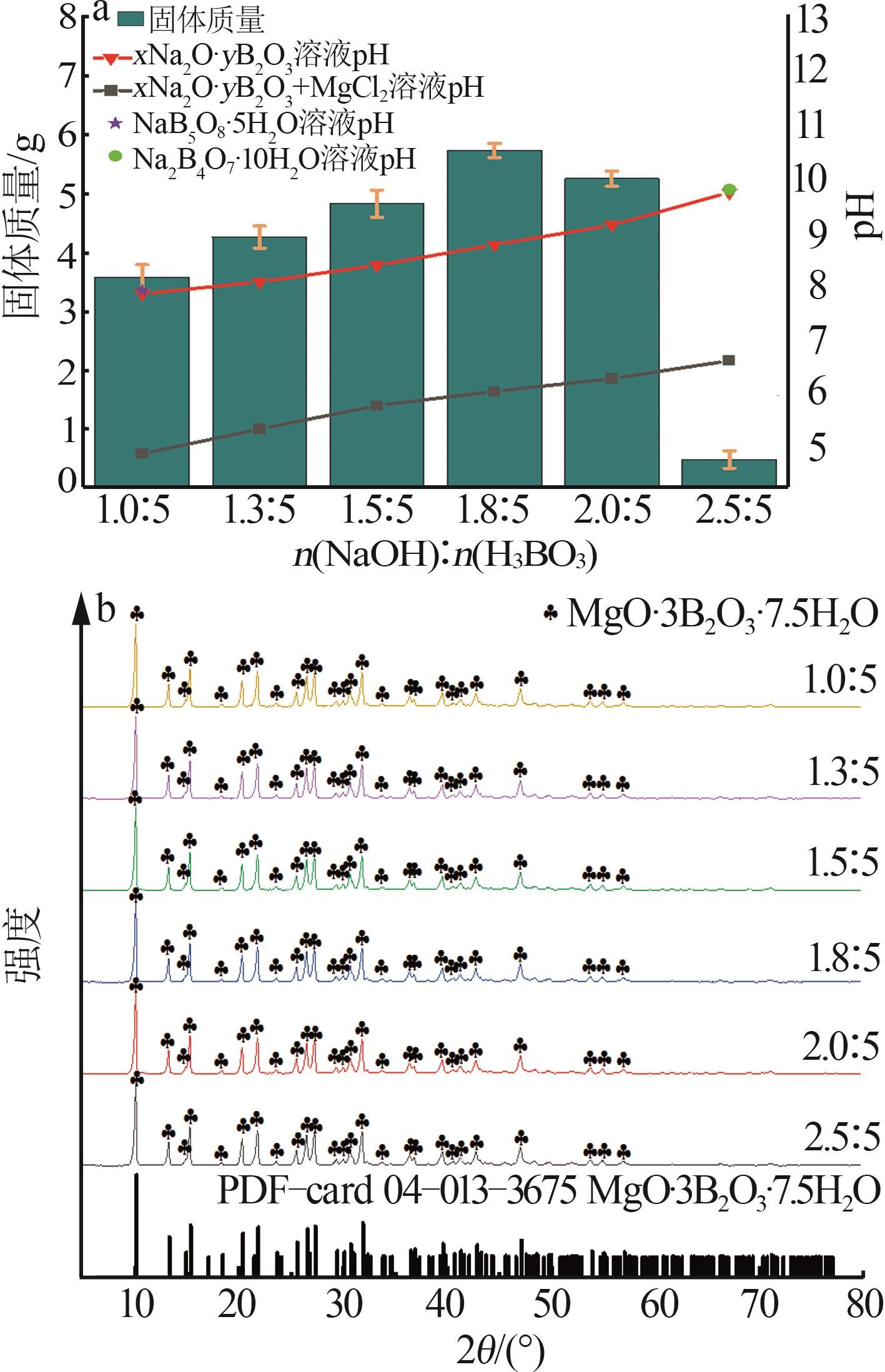
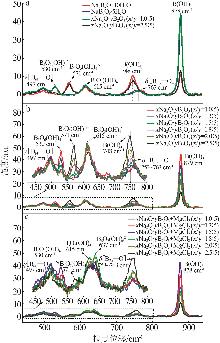
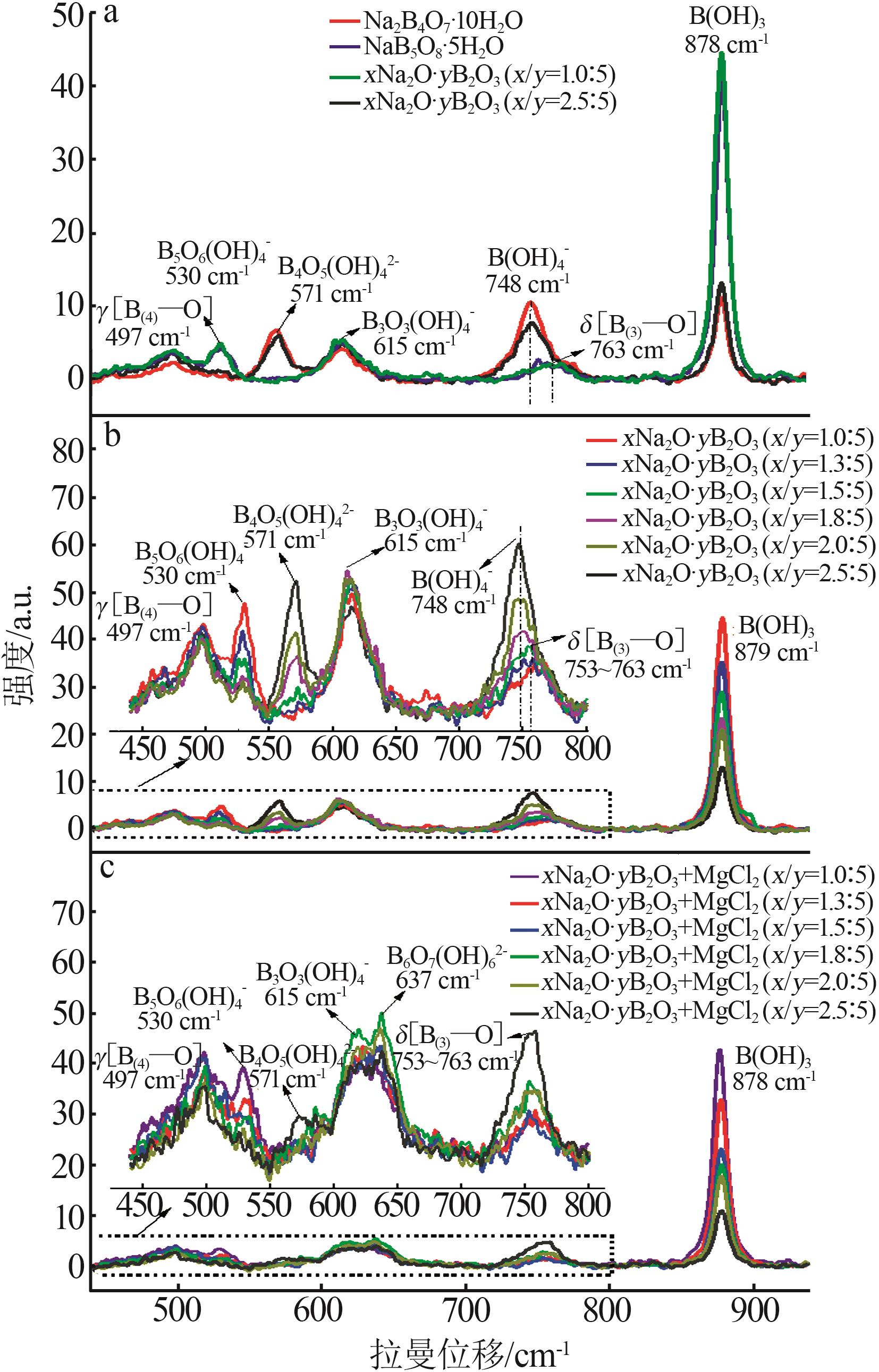
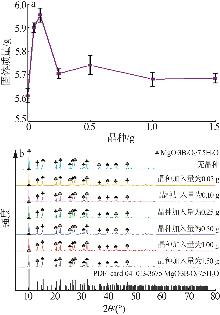
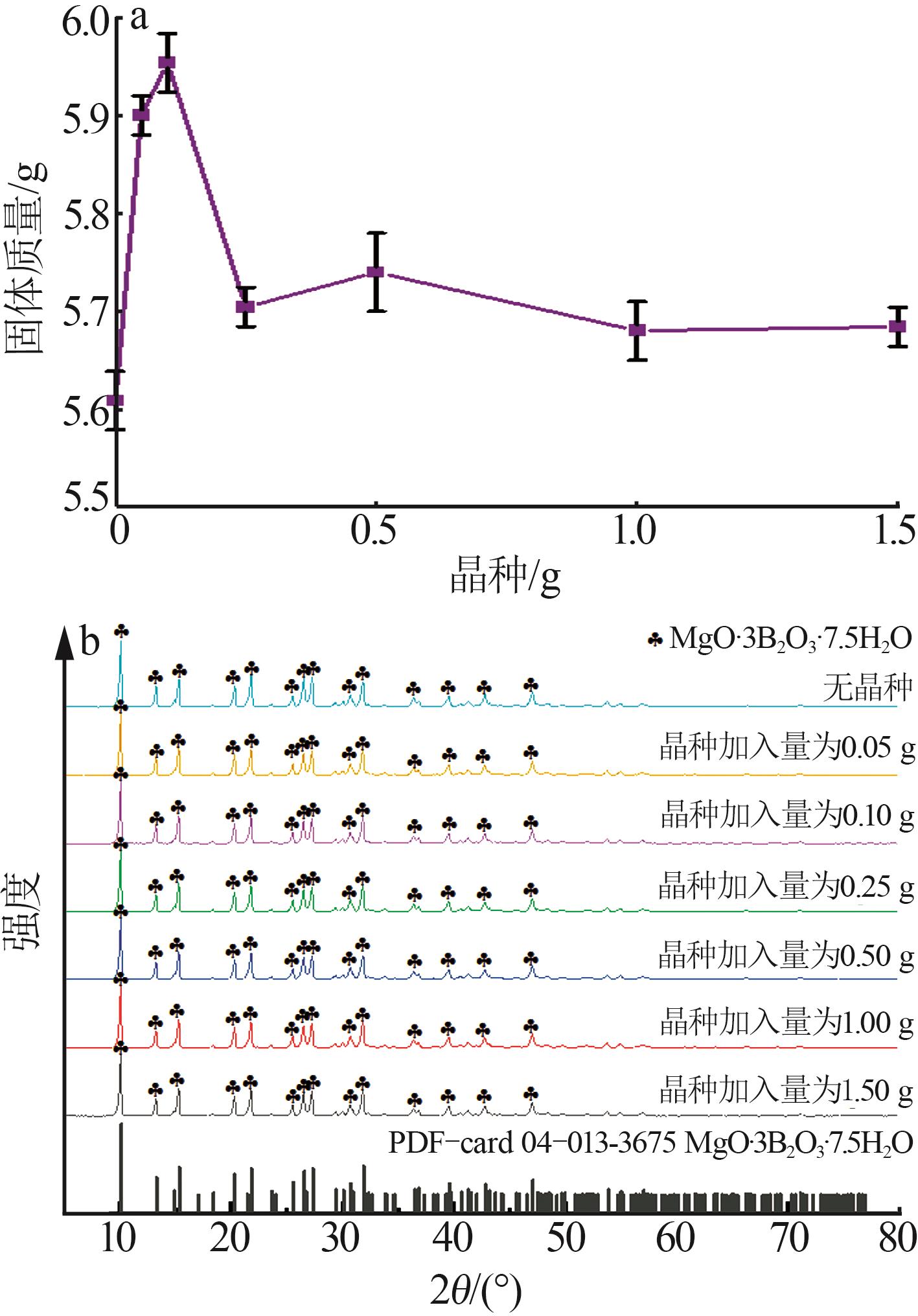
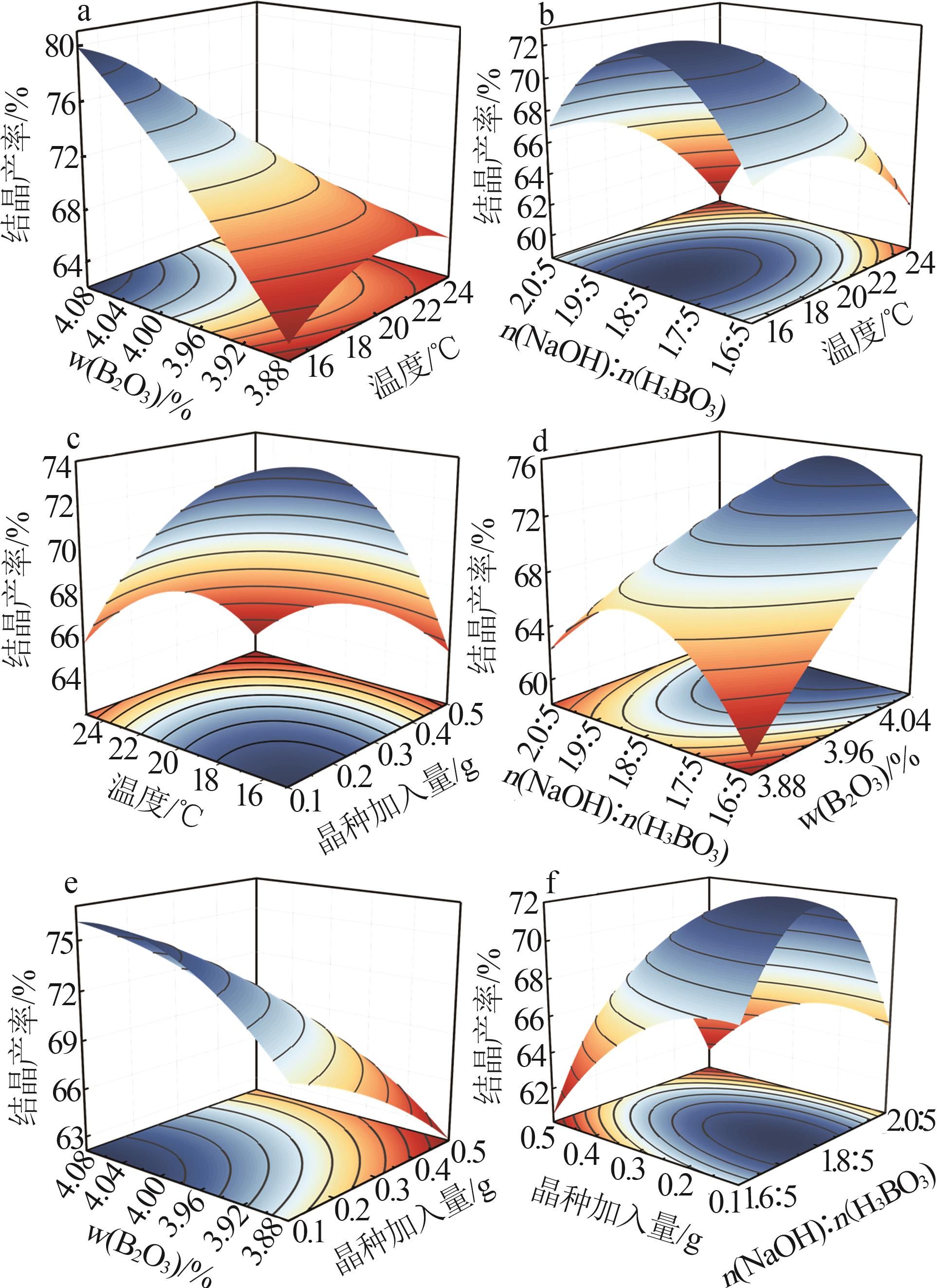
Table 2
BBD experimental design and results"
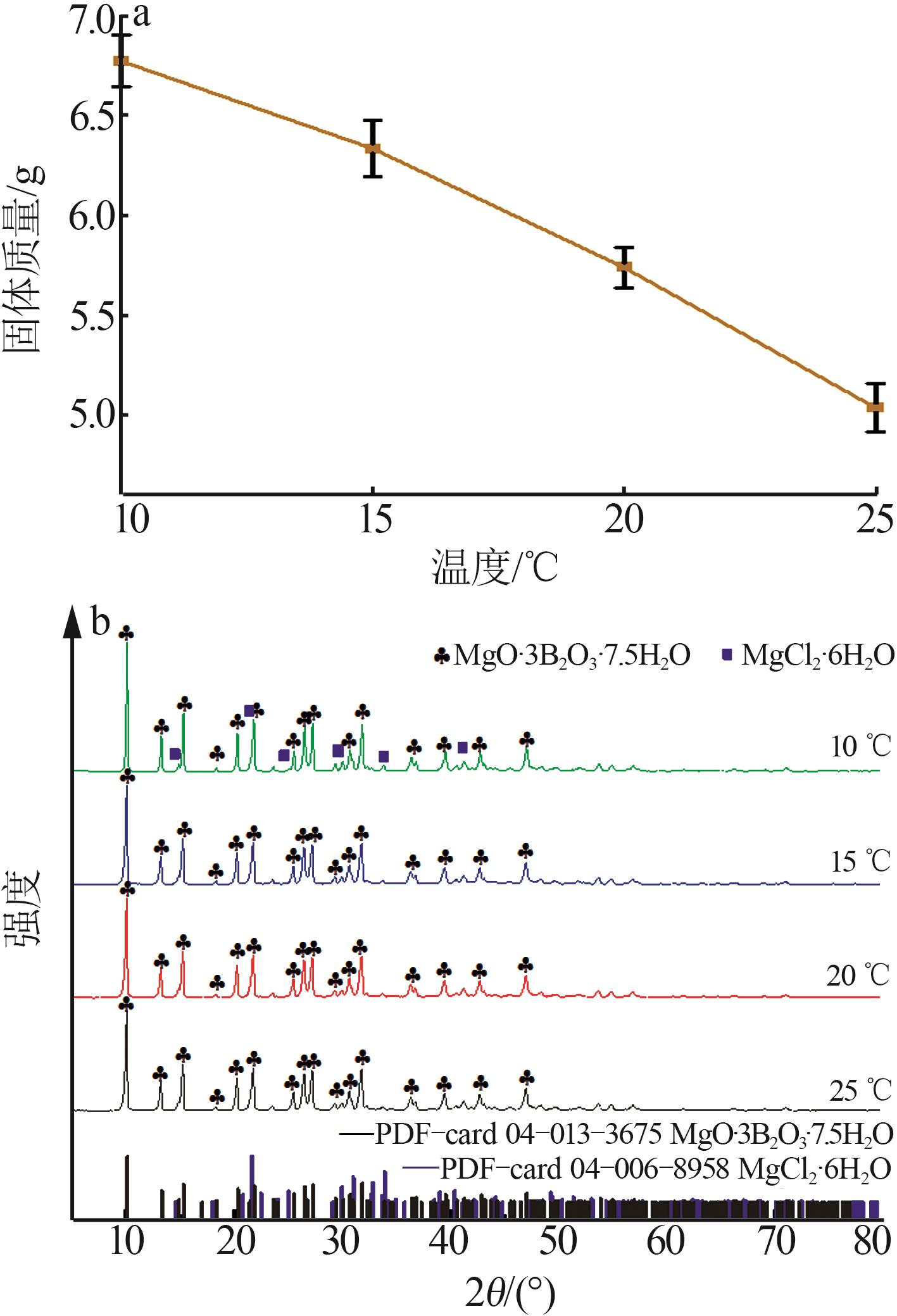
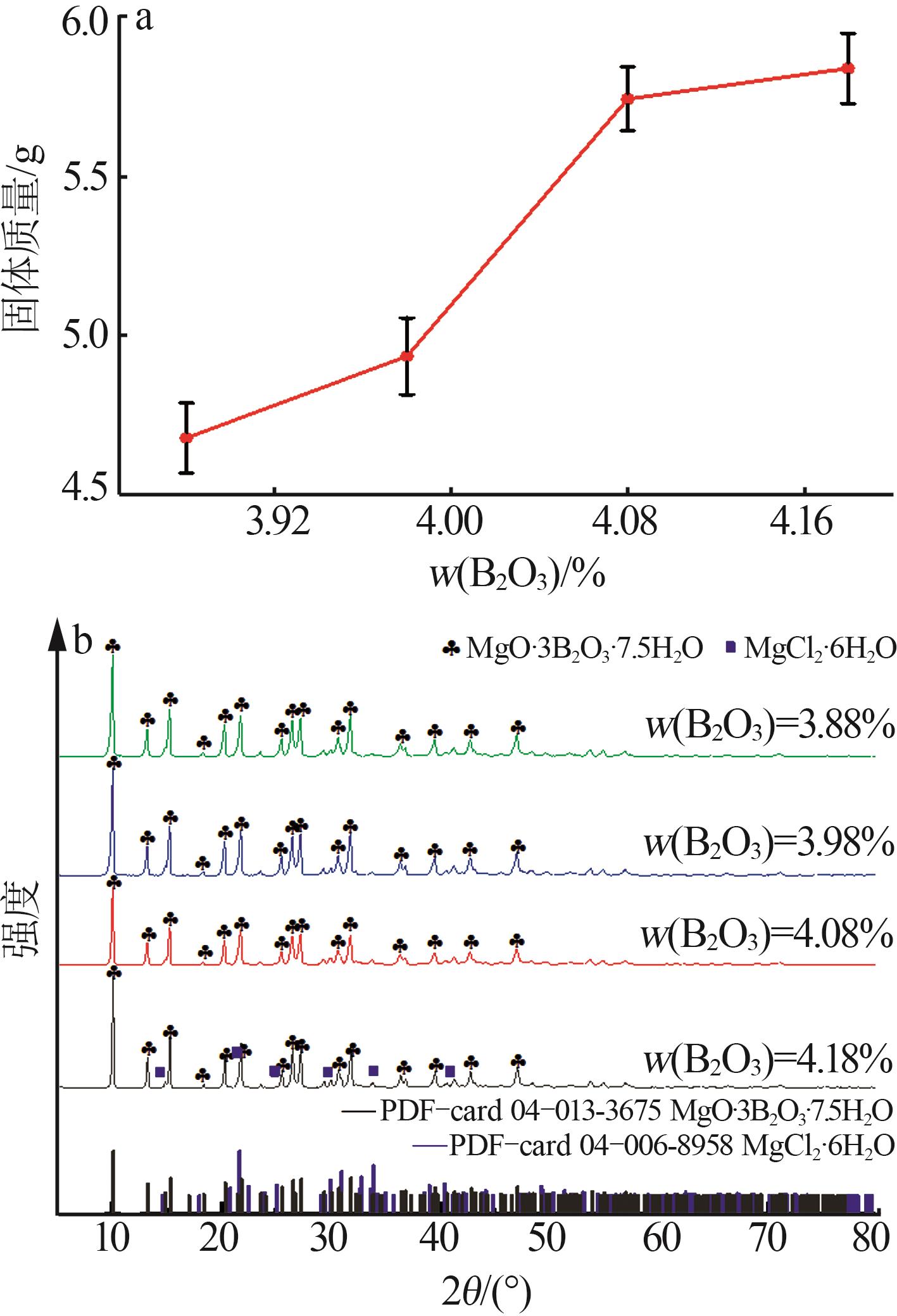
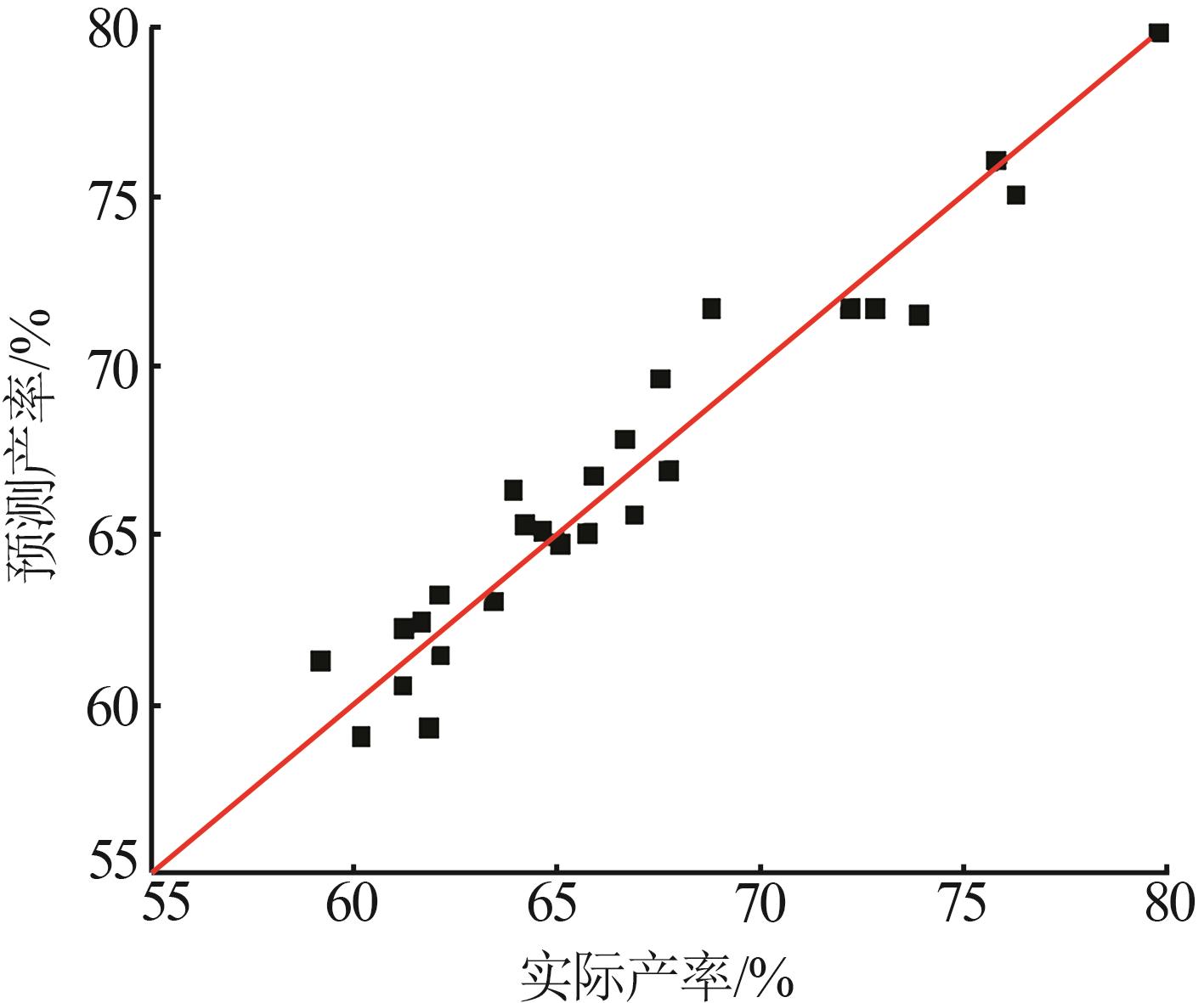
实验 号 | 温度(X1)/℃ | w(B2O3)(X2)/% | n(NaOH): n(H3BO3)(X3) | 晶种加入 量(X4)/g | 结晶产率/% | |
|---|---|---|---|---|---|---|
| 实际值 | 预测值 | |||||
| 1 | 15 | 3.88 | 1.8:5 | 0.30 | 62.08 | 63.21 |
| 2 | 20 | 3.88 | 2.0:5 | 0.30 | 61.82 | 59.28 |
| 3 | 25 | 4.08 | 1.8:5 | 0.30 | 65.73 | 65.05 |
| 4 | 20 | 3.88 | 1.8:5 | 0.50 | 61.21 | 62.22 |
| 5 | 20 | 3.98 | 1.6:5 | 0.10 | 64.18 | 65.29 |
| 6 | 20 | 3.98 | 1.6:5 | 0.50 | 62.11 | 61.42 |
| 7 | 20 | 3.98 | 1.8:5 | 0.30 | 72.24 | 71.68 |
| 8 | 20 | 4.08 | 1.8:5 | 0.50 | 67.54 | 69.61 |
| 9 | 20 | 3.98 | 1.8:5 | 0.30 | 72.24 | 71.68 |
| 10 | 20 | 3.88 | 1.8:5 | 0.10 | 67.75 | 66.92 |
| 11 | 25 | 3.88 | 1.8:5 | 0.30 | 64.63 | 65.11 |
| 12 | 20 | 3.88 | 1.6:5 | 0.30 | 61.65 | 62.40 |
| 13 | 15 | 3.98 | 2.0:5 | 0.30 | 63.92 | 66.30 |
| 14 | 20 | 4.08 | 2.0:5 | 0.30 | 73.94 | 71.50 |
| 15 | 15 | 3.98 | 1.8:5 | 0.50 | 66.91 | 65.59 |
| 16 | 20 | 3.98 | 2.0:5 | 0.50 | 61.19 | 60.53 |
| 17 | 25 | 3.98 | 2.0:5 | 0.30 | 59.15 | 61.26 |
| 18 | 15 | 4.08 | 1.8:5 | 0.30 | 79.87 | 79.84 |
| 19 | 20 | 4.08 | 1.6:5 | 0.30 | 65.89 | 66.74 |
