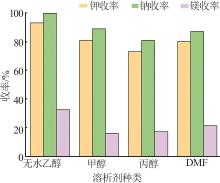
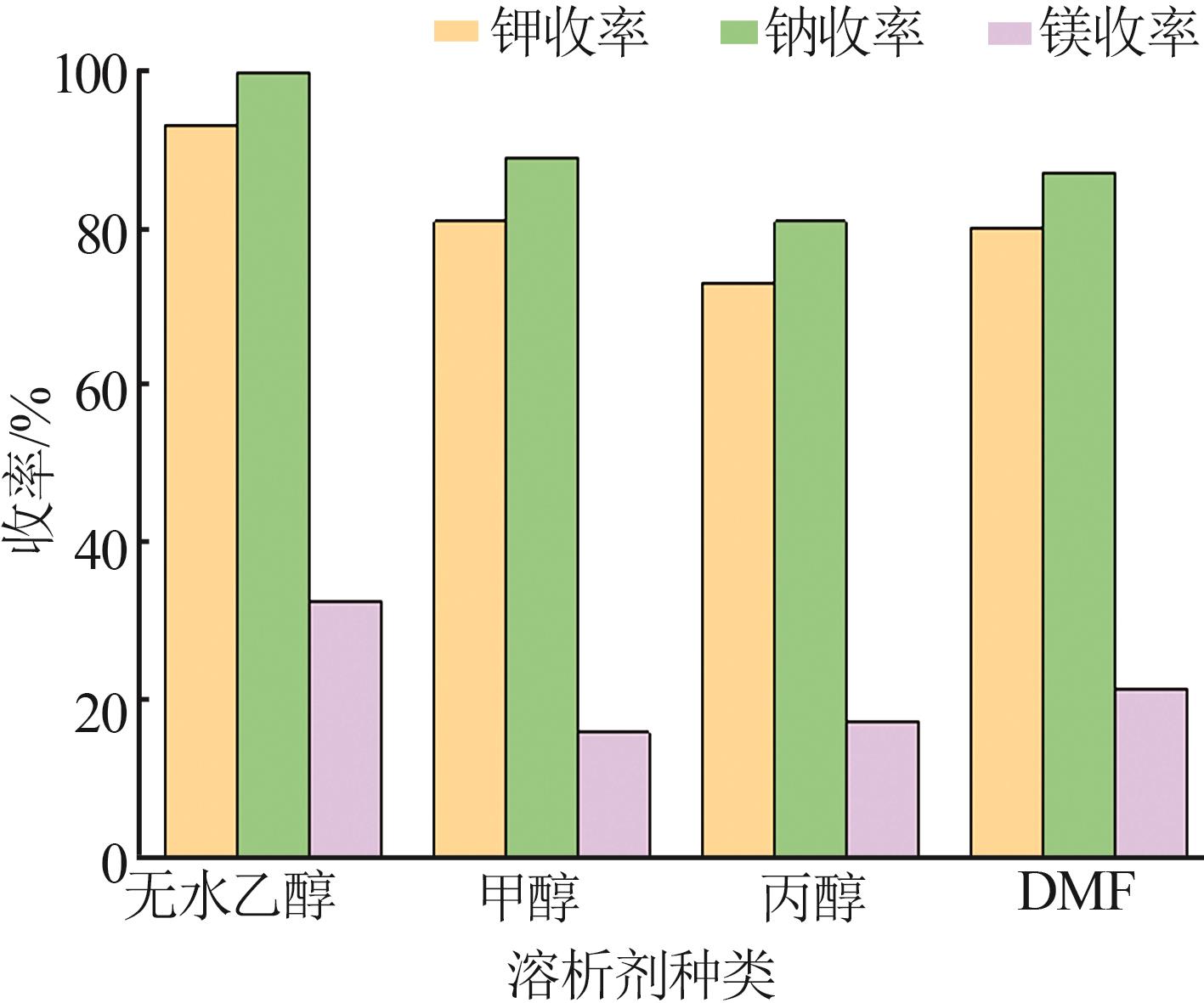
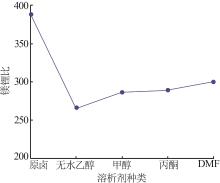
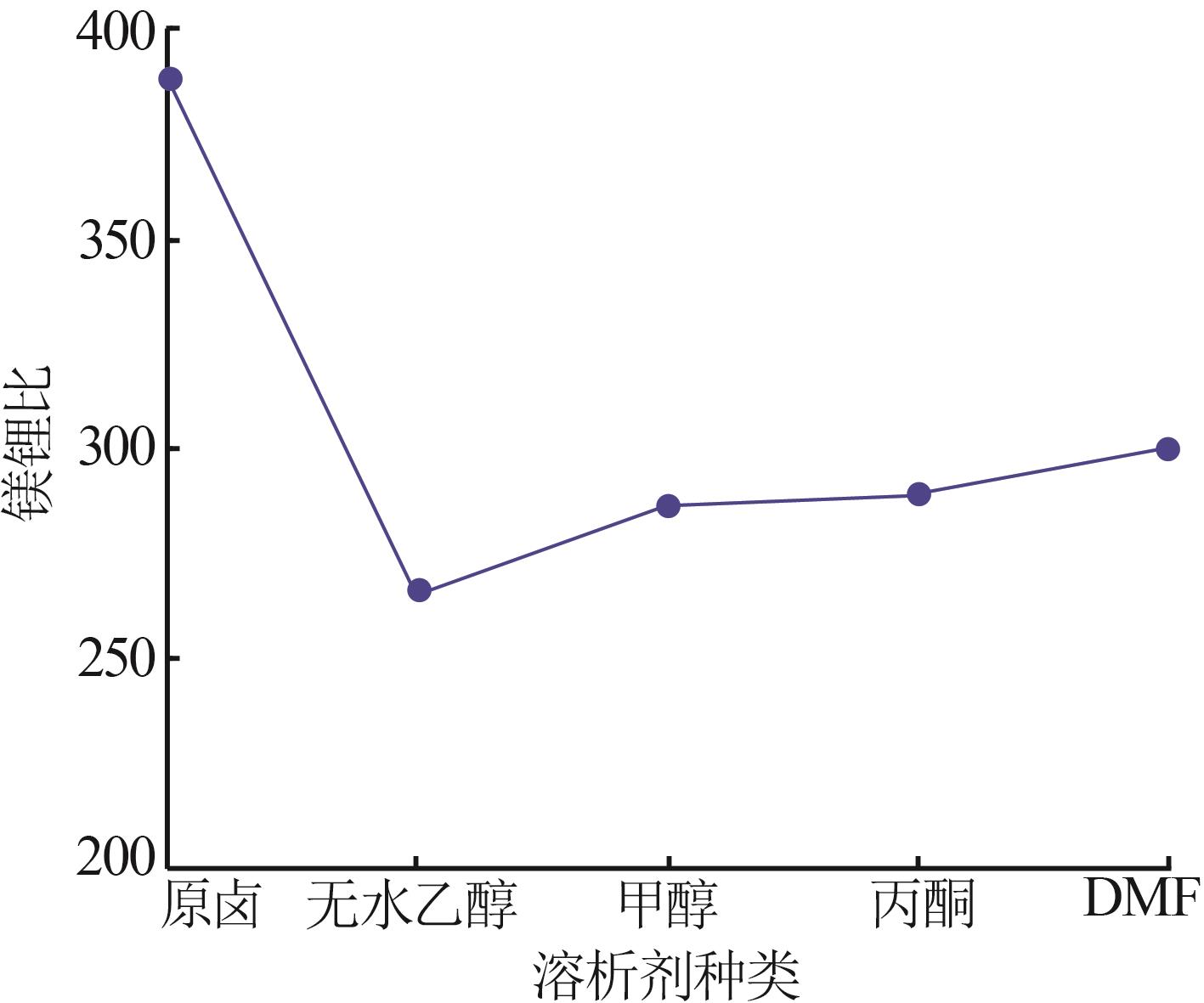
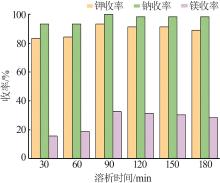
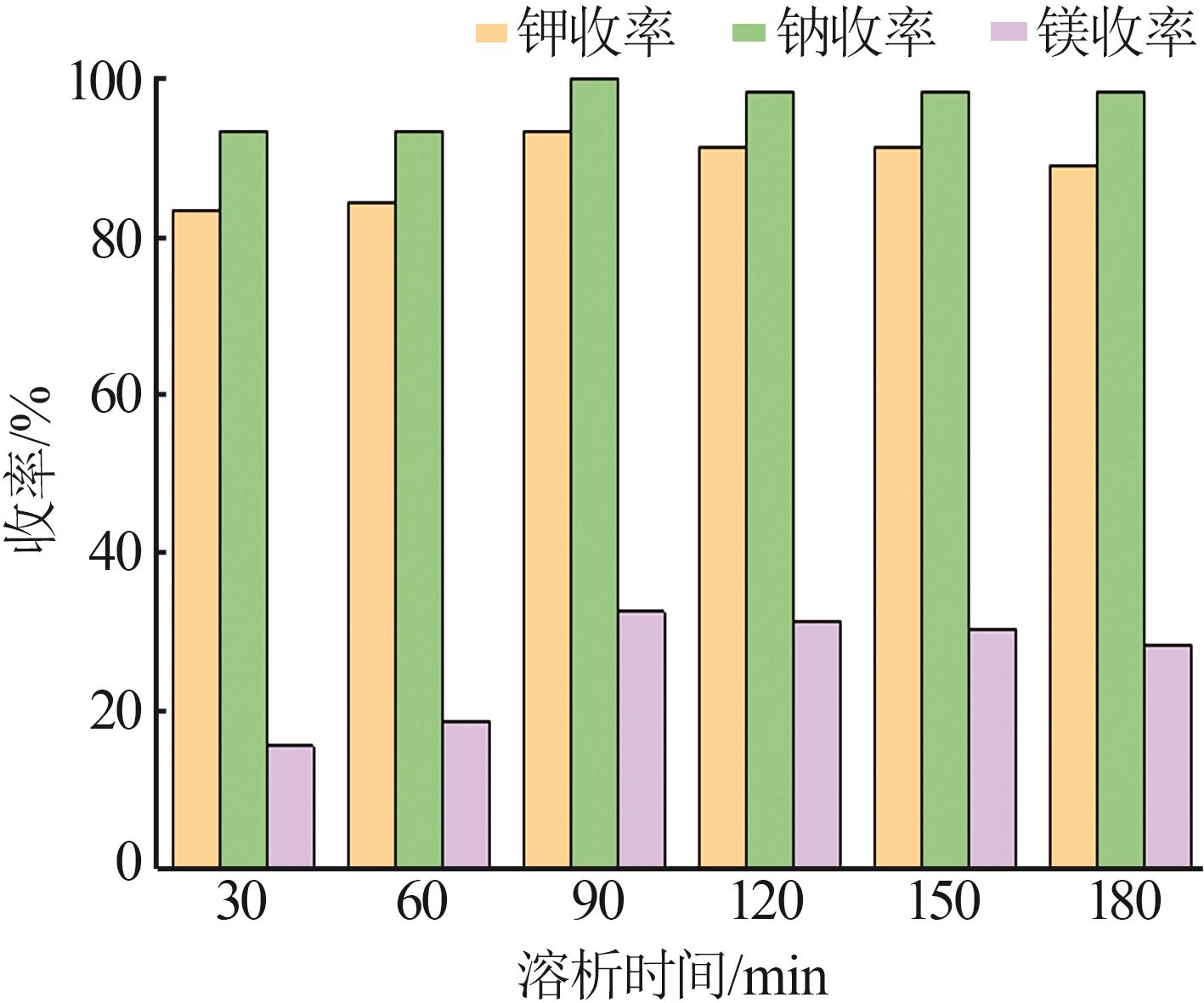
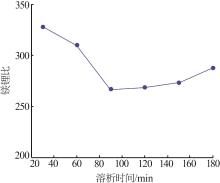
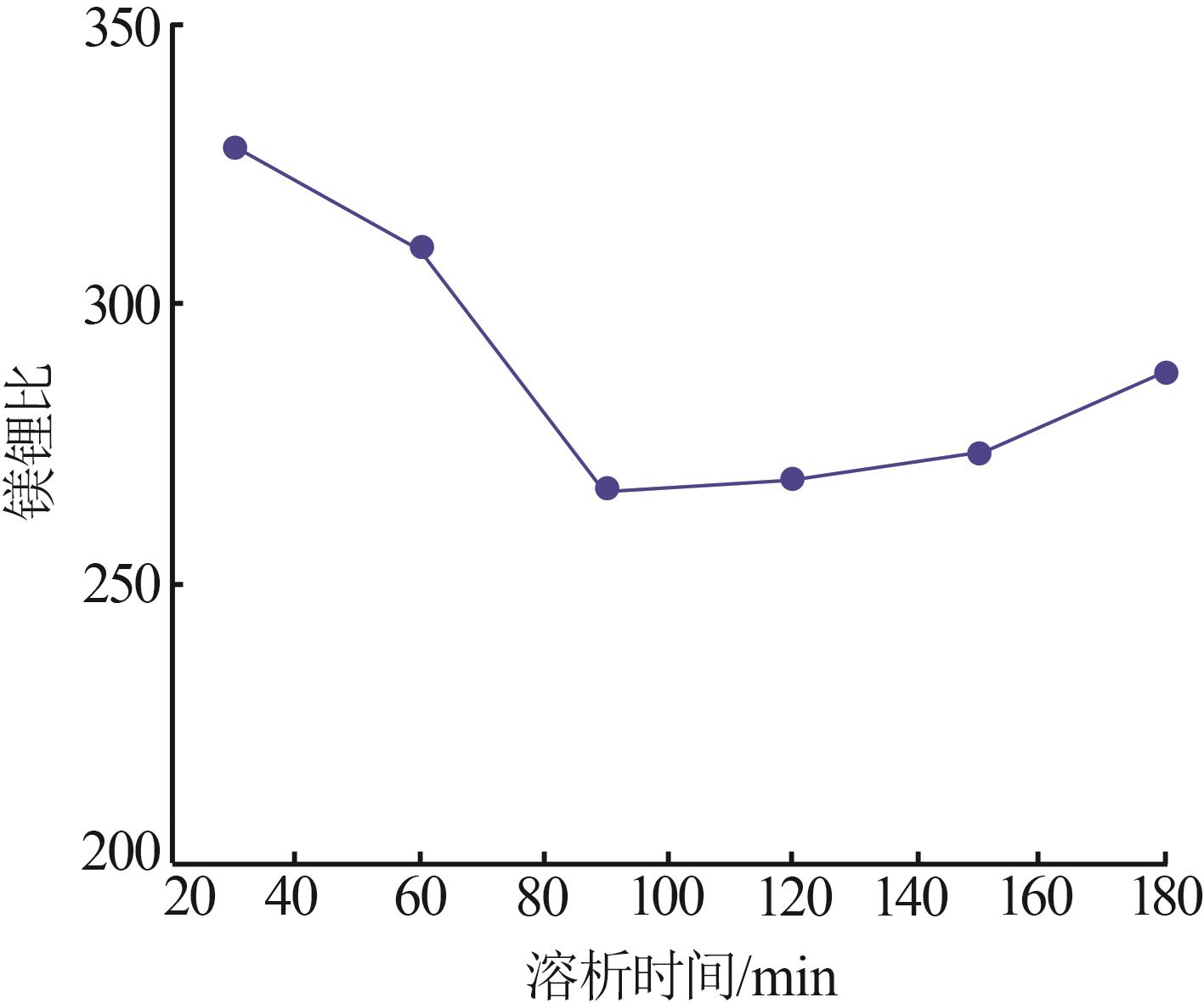
| 1 |
