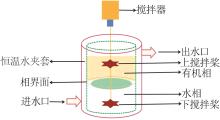
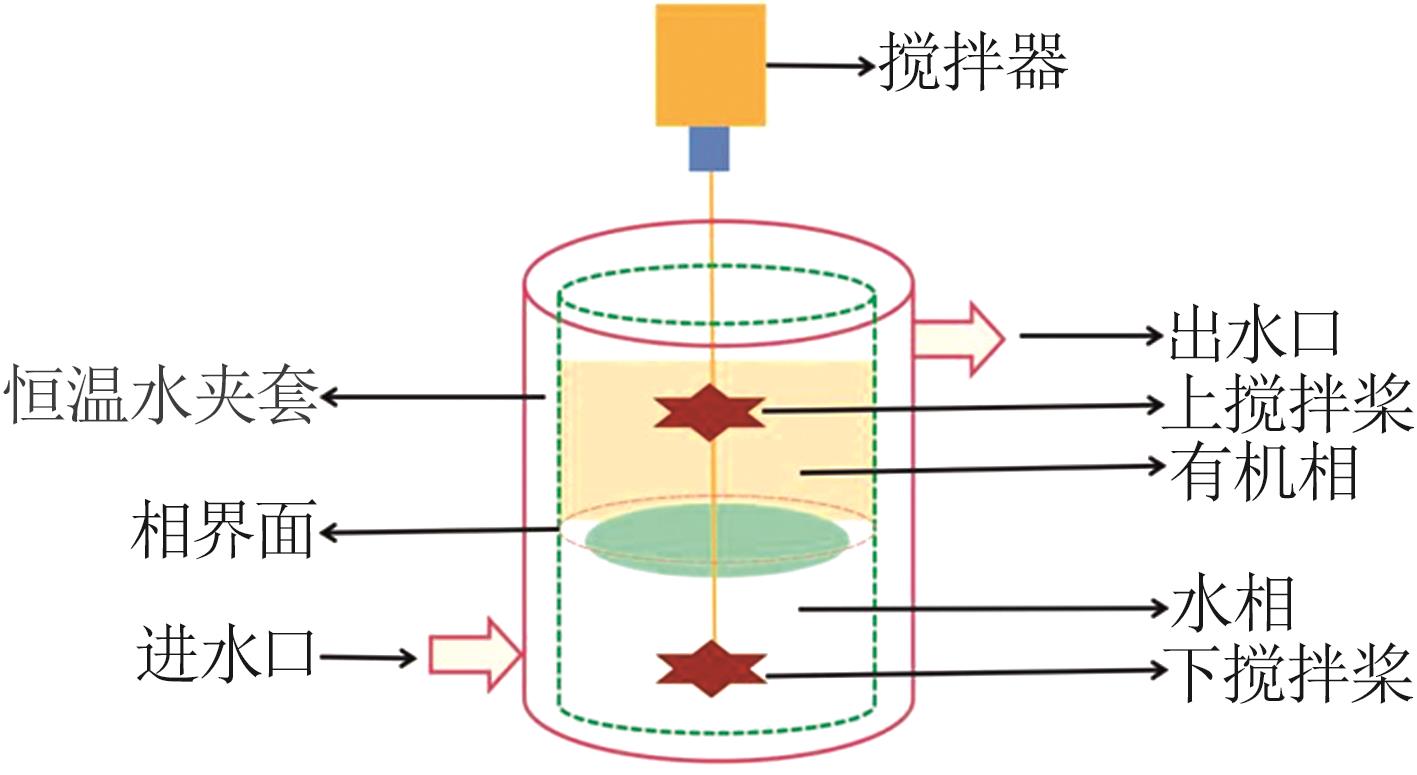
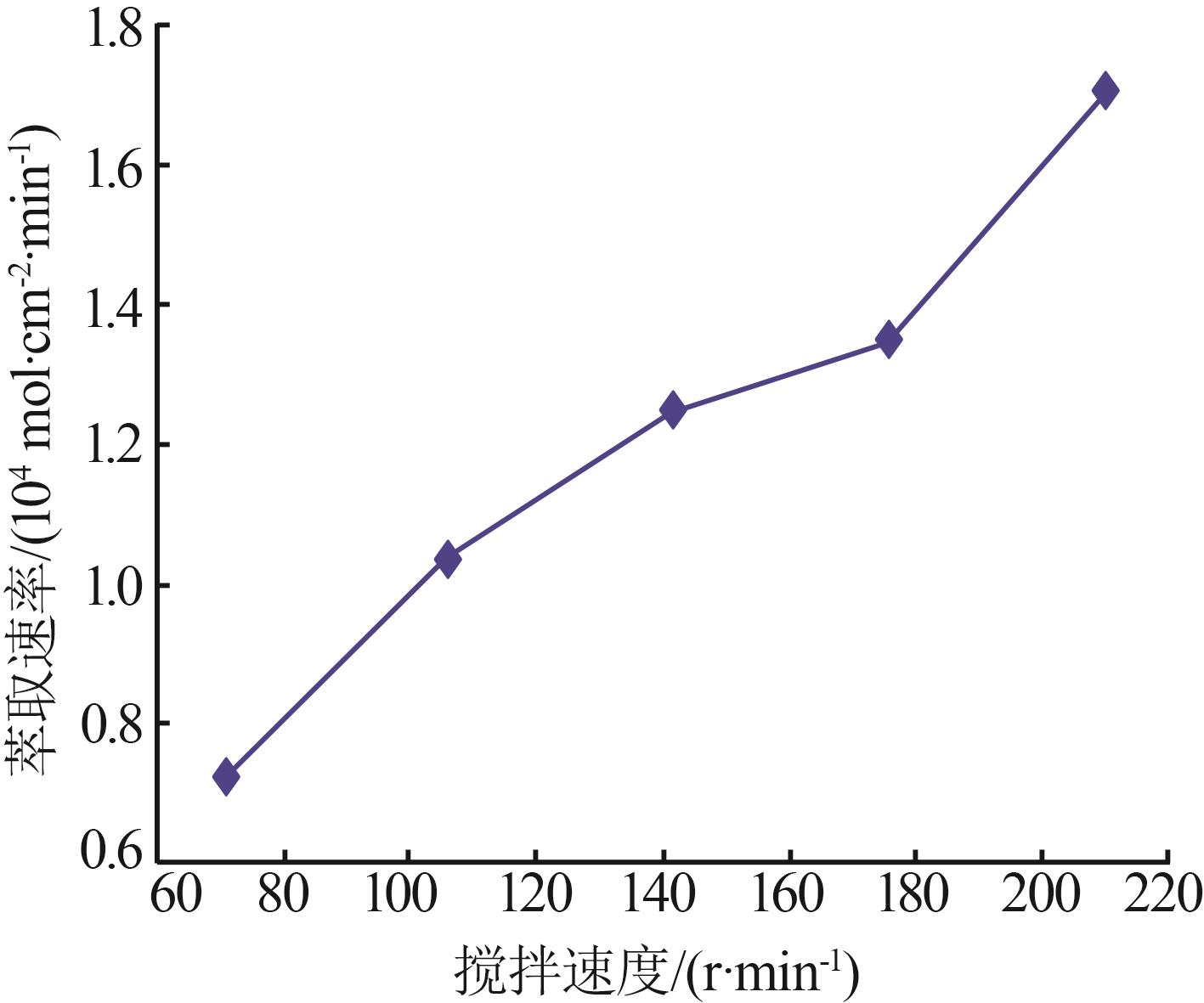
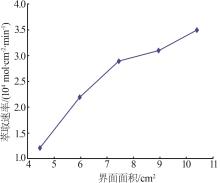
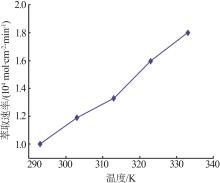
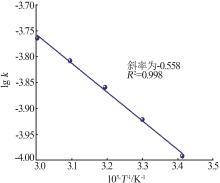
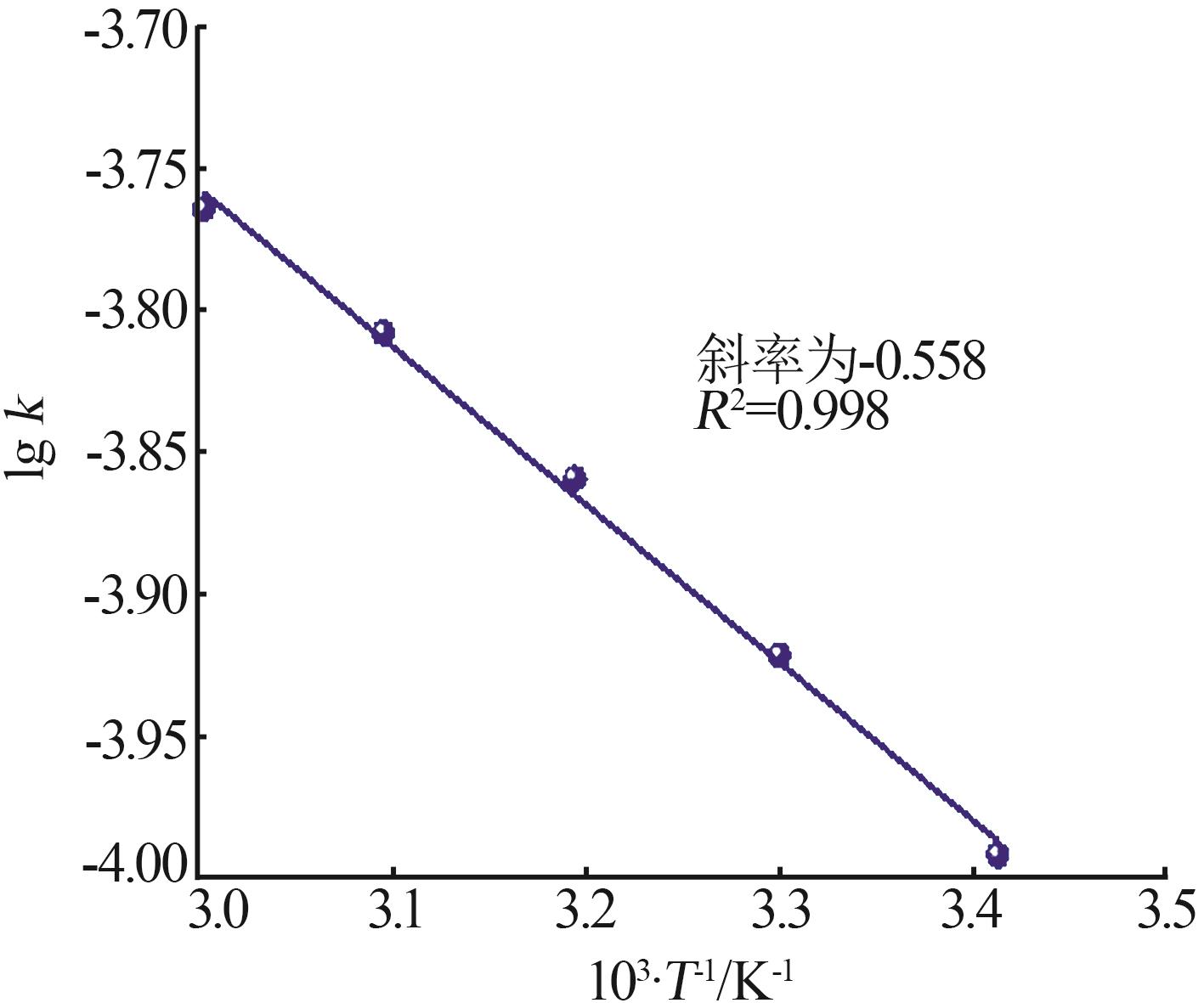
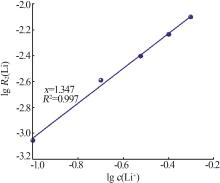
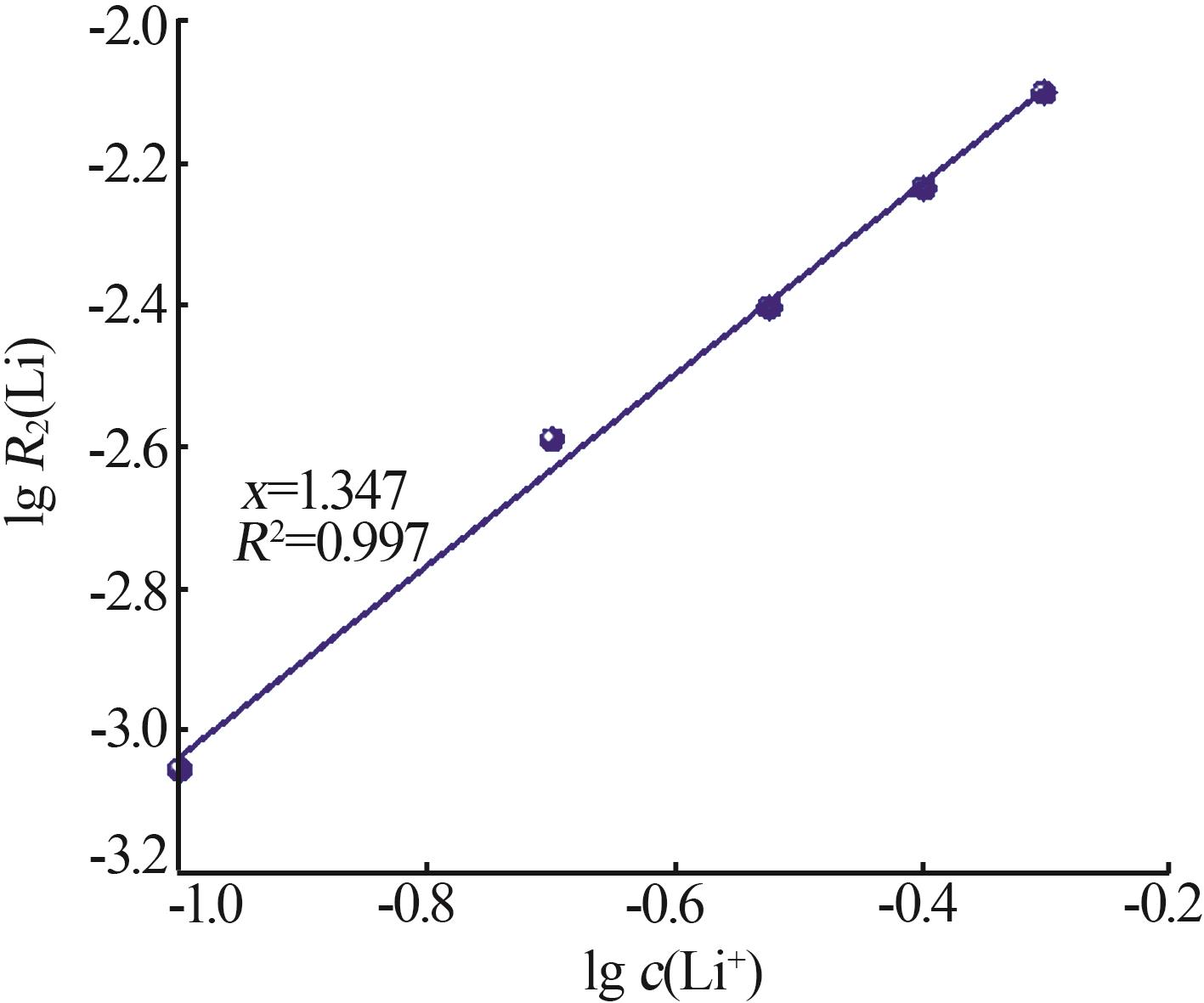
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (9): 60-66.doi: 10.19964/j.issn.1006-4990.2023-0560
• Research & Development • Previous Articles Next Articles
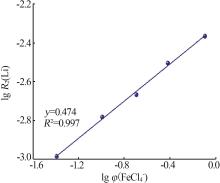
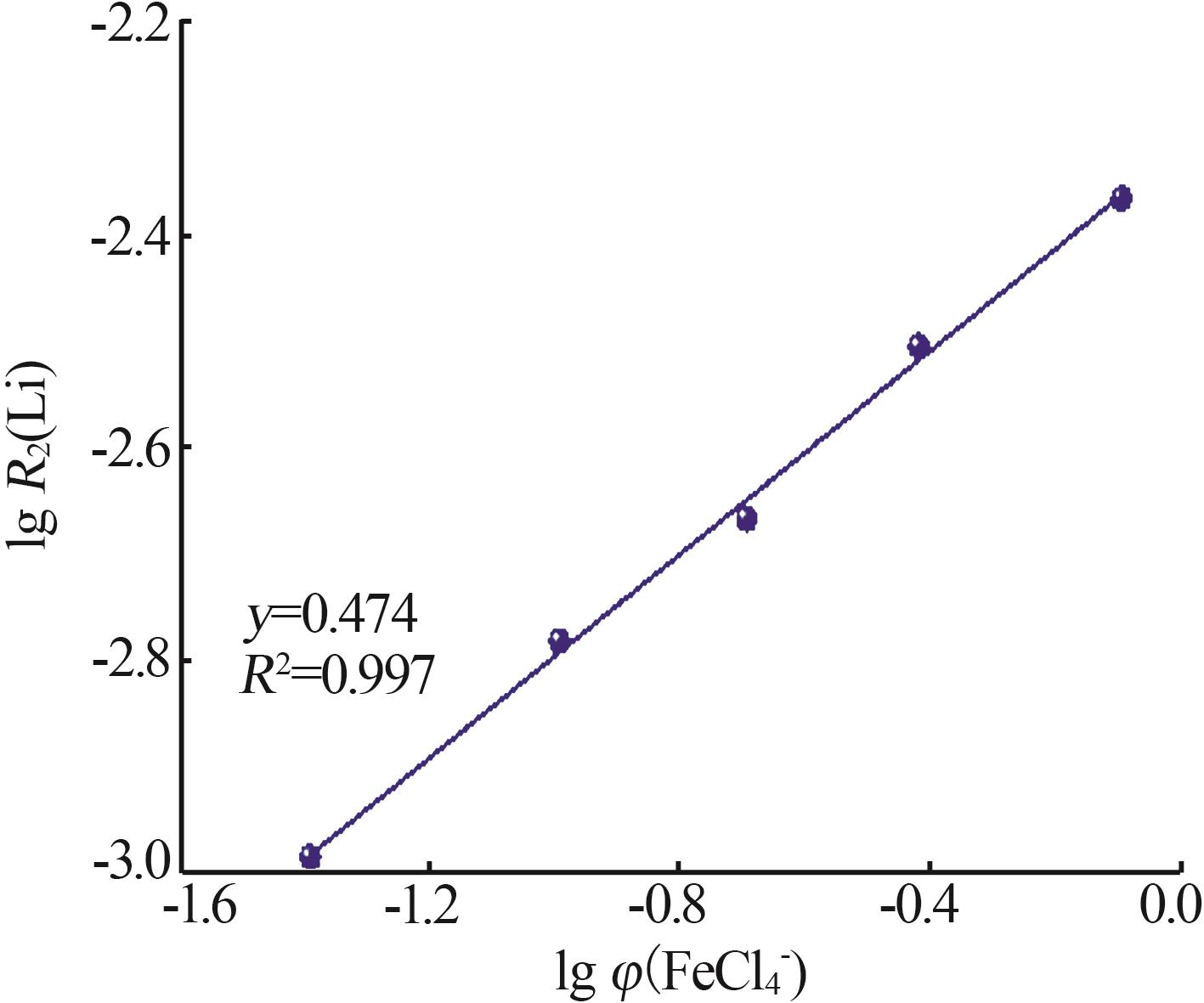
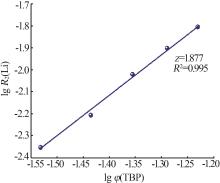
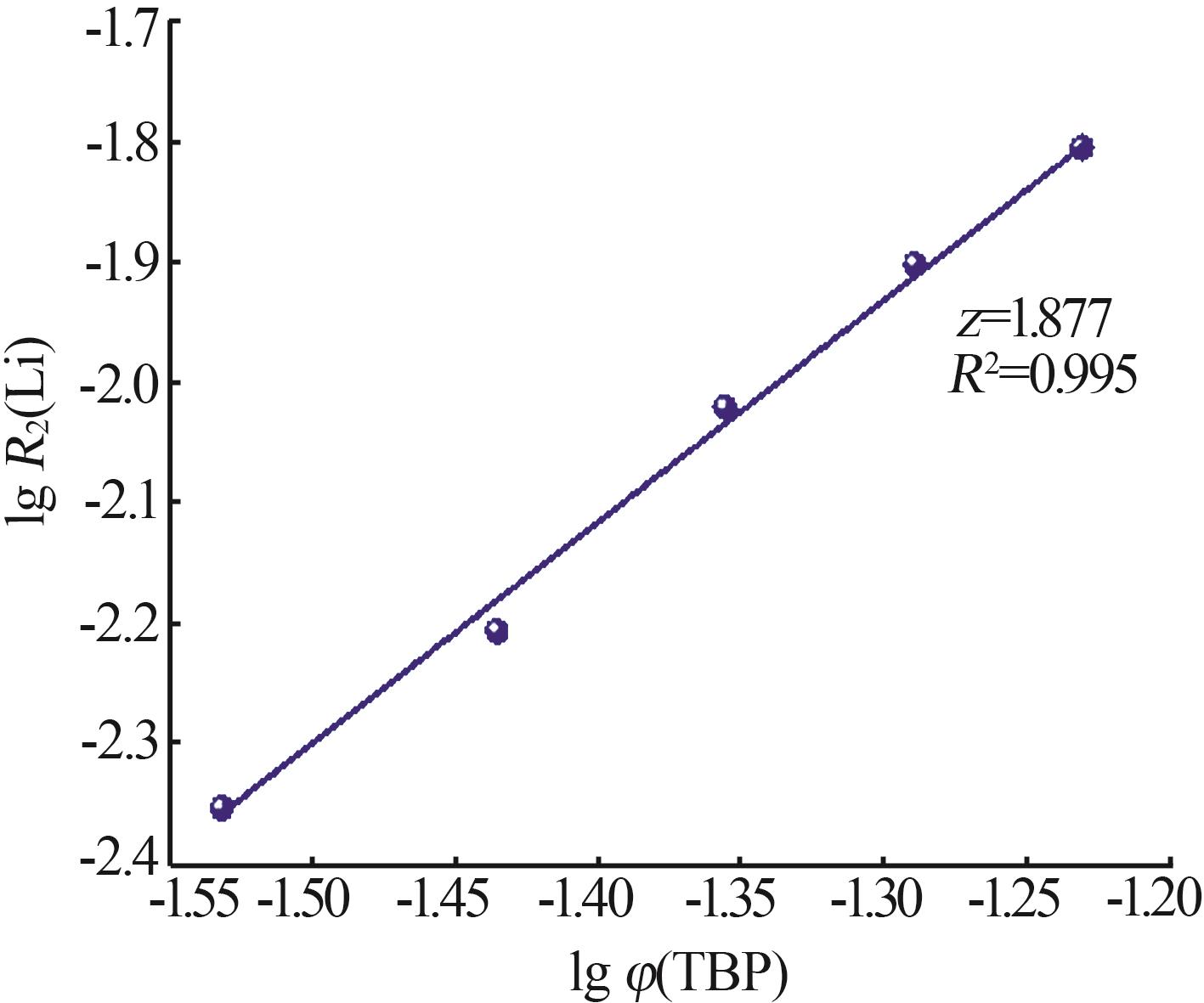
Kinetic study of lithium extraction from solution with iron-based ionic liquid system
MA Shuqing1,2( ), LI Changwen1,2, SHI Chenglong1,2(
), LI Changwen1,2, SHI Chenglong1,2( ), QIN Yaru1,3(
), QIN Yaru1,3( )
)
- 1.School of Chemistry and Chemical Engineering,Qinghai Minzu University,Xining 810007,China
2.Key Laboratory of Resource Chemistry and Eco-environmental Protection in Tibetan Plateau,State Ethnic Affairs Commission,Xining 810007,China
3.College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
-
Received:2023-11-26Online:2024-09-10Published:2024-09-26 -