| 20 | 25 | 3.98 | 1.8:5 | 0.10 | 65.09 | 64.72 |
| 21 | 20 | 3.98 | 1.8:5 | 0.30 | 68.81 | 71.68 |
| 22 | 15 | 3.98 | 1.8:5 | 0.10 | 76.33 | 75.05 |
| 23 | 20 | 4.08 | 1.8:5 | 0.10 | 75.85 | 76.08 |
| 24 | 20 | 3.98 | 1.8:5 | 0.30 | 72.86 | 71.68 |
| 25 | 15 | 3.98 | 1.6:5 | 0.30 | 67.75 | 66.88 |
| 26 | 25 | 3.98 | 1.6:5 | 0.30 | 60.16 | 59.02 |
| 27 | 20 | 3.98 | 1.8:5 | 0.30 | 72.23 | 71.68 |
| 28 | 25 | 3.98 | 1.8:5 | 0.50 | 63.43 | 63.02 |
| 29 | 20 | 3.98 | 2.0:5 | 0.10 | 66.68 | 67.83 |
Table 3
Variance analysis of regression model"
| 变异来源 | 平方和 | 自由度 | 均方 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 770.56 | 14 | 55.04 | 14.94 | <0.000 1 | 极显著 |
| X1 | 124.61 | 1 | 124.61 | 33.83 | <0.000 1 | 极显著 |
| X2 | 205.68 | 1 | 205.68 | 55.83 | <0.000 1 | 极显著 |
| X3 | 2.05 | 1 | 2.05 | 0.56 | 0.468 0 | 不显著 |
| X4 | 93.47 | 1 | 93.47 | 25.37 | 0.000 2 | 极显著 |
| X1X2 | 69.64 | 1 | 69.64 | 18.90 | 0.000 7 | 极显著 |
| X1X3 | 1.99 | 1 | 1.99 | 0.54 | 0.474 7 | 不显著 |
| X1X4 | 15.05 | 1 | 15.05 | 4.09 | 0.062 8 | 不显著 |
| X2X3 | 15.52 | 1 | 15.52 | 4.21 | 0.059 3 | 不显著 |
| X2X4 | 0.78 | 1 | 0.78 | 0.21 | 0.651 8 | 不显著 |
| X3X4 | 2.92 | 1 | 2.92 | 0.79 | 0.388 0 | 不显著 |
| X12 | 40.30 | 1 | 40.30 | 10.94 | 0.005 2 | 极显著 |
| X22 | 5.01 | 1 | 5.01 | 1.36 | 0.263 0 | 不显著 |
| X32 | 219.62 | 1 | 219.62 | 59.61 | <0.000 1 | 极显著 |
| X42 | 28.34 | 1 | 28.34 | 7.69 | 0.014 9 | 显著 |
| 残差 | 51.58 | 14 | 3.68 | |||
| 失拟项 | 41.02 | 10 | 4.10 | 1.55 | 0.356 2 | 不显著 |