许志琴,朱文斌,郑碧海,等.新能源锂矿战略与大陆动力学研究:纪念南京大学地球科学与工程学院100周年华诞[J].地质学报,2021,95(10):2937-2954.
|
|
XU Zhiqin, ZHU Wenbin, ZHENG Bihai,et al.New energy strategy for lithium resource and the continental dynamics research:Celebrating the centenary of the School of Earth Sciences and Engineering,Nanjing University[J].Acta Geologica Sinica,2021, 95(10):2937-2954.
|
| 2 |
漆贵财,海春喜,周园.锰氧化物锂离子筛型吸附剂提锂进展[J].功能材料,2018,49(11):11023-11032.
|
|
QI Guicai, Chunxi HAI, ZHOU Yuan.Progress of extracting lithium by manganese oxide ion-sieve adsorbent[J].Journal of Functional Materials,2018,49(11):11023-11032.
|
| 3 |
GAO Tianming, FAN Na, CHEN Wu,et al.Lithium extraction from hard rock lithium ores(spodumene,lepidolite,zinnwaldite,petalite):Technology,resources,environment and cost[J].China Geology,2023(6):137-153.
|
| 4 |
HU Bin, SHANG Xiaohong, NIE Pengfei,et al.Lithium ion sieve modified three-dimensional graphene electrode for selective extraction of lithium by capacitive deionization[J].Journal of Colloid and Interface Science,2022,612:392-400.
