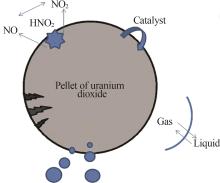
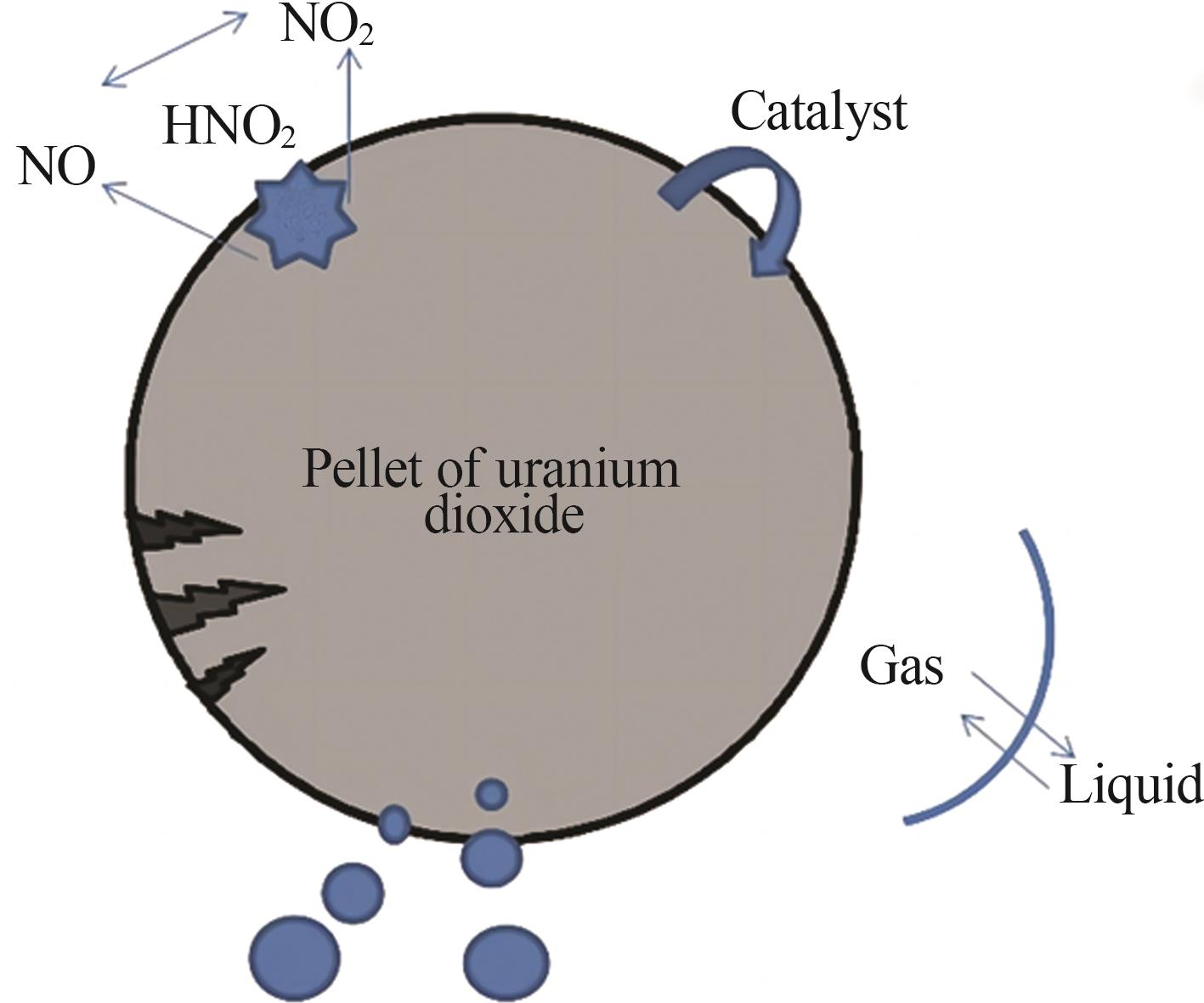
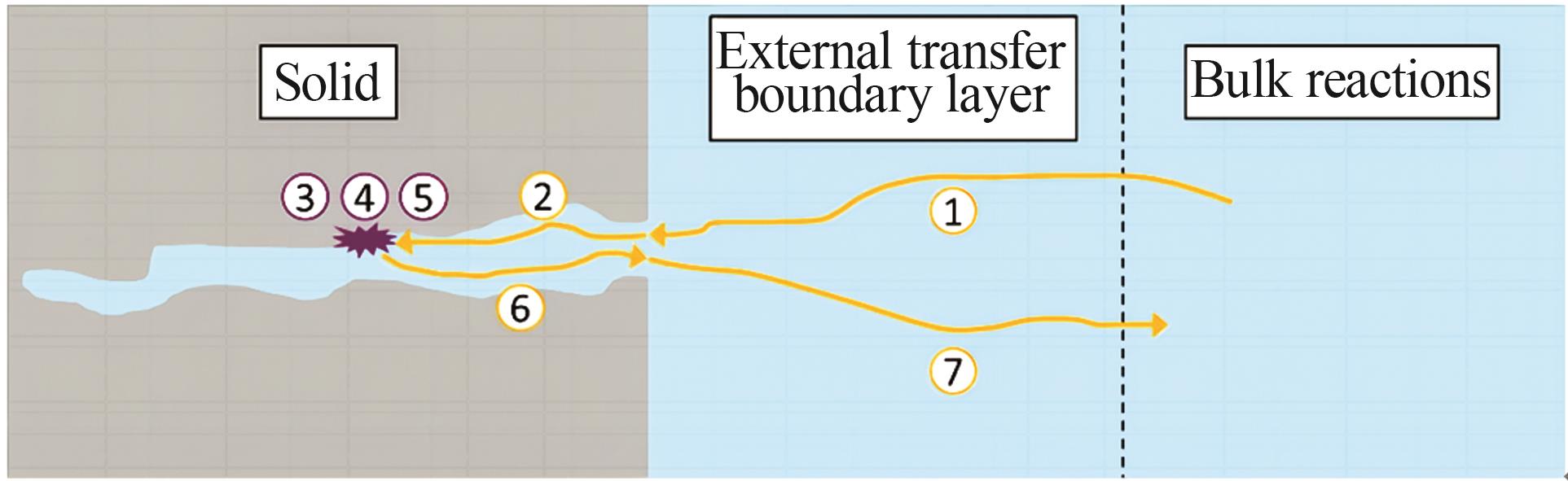
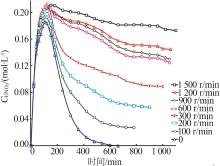
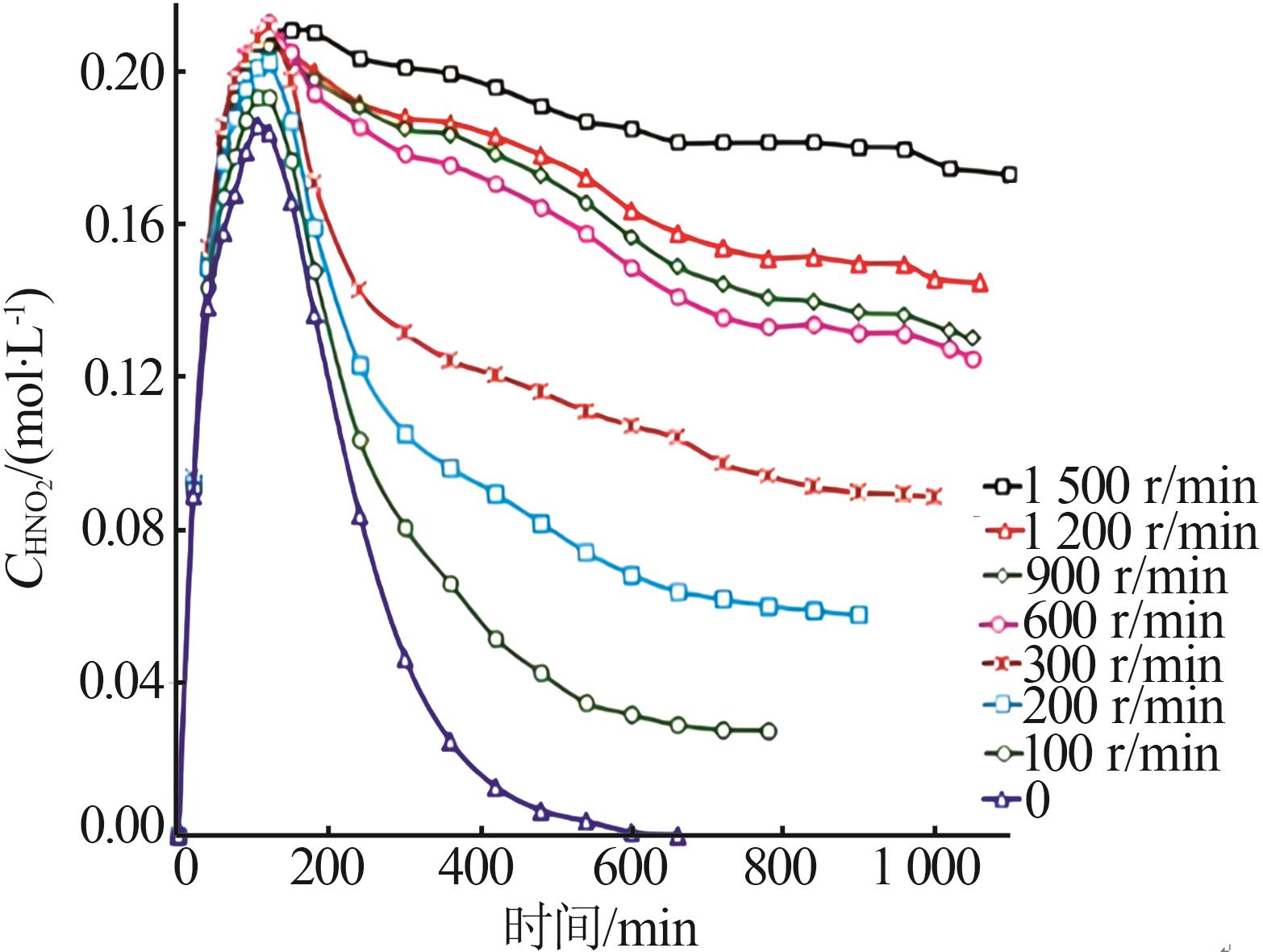
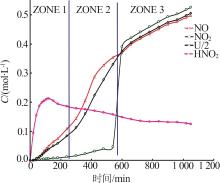