| 纯误差 | 10.56 | 4 | 2.64 | |||
| 总和 | 822.14 | 28 |
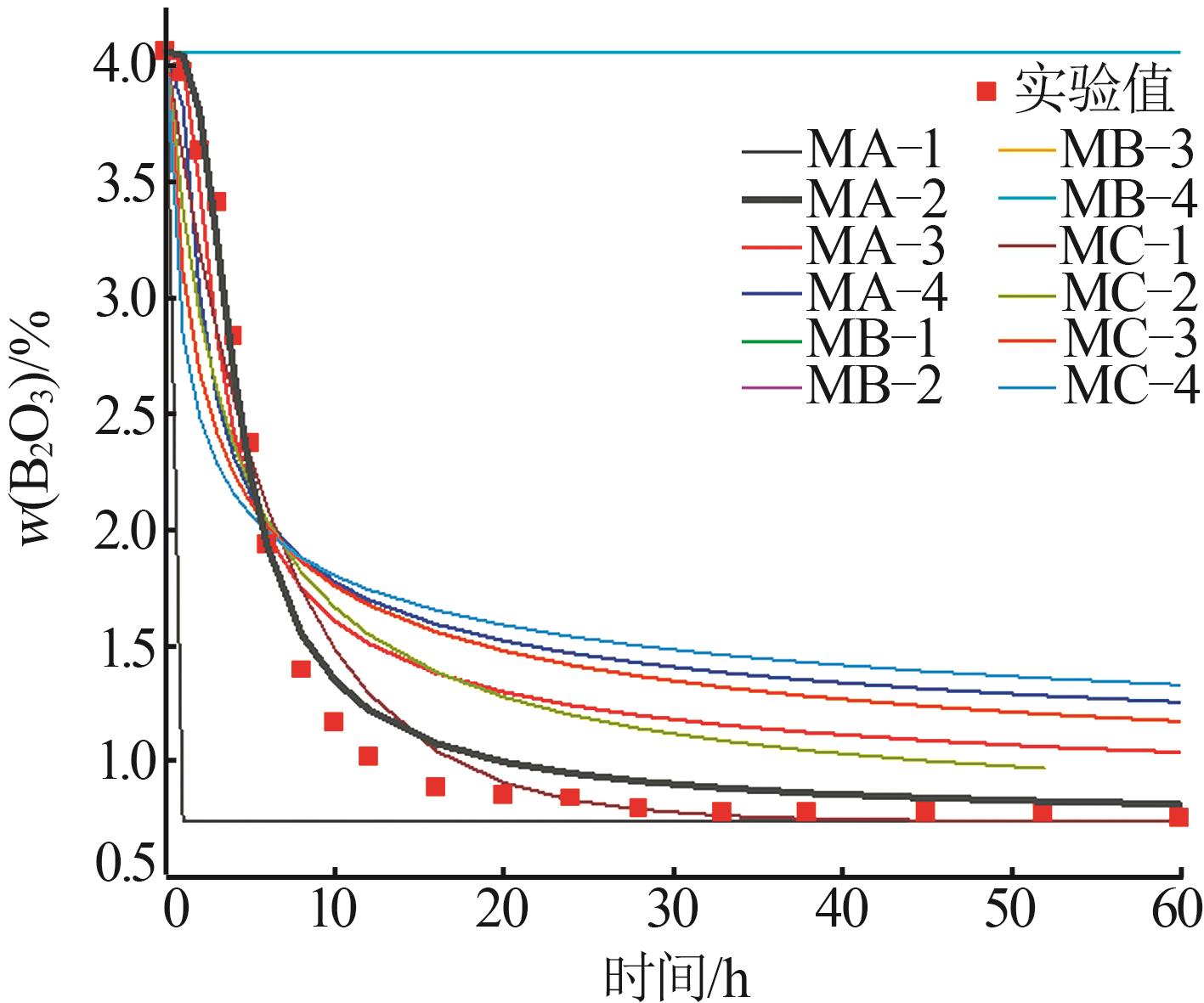
Table 5
Fitting results of model for crystallization kinetics of macallisterite at 15 ℃"
| 动力学模型 | 残差平方和S | 速率常数k | 拟合优度R2 |
|---|---|---|---|
| MA-1 | 35.165 4 | 5.761 0 | 0.279 3 |
| MA-2 | 0.305 4 | 0.110 7 | 0.984 8 |
| MA-3 | 2.398 6 | 0.049 3 | 0.850 8 |
| MA-4 | 5.698 8 | 0.022 3 | 0.621 7 |
| MB-1 | 129.653 8 | 0.001 0 | 0.454 7 |
| MB-2 | 129.653 8 | 0.001 0 | 0.454 7 |
| MB-3 | 129.653 8 | 0.001 0 | 0.454 7 |
| MB-4 | 129.653 8 | 0.001 0 | 0.454 7 |
| MC-1 | 1.064 6 | 0.149 7 | 0.936 2 |
| MC-2 | 3.437 2 | 0.078 4 | 0.731 1 |
| MC-3 | 6.663 6 | 0.044 0 | 0.462 9 |
| MC-4 | 9.610 3 | 0.027 1 | 0.281 3 |
| [1] | 贺茂勇,张宁,文雪琴,等.硼同位素高精度测定进展、应用及挑战[J].东华理工大学学报(自然科学版),2023,46(6):597- 607. |
| HE Maoyong, ZHANG Ning, WEN Xueqin,et al.Progress,applications,and challenges in high precision measurement of boron isotopes[J].Journal of East China University of Technology(Natural Science),2023,46(6):597-607. | |
| [2] | INKRATAITE G, SKRUODIENE M, SKAUDZIUS R.Synthesis and investigation of novel boron- and magnesium-doped YAG:Ce and LuAG:Ce phosphor ceramics[J].Luminescence,2024,39(1):e4673. |
| [3] | LIU Jingfei, LI Yuanbing, YIN Bo,et al.Novel magnesium borate ceramic matrix composites with glass fiber reinforcement[J].Ceramics International,2023,49(7):11197-11203. |