Contact:SHI Chenglong, QIN Yaru E-mail:18297180853@163.com;qhislscl@126.com;szdxqyr@126.com
CLC Number:
Cite this article
MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru. Kinetic study of lithium extraction from solution with iron-based ionic liquid system[J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66.
share this article
| 1 | 孙佳楠,陈可可,薛立波,等.一种高性能盐湖提锂纳滤膜的制备及研究[J].工业水处理,2023,43(11):120-125. |
| SUN Jianan, CHEN Keke, XUE Libo,et al.Preparation and research of a high performance nanofiltration membrane for lithium extraction from salt lake[J].Industrial Water Treatment,2023,43(11):120-125. | |
| 2 | 康乐,景茂祥,李东红,等.铝酸锂纳米棒改性固态电解质的制备及电化学性能研究[J].无机盐工业,2023,55(8):65-70. |
| KANG Le, JING Maoxiang, LI Donghong,et al.Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte[J].Inorganic Chemicals Industry,2023,55(8):65-70. | |
| 3 | 付煜,邓觅,黄冬根,等.盐湖卤水提锂技术研究进展[J].无机盐工业,2023,55(9):9-16,65. |
| FU Yu, DENG Mi, HUANG Donggen,et al.Research progress of lithium extraction technology from salt lake brine[J].Inorganic Chemicals Industry,2023,55(9):9-16,65. | |
| 4 | 卢娜娜,秦亚茹,马淑清,等.吡啶类离子液体体系对模拟盐湖老卤中锂的萃取研究[J].无机盐工业,2023,55(7):45-50. |
| LU Nana, QIN Yaru, MA Shuqing,et al.Study on extraction of lithium from simulated old brine of salt lake by pyridine ionic liquid system[J].Inorganic Chemicals Industry,2023,55(7):45-50. | |