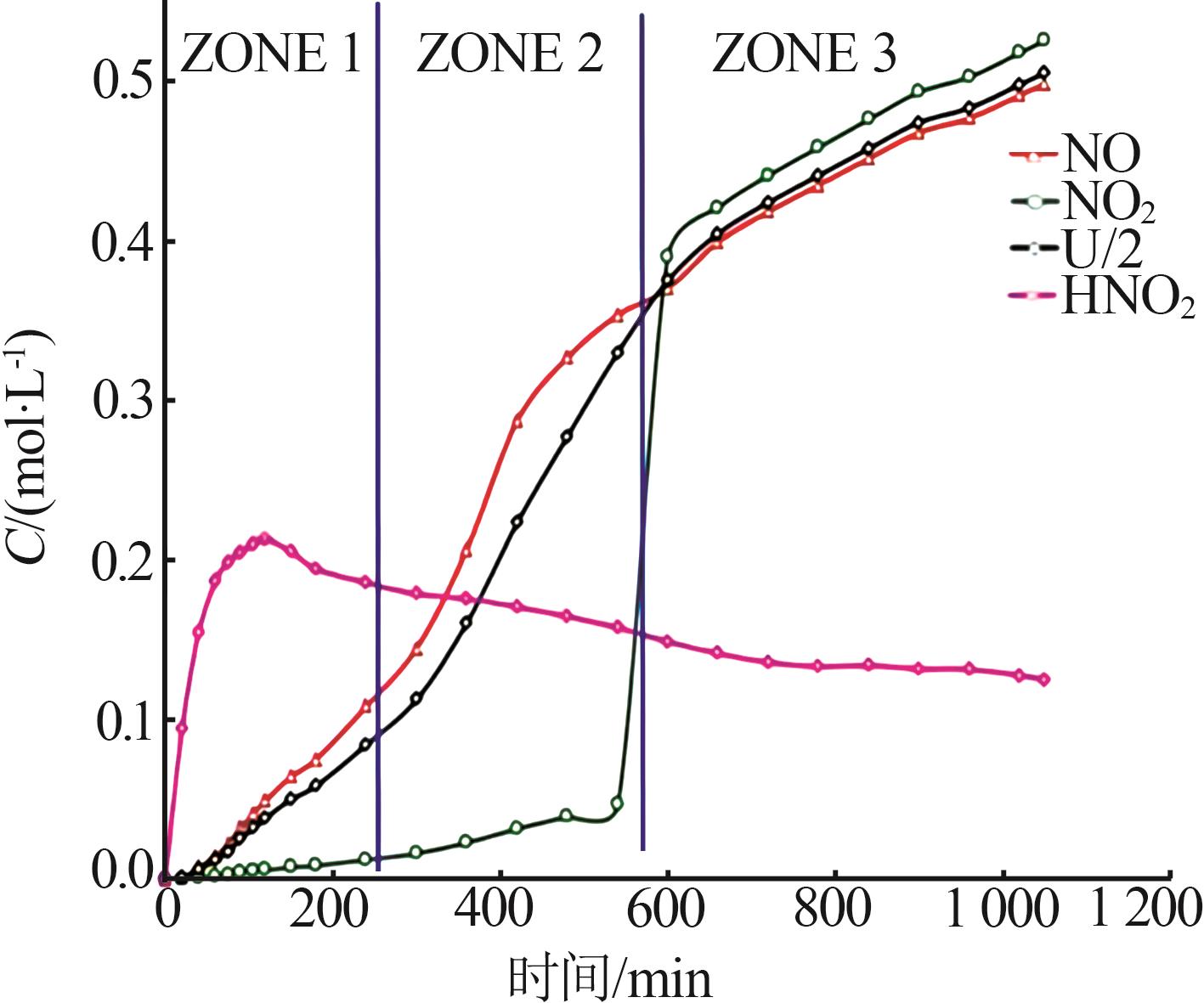
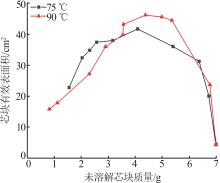
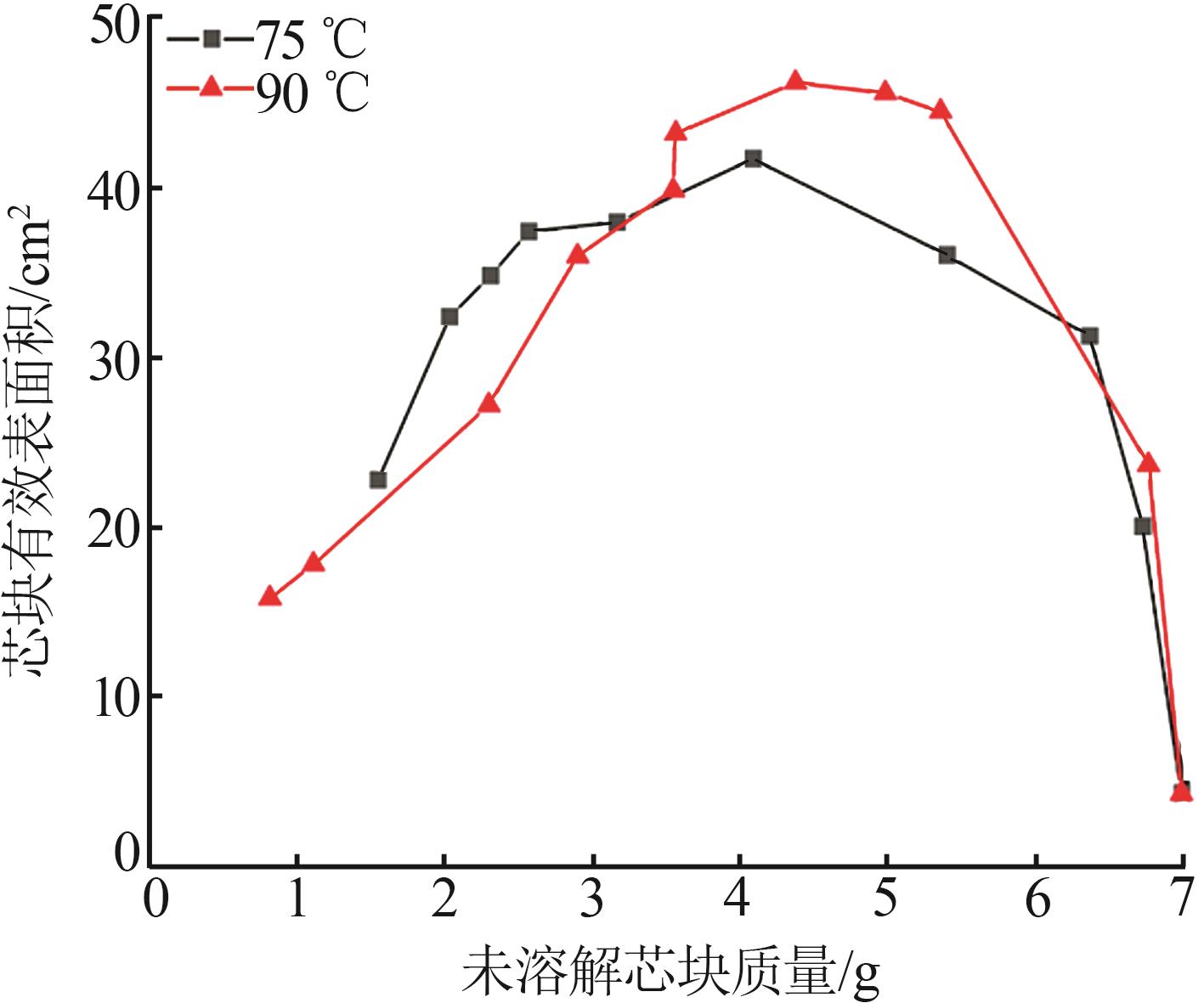
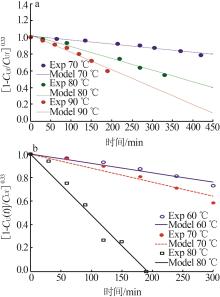
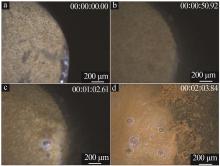
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (9): 34-43.doi: 10.19964/j.issn.1006-4990.2024-0030
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid
FANG Fan( ), YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui(
), YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui( )
)
- Department of Radiochemistry,China Institute of Atomic Energy,Beijing 102413,China
-
Received:2024-01-15Online:2024-09-10Published:2024-09-26 -
Contact:HE Hui E-mail:fan.fang001outlook.com;hehui401@126.com
CLC Number:
Cite this article
FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui. Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid[J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43.