| [4] | ALEXEY S, VITA V.Investigation of BaSO4-KPO3-Na2B4O7 low-melting glass system as a basis for synthesis of a glass-solder material[J].Key Engineering Materials,2021,6152:60-64. |
| [5] | 赵鋆,周桓.盐湖卤水体系硼的存在形态及其分布规律的研究进展[J].盐科学与化工,2024,53(1):1-7. |
| ZHAO Yun, ZHOU Huan.Research progress on the existing forms and distribution patterns of boron in salt lake brine systems[J].Journal of Salt Science and Chemical Industry,2024,53(1):1-7. | |
| [6] | CHI Mengjiao, LIU Yahan, ZHOU Tao,et al.Ni doping improving magnesium borate microwave dielectric ceramic for LTCC via cold sintering and post-annealing process[J].Journal of Materials Science:Materials in Electronics,2023,34(3):235. |
| [7] | YANG Rui, JIA Qingchao, ZHANG Liangzhu,et al.Unveiling the effect of phase separation on crystallization of calcium borosilicate glass-ceramics[J].Ceramics International,2024,50(7):11003-11011. |
| [8] | 孟令宗,邓天龙,段超文,等.三方硼镁石合成方法研究[J].世界科技研究与发展,2010,32(6):825-826. |
| MENG Lingzong, DENG Tianlong, DUAN Chaowen,et al.A synthetic method for mcallisterite[J].World Sci-Tech R&D,2010,32(6):825-826. | |
| [9] | HABERMANN F, BURKMANN K, KRAUS J,et al.Thermodynamic and kinetic study of the thermal dehydrogenation of Sr(AlH4)2 taking into account the by-products NaCl and LiCl[J].Journal of Alloys and Compounds,2024,980:173476. |
| [10] | 刘志宏,胡满成,高世扬.2MgO·2B2O3·MgCl2·14H2O-7.8%H3BO3-H2O体系多温相关系研究[J].高等学校化学学报,2003,24(2):189-194. |
| LIU Zhihong, HU Mancheng, GAO Shiyang.Phase relation of 2MgO·2B2O3·MgCl2·14H2O-7.8%H3BO3-H2O system at various temperatures[J].Chemical Research in Chinese Universities,2003,24(2):189-194. | |