| 5 | 乔小斌,李直,徐梓馥,等.盐湖提锂副产镁渣的炼镁工艺研究[J].现代化工,2023,43(S2):136-140. |
| QIAO Xiaobin, LI Zhi, XU Zifu,et al.Study on magnesium smelting process of by-product magnesium slag from lithium extraction in salt lake[J].Modern Chemical Industry,2023,43(S2):136-140. | |
| 6 | 李燕,王敏,赵有璟,等.盐湖卤水锂资源提取技术及开发现状[J].盐湖研究,2023,31(2):71-80. |
| LI Yan, WANG Min, ZHAO Youjing,et al.Technology and development of lithium extraction from salt lake brine[J].Journal of Salt Lake Research,2023,31(2):71-80. | |
| 7 | 张婷婷,桑世华,傅超,等.三元体系Li2B4O7-K2B4O7-H2O 273 K稳定相平衡研究[J].化学工程,2017,45(3):37-41. |
| ZHANG Tingting, SANG Shihua, FU Chao,et al.Phase equilibrium in Li2B4O7-K2B4O7-H2O aqueous ternary system at 273 K[J].Chemical Engineering(China),2017,45(3):37-41. | |
| 8 | 陈国蓬,李斌,雷鸣,等.288 K和323 K下三元体系LiBr-Li2SO4-H2O相平衡研究[J].化学工程,2022,50(4):30-34. |
| CHEN Guopeng, LI Bin, LEI Ming,et al.Phase equilibria in ternary system LiBr-Li2SO4-H2O at 288 K and 323 K[J].Chemical Engineering(China),2022,50(4):30-34. | |
| 9 | 毛强,李敬.锰基吸附剂用于新疆某盐湖卤水提锂工艺[J].化学工程,2023,51(6):24-28. |
| MAO Qiang, LI Jing.Lithium extraction process with manganese based adsorbent from a salt lake in Xinjiang[J].Chemical Engineering(China),2023,51(6):24-28. | |
| 10 | 靳佳奇,李岩,林森.盐湖卤水吸附提锂技术研究进展[J].化学工程,2023,51(5):20-25. |