|
| 5 |
乜贞,伍倩,丁涛,等.中国盐湖卤水提锂产业化技术研究进展[J].无机盐工业,2022,54(10):1-12.
|
|
NIE Zhen, WU Qian, DING Tao,et al.Research progress on industrialization technology of lithium extraction from salt lake brine in China[J].Inorganic Chemicals Industry,2022,54(10):1-12.
|
| 6 |
邢凯,朱清,任军平,等.全球锂资源特征及市场发展态势分析[J].地质通报,2023,42(8):1402-1421.
|
|
XING Kai, ZHU Qing, REN Junping,et al.Research on the characteristics and market development trend of global lithium resourc-es[J].Geological Bulletin of China,2023,42(8):1402-1421.
|
| 7 |
钟财富,刘坚,吕斌,等.我国新能源汽车产业锂资源需求分析及政策建议[J].中国能源,2018,40(10):12-15,24.
|
|
ZHONG Caifu, LIU Jian, Bin LÜ,et al.Demand analysis and policy suggestions for lithium resources in China′s new energy automobile industry[J].Energy of China,2018,40(10):12-15, 24.
|
| 8 |
伍倩,刘喜方,郑绵平,等.我国盐湖锂资源开发现状、存在问题及对策[J].现代化工,2017,37(5):1-5.
|
|
WU Qian, LIU Xifang, ZHENG Mianping,et al.Present situation,existing problems and countermeasures of development of salt lake lithium resources in China[J].Modern Chemical Industry,2017,37(5):1-5.
|
| 9 |
PENG Huawen, ZHAO Qiang.A nano-heterogeneous membrane for efficient separation of lithium from high magnesium/lithium ratio brine[J].Advanced Functional Materials,2021,31(14):2009430.
|
| 10 |
王兴富,王石军,田红斌,等.青海盐湖提钾技术进展与我国钾肥工业的发展[J].化工矿物与加工,2017,46(11):48- 52.
|
|
WANG Xingfu, WANG Shijun, TIAN Hongbin,et al.Potassium extraction technology progress in Qinghai Salt Lake and development of potash fertilizer industry in China[J].Industrial Minerals & Processing,2017,46(11):48-52.
|
| 11 |
吴军.乙醇溶析结晶法从棉籽壳水解母液制备木糖的优化研究[D].天津:天津大学,2010.
|
|
WU Jun.Studies on the optimization of preparation of xylose from the hydrolysis mother liquor of cottonseed hulls by ethanol solventing-out crystallization[D].Tianjin:Tianjin University,2010.
|
| 12 |
刘琴.聚合物包合膜法和溶析结晶法提取锂的研究[D].无锡:江南大学,2012.
|
|
LIU Qin.The study of extraction of lithium with polymer inclusion membrane and drowning-out crystallization methods[D].Wuxi:Jiangnan University,2012.
|
| 13 |
李珍爱,李海军,胡红涛,等.四氢嘧啶溶析结晶工艺的研究[J].现代化工,2022,42(11):207-210.
|
|
LI Zhenai, LI Haijun, HU Hongtao,et al.Study on elution crystallization process of tetrahydropyrimidine[J].Modern Chemical Industry,2022,42(11):207-210.
|
| 14 |
李朝荣,苏殊,许德华,等.磷酸二氢钙在硝酸钙-磷酸-水溶液体系中溶析结晶工艺[J].高校化学工程学报,2021,35(4):608-615.
|
|
LI Chaorong, SU Shu, XU Dehua,et al.Antisolvent crystallization of monocalcium phosphate in calcium nitratephosphoric acid-water solution system[J].Journal of Chemical Engineering of Chinese Universities,2021,35(4):608-615.
|
| 15 |
KSHIRSAGAR S, LAKSHMI R S N, RAMAKRISHNAN S,et al.Process intensification of atorvastatin calcium crystallization for target polymorph development via continuous combined cooling and antisolvent crystallization using an oscillatory baffled crystallizer[J].International Journal of Pharmaceutics,2022,627:122172.
|
| 16 |
NOROUZI M, TAHERNEJAD M, SG-HORBAN H S,et al.Taguchi optimization of solvent-antisolvent crystallization to prepare ammonium perchlorate particles[J].Chemical Engineering & Technology,2020,43(11):2215-2223.