| [11] | KIPCAK A S, YILDIRIM M, AYDIN YUKSEL S,et al.The synthesis and physical properties of magnesium borate mineral of admontite synthesized from sodium borates[J].Advances in Materials Science and Engineering,2014,2014:819745. |
| [12] | YILDIRIM M, KIPCAK A S, DERUN E M.Sonochemical-assisted magnesium borate synthesis from different boron sources[J].Polish Journal of Chemical Technology,2017,19(1):81-88. |
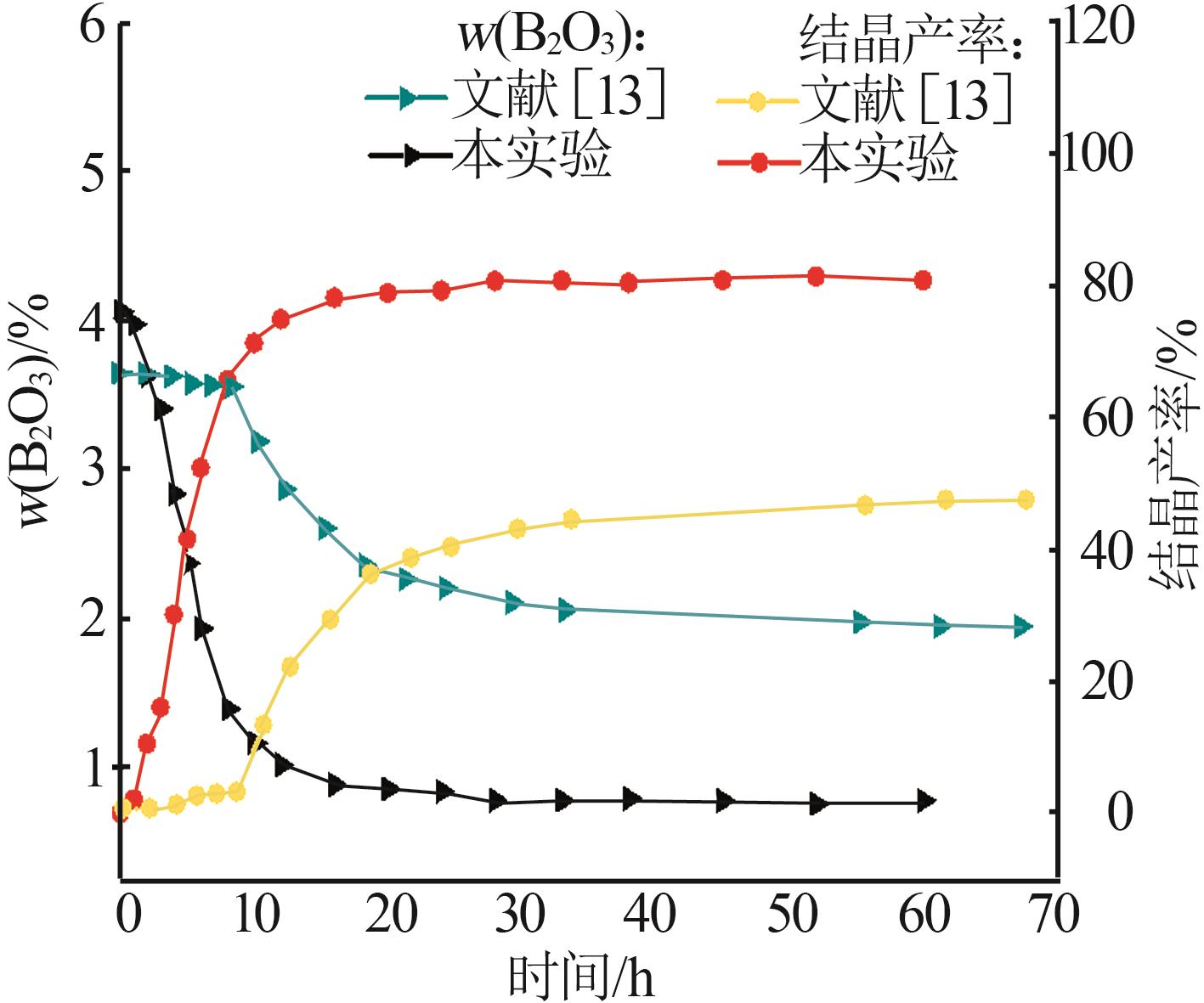
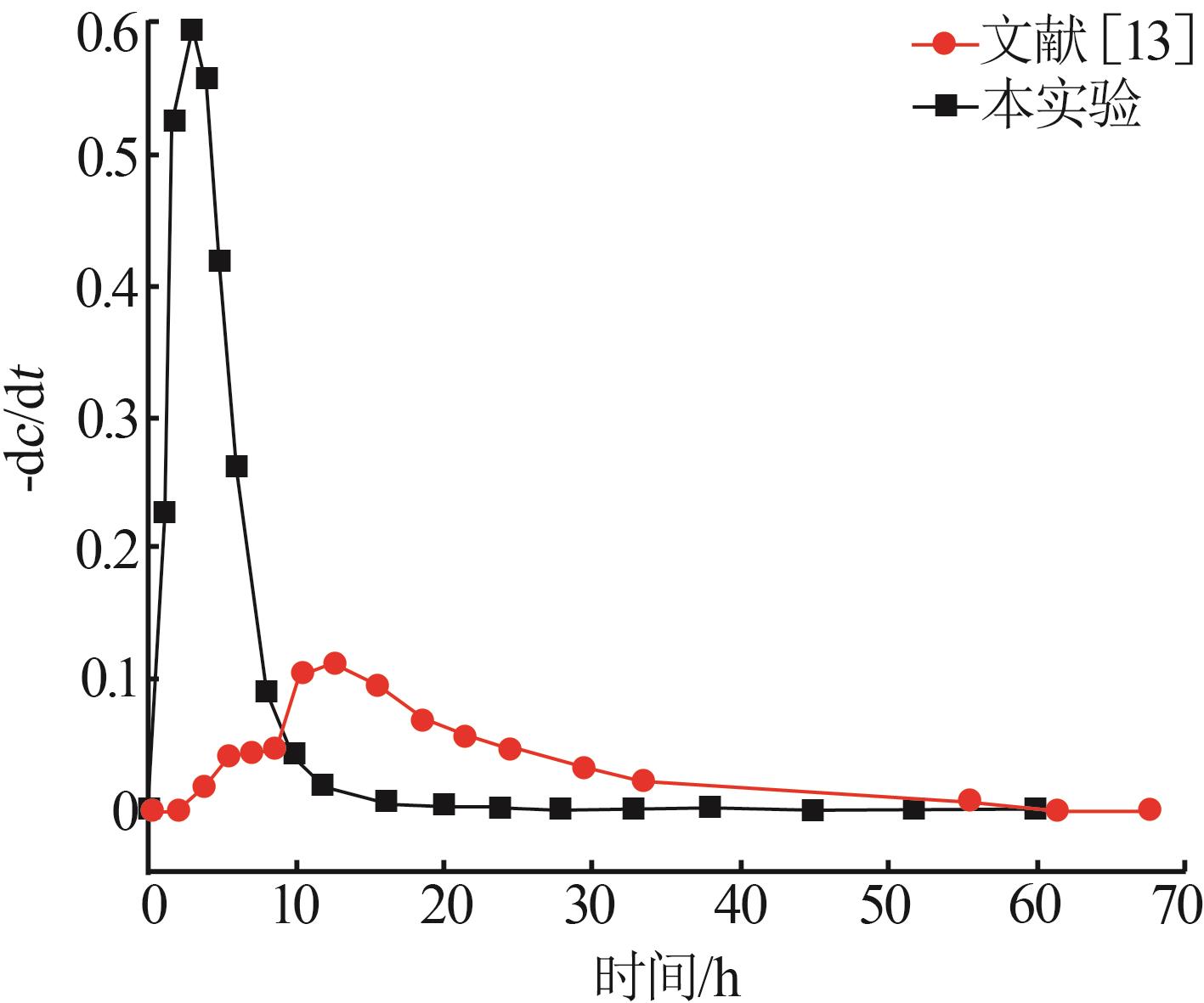
| [13] | 彭姣玉,谈钰琴,杨克利,等.盐湖水氯镁石合成三方硼镁石的结晶机理及动力学研究[J].无机盐工业,2023,55(10):56-62. |
| PENG Jiaoyu, TAN Yuqin, YANG Keli,et al.Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake[J].Inorganic Chemicals Industry,2023,55(10):56-62. | |
| [14] | LIN Yong, GYAKWAA F, KOKKONEN T,et al.Investigation on crystallization of CaO-Al2O3-B2O3-BaO slag using differential scanning calorimeter and in situ high-temperature Raman spectroscopy[J].Steel Research International,2024,24(4):2400697. |
| [15] | JESSEN C, KORNATH A J.Synthesis and structure of protonated sulfur dioxide[J].Angewandte Chemie International Edition,2024,63(20):e202401953. |
| [16] | KOROLEVA O N, NEVOLINA L A, KRIVENKO A P.Crystallization of Na- and Cs-bearing borosilicate melts:Results of Raman spectroscopy[J].Geochemistry International,2024,62(10):1057-1064. |
| [17] | 彭姣玉,多杰卓么,黄保坤,等.拉曼积分球光谱仪对盐水溶液中B(OH)3的定量分析[J].盐湖研究,2024,32(2):30-37. |