| JIN Jiaqi, LI Yan, LIN Sen.Research progress of lithium extraction from salt lake brine by adsorption[J].Chemical Engineering(China),2023,51(5):20-25. | |
| 11 | 乜贞,伍倩,丁涛,等.中国盐湖卤水提锂产业化技术研究进展[J].无机盐工业,2022,54(10):1-12. |
| NIE Zhen, WU Qian, DING Tao,et al.Research progress on industrialization technology of lithium extraction from salt lake brine in China[J].Inorganic Chemicals Industry,2022,54(10):1-12. | |
| 12 | WANG Yong, LIU Haotian, FAN Jiahui,et al.Recovery of lithium ions from salt lake brine with a high magnesium/lithium ratio using heteropolyacid ionic liquid[J].ACS Sustainable Chemistry & Engineering,2019,7(3):3062-3072. |
| 13 | CAI Chunqing, HANADA T, FAJAR A T N,et al.An ionic liquid extractant dissolved in an ionic liquid diluent for selective extraction of Li(I) from salt lakes[J].Desalination,2021,509:115073. |
| 14 | LI Rujie, WANG Wanhang, WANG Yangyang,et al.Novel ionic liquid as co-extractant for selective extraction of lithium ions from salt lake brines with high Mg/Li ratio[J].Separation and Purification Technology,2021,277:119471. |
| 15 | ZHENG Guangya, XIA Jupei, LIU Chenglong,et al.Kinetics of aluminum extraction from aluminum ash by leaching with sulfuric acid[J].Phosphorus,Sulfur,and Silicon and the Related Elements,2020,195(7):536-543. |