|
| 17 |
SAVVOPOULOS S V, HUSSAIN M N, JORDENS J,et al.A mathematical model of the ultrasound-assisted continuous tubular crystallization of aspirin[J].Crystal Growth & Design,2019,19(9):5111-5122.
|
| 18 |
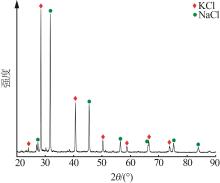
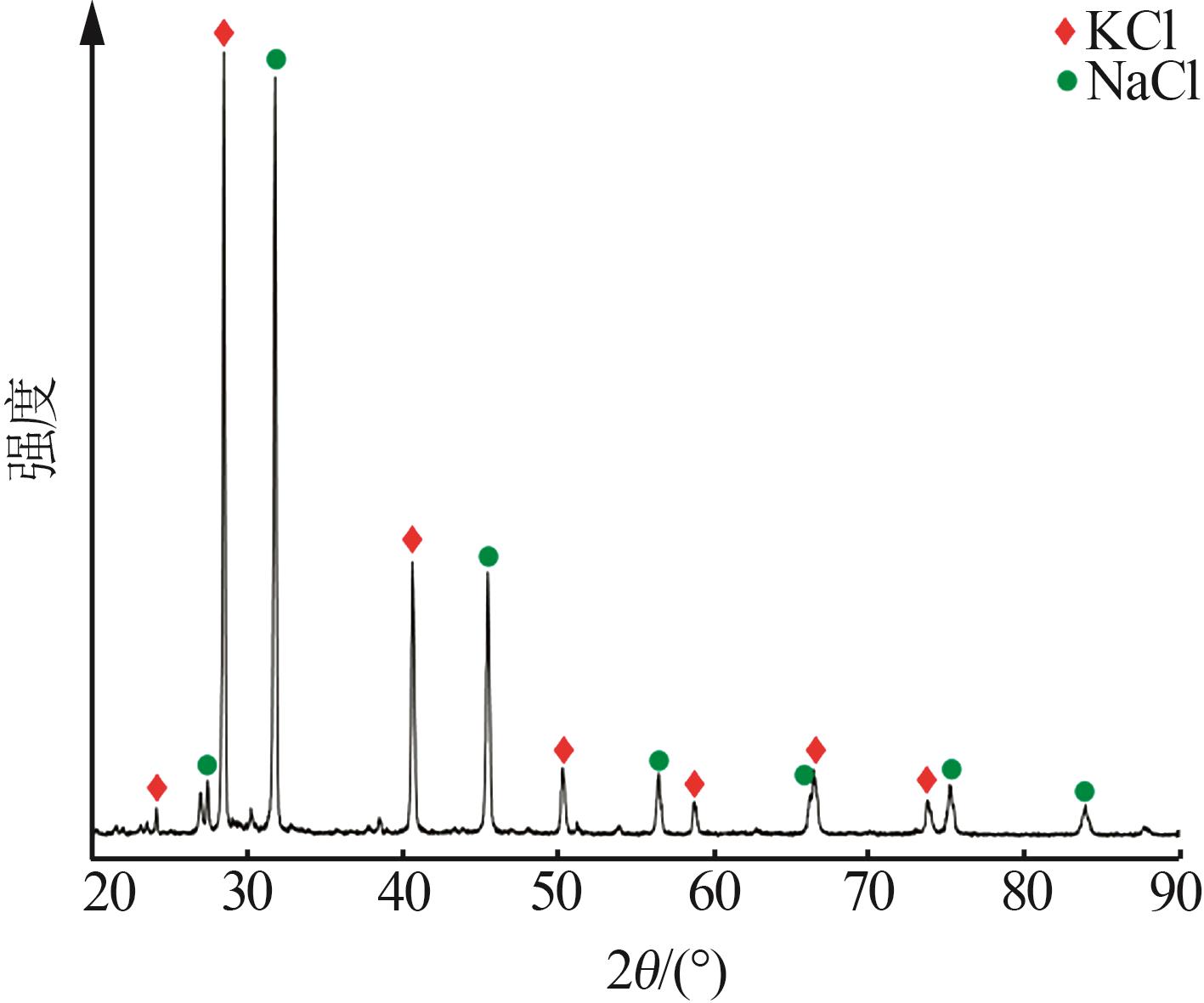
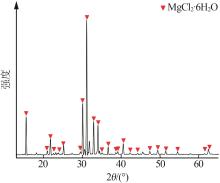
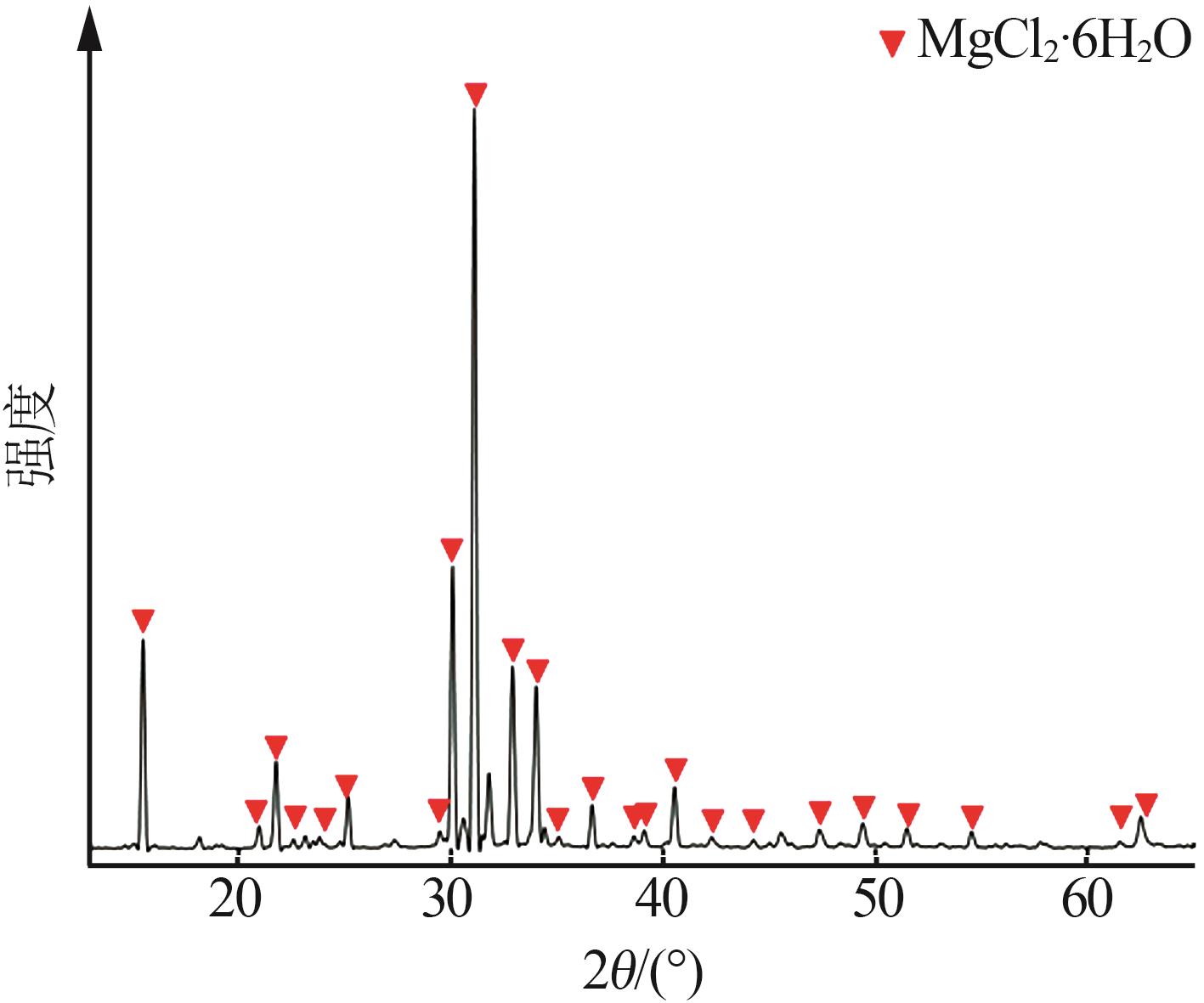
吴然昊,程卓,李春丽,等.溶析结晶制备碳酸锂的实验研究[J].中国井矿盐,2023,54(3):30-32.
|
|
WU Ranhao, CHENG Zhuo, LI Chunli,et al.Experimental study on preparation of lithium carbonate by anti-solvent crystallizati-on[J].China Well and Rock Salt,2023,54(3):30-32.
|
 ), LI Yulong2,3,4, ZHANG Zhiqiang1, LIU Xuejing1
), LI Yulong2,3,4, ZHANG Zhiqiang1, LIU Xuejing1