| PENG Jiaoyu, DUOJIE Zhuome, HUANG Baokun,et al.Quantitative analysis of boric acid(B(OH)3)in aqueous salt solution by simplified Raman integrating sphere[J].Journal of Salt Lake Research,2024,32(2):30-37. | |
| [18] | 贾艳萍,马艳菊,管文昕,等.响应面法优化Fe0/H2O2体系降解染料废水的工艺条件及机理[J].化工学报,2025,76(1):348-362. |
| JIA Yanping, MA Yanju, GUAN Wenxin,et al.Process conditions optimization and degradation mechanism of dye wastewater by Fe0/H2O2 system using response surface methodology[J].CIESC Journal,2025,76(1):348-362. | |
| [19] | FERREIRA N, VIANA T, HENRIQUES B,et al.Application of response surface methodology and box-behnken design for the optimization of mercury removal by Ulva sp[J].Journal of Hazardous Materials,2023,445:130405. |
| [20] | 郭元亨,皮冬伟,陶进,等.响应曲面法优化赤藓糖醇结晶工艺[J].食品与发酵工业,2024,50(9):56-63. |
| GUO Yuanheng, PI Dongwei, TAO Jin,et al.Crystallization process optimization of erythritol by response surface methodology[J].Food and Fermentation Industries,2024,50(9):56-63. | |
| [21] | POLAT S, SAYAN P.Box-behnken experimental design for zinc borate Zn2B6O11·7H2O[J].Journal of Boron,2020:5(3):152- 161. |
| [22] | KALASHGRANI M, BABAPOOR A, MOUSAVI S,et al.Synthesis of isoreticular metal organic framework-3 (IRMOF-3) porous nanostructure and its effect on naphthalene adsorption:Optimized by response surface methodology[J].Separations,2023,10(4):261. |
| [23] | 彭姣玉,张波,陈婧,等.大柴旦富硼浓缩盐卤中硼酸镁盐稀释结晶动力学[J].无机化学学报,2019,35(10):1821-1833. |