| 16 | SONG Qiang, ZHANG Wenjie, TONG Xiong,et al.Extraction kinetics of lanthanum and cerium in bis(2-ethylhexyl) phosphate (HDEHP)-lactic acid complex system using Lewis cell[J].Transactions of Nonferrous Metals Society of China,2023,33(6):1943-1952. |
| 17 | HE Jingui, LI Yong, TAO Wenju,et al.Study on reduction stripping kinetics of Ce4+ using a constant interfacial area cell with laminar flow[J].Metals,2022,12(4):664. |
| 18 | SONG Lianjun, LIU Ying, DING Songdong,et al.Extraction kinetics of uranium(Ⅵ) and thorium(Ⅳ) with di(1-methyl-heptyl)methyl phosphonate from nitric acid medium using a Lewis cell[J].Separation and Purification Technology,2019,217:258-264. |
| 19 | 余静,姜德彬,郭俊元,等.应用恒界面池研究反胶团萃取废水中锌的动力学[J].环境科学学报,2015,35(8):2435-2441. |
| YU Jing, JIANG Debin, GUO Junyuan,et al.Kinetics of reverse micelles extraction of zinc from wastewater using lewis cell[J].Acta Scientiae Circumstantiae,2015,35(8):2435-2441. | |
| 20 | YANG Xiuli, ZHANG Junwei, FANG Xihui.Extraction kinetics of niobium by tertiary amine N235 using Lewis cell[J].Hydrometallurgy,2015,151:56-61. |
| 21 | 彭小五,李丽娟,时东,等.2-丁基-1-正辛醇萃取硼酸的动力学研究[J].盐湖研究,2020,28(3):79-84. |
| PENG Xiaowu, LI Lijuan, SHI Dong,et al.Kinetics of extraction of boric acid with 2-butyl-1-n-octanol[J].Journal of Salt Lake Research,2020,28(3):79-84. | |