| PENG Jiaoyu, ZHANG Bo, CHEN Jing,et al.Crystallization kinetics of Mg-borates precipitating from diluted boron-containing brine of da Qaidam saline lake[J].Chinese Journal of Inorganic Chemistry,2019,35(10):1821-1833. | |
| [24] | ZHANG Ye, YAN Xiao, WANG Li,et al.Forsterite refractory preparation using magnesium resources from salt lake brines[J].Minerals Engineering,2023,203:108333. |
| [25] | FUKUDA H, TSUCHIYA K, TOBA Y,et al.Rapid boron removal from wastewater using low-crystalline magnesium oxide[J].Journal of Environmental Chemical Engineering,2020,8(5):104171. |
| [1] | CHEN Mengmeng, XU Dekan, HUANG Jilong, TANG Zhilan, ZHANG Xu, TAN Chao, WANG Xiaohu, PENG Wenbo. Study on preparation and performance of Ni-modified titanium-based lithium ion sieve [J]. Inorganic Chemicals Industry, 2025, 57(9): 37-45. |
| [2] | SHOU Zhixin, XU Dehua, LIU Yujia, YANG Wengong, WANG Xinlong. Study on thermal decomposition kinetics of sodium fluosilicate [J]. Inorganic Chemicals Industry, 2025, 57(8): 28-34. |
| [3] | WU Mingsong, ZOU Zhigang, WANG He, CHEN Yu, ZHENG Jiaxing. Study on catalytic reaction kinetics for preparation of chlorine dioxide from electrolyzed sodium chlorite [J]. Inorganic Chemicals Industry, 2025, 57(7): 44-49. |
| [4] | KONG Lingjie, LI Guangbi, XIE Jiahao, YANG Xinhui, BAI Xiaoqin. Research progress on lithium extraction technology from salt lake brine [J]. Inorganic Chemicals Industry, 2025, 57(1): 14-26. |
| [5] | ZENG Yijun, JIANG Ziwen, JIAN Chengzong, QUAN Xuejun. Study on deep extraction of chromium from calcium-free roasting slag of chromite ore [J]. Inorganic Chemicals Industry, 2025, 57(1): 90-96. |
| [6] | MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru. Kinetic study of lithium extraction from solution with iron-based ionic liquid system [J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66. |
| [7] | FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui. Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid [J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43. |
| [8] | ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. |
| [9] | CHENG Ziyang, CHEN Guofu. Early hydration kinetics research of nano-SiO2 and cement composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 80-87. |
| [10] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [11] | LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing. Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72. |
| [12] | LI Chunli, ZHANG Huanhuan, CHENG Zhuo, TANG Xiuhua, ZHANG Fengzhen, YE Yuling. Anti-solvent crystallization process of NH4VO3 in NaVO3-NH4Cl-H2O solution system [J]. Inorganic Chemicals Industry, 2024, 56(5): 39-44. |
| [13] | ZHANG Yu, ZHAO Guiyan, TIAN Yongchang, QIU Xiaokui, SUN Jiali, XU Lixin. Reaction kinetics of ethylenediamine hydrochloride with calcium hydroxide [J]. Inorganic Chemicals Industry, 2024, 56(5): 64-69. |
| [14] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [15] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||