| 22 | ZHANG Wenjie, XIE Xian, TONG Xiong,et al.Extraction kinetics of lanthanum(Ⅲ) by bis(2-ethylhexyl) phosphate and dimethylheptyl methyl phosphate in the presence of sulfonated kerosene using a Lewis cell[J].Chemical Physics Letters,2021,778:138744. |
| 23 | YIN Shaohua, LI Shiwei, ZHANG Bo,et al.Mass transfer kinetics of lanthanum(Ⅲ) extraction in the presence of two complexing agents by D2EHPA using a constant interfacial area cell with laminar flow[J].Chemical Engineering Research and Design,2015,104:92-97. |
| 24 | WANG Liangshi, YU Ying, HUANG Xiaowei,et al.Thermodynamics and kinetics of thorium extraction from sulfuric acid medium by HEH(EHP)[J].Hydrometallurgy,2014,150:167-172. |
| 25 | WU Dongbei, WANG Xianglan, LI Deqian.Extraction kinetics of Sc(Ⅲ),Y(Ⅲ),La(Ⅲ) and Gd(Ⅲ) from chloride medium by Cyanex 302 in heptane using the constant interfacial cell with laminar flow[J].Chemical Engineering and Processing:Process Intensification,2007,46(1):17-24. |
| 26 | 王兴权.疏水性离子液体萃取体系的构建及其对盐湖卤水中锂的分离研究[D].西宁:中国科学院大学(中国科学院青海盐湖研究所),2018. |
| WANG Xingquan.Fabriction of hydrophobic ionic liquid extraction system and its applications in separation of lithium form salt lake brines[D].Xining:Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,2018. | |
| 27 | LI Huifang, LI Lijuan, SHI Dong,et al.Kinetics of lithium extraction with tri-n-butyl phosphate from salt lake brine by a single drop method[J].Chemistry Letters,2015,44(7):987-988. |
| 28 | LI Huifang, LI Lijuan, LI Wu,et al.The kinetics and thermodynamics investigation of lithium extraction from brine by TBP using Lewis cell technique[J].Chemical Physics Letters,2020,754:137692. |
| [1] | ZENG Yijun, JIANG Ziwen, JIAN Chengzong, QUAN Xuejun. Study on deep extraction of chromium from calcium-free roasting slag of chromite ore [J]. Inorganic Chemicals Industry, 2025, 57(1): 90-96. |
| [2] | FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui. Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid [J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43. |
| [3] | ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. |
| [4] | CHENG Ziyang, CHEN Guofu. Early hydration kinetics research of nano-SiO2 and cement composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 80-87. |
| [5] | ZHANG Yu, ZHAO Guiyan, TIAN Yongchang, QIU Xiaokui, SUN Jiali, XU Lixin. Reaction kinetics of ethylenediamine hydrochloride with calcium hydroxide [J]. Inorganic Chemicals Industry, 2024, 56(5): 64-69. |
| [6] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [7] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [8] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [9] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [10] | ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction [J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84. |
| [11] | DING Ning, ZHANG Jian, PING Qingwei, SHENG Xueru, LI Na. Study on adsorption and release properties of matrine by magnesium-modified diatomite [J]. Inorganic Chemicals Industry, 2023, 55(11): 37-46. |
| [12] | LI Xiyan, ZHANG Hong, LIU Xuejing, YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen. Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere [J]. Inorganic Chemicals Industry, 2023, 55(10): 50-55. |
| [13] | PENG Jiaoyu, TAN Yuqin, YANG Keli, DONG Yaping, ZHANG Bo, LI Wu. Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake [J]. Inorganic Chemicals Industry, 2023, 55(10): 56-62. |
| [14] | WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun. Study on decomposition process and thermal decomposition kinetics of ammonium sulfate [J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92. |
| [15] | ZHU Yue,QIU Shengbo,LIU Chenglin,YU Jianguo. Study on enhancement of spodumene phase reconstruction process by mechanical activation [J]. Inorganic Chemicals Industry, 2023, 55(1): 81-86. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||