share this article
Table 3
Quantity of NO x gas generated during dissolution of 1 mol UO2 under different concentrations of nitric acid[25]"
芯块 质量/g | c(HNO3)/ (mol·L-1) | 溶解1mol UO2产生NO x 的气体量/mol | ||
|---|---|---|---|---|
| n(NO x ) | n(NO) | n(NO2) | ||
| 1.019 5 | 3.4 | 0.71 | 0.64 | 0.07 |
| 1.005 0 | 4.5 | 0.70 | 0.62 | 0.08 |
| 0.990 4 | 6.7 | 0.74 | 0.61 | 0.13 |
| 1.005 7 | 8.1 | 0.98 | 0.54 | 0.43 |
| 0.940 6 | 12.5 | 1.42 | 0.36 | 1.06 |
Table 4
Activation energy and order of reaction under different experimental conditions"
| UO2形态 | 活化能/ (kJ·mol-1) | 级数 | c(HNO3)/ (mol·L-1) |
|---|---|---|---|
| 芯块[ | 47.99 | 2.27 | 3~10 |
| 芯块[ | 2.3 | 2~10 | |
| 粉末[ | 30.67 | 1.21 | 0.5~2 |
| 芯块[ | 107.11a | 3.3 | 4~10 |
| 粉末[ | 70.88 | 1.9 | 4~10 |
| 芯块[ | 3.2 | 7.3 | |
| 粉末[ | 59.50 | 2.74 | 6~9.5 |
| 芯块[ | 59.71 | 2.13 | 5 |
| 粉末[ | 73.20 | 1.58 | 4~8 |
| 芯块[ | 24.35 | 4.44 | 0.1~4 |
| 1 | MONDAL S, DAS S N, SIVAKUMAR D,et al.Determination of concentration of formaldehyde in high level waste during the destruction of nitric acid in PUREX process[J].Journal of Radioanalytical and Nuclear Chemistry,2024,333(2):971-977. |
| 2 | 伍思达,林如山,张磊,等.干法后处理废盐中活泼裂片元素的净化工艺研究进展[J].无机盐工业,2022,54(4):81-87. |
| WU Sida, LIN Rushan, ZHANG Lei,et al.Research progress on purification process for active crack elements in waste salt by dry post-treatment[J].Inorganic Chemicals Industry,2022,54(4):81-87. | |
| 3 | 衣峰,周文涛,王德忠.乏燃料熔盐电解后处理动力学模型研究[J].无机盐工业,2022,54(11):45-51. |
| YI Feng, ZHOU Wentao, WANG Dezhong.Study on kinetic model of electrorefining reprocessing of used nuclear fuel molten salt[J].Inorganic Chemicals Industry,2022,54(11):45-51. | |
| 4 | GEORGE K, MASTERS A J, LIVENS F R,et al.A review of technetium and zirconium extraction into tributyl phosphate in the PUREX process[J].Hydrometallurgy,2022,211:105892. |
| 5 | MISHRA S, ANAND P V, PATRA C,et al.Solvent wash studies for the removal of di-butyl phosphate from spent solvent under simulated PUREX condition[J].Journal of Radioanalytical and Nuclear Chemistry,2023,332(2):343-353. |
| 6 | MISHRA S, RAMA SWAMI K, RAJESH P,et al.Effect of di-butyl phosphate on the distribution behavior of uranyl ions in PUREX solvent[J].Separation Science and Technology,2024,59(1):112-121. |
| 7 | 叶国安,郑卫芳,何辉,等.我国核燃料后处理技术现状和发展[J].原子能科学技术,2020,54(S1):75-83. |
| YE Guoan, ZHENG Weifang, HE Hui,et al.Present situation and development of nuclear fuel reprocessing technology in China[J].Atomic Energy Science and Technology,2020,54(S1):75-83. | |
| 8 | YAN Ruihan, GAO Yang, ZHOU Yu,et al.An integrated model combining mass transfer and chemical reaction for co-decontamination extraction step of PUREX process in a pulsed extraction column[J].Solvent Extraction and Ion Exchange,2023,41(7):905-939. |
| 9 | KIM Y H, CHO Y Z.Analyzing design considerations for disassembly of spent nuclear fuel during head-end process of pyroprocessing[J].Science and Technology of Nuclear Installations,2020,2020:8868444. |
| 10 | TAYLOR R, MATHERS G, BANFORD A.The development of future options for aqueous recycling of spent nuclear fuels[J].Progress in Nuclear Energy,2023,164:104837. |
| 11 | GONDA K,OKA K, NEMOTO T.Characteristics and behavior of emulsion at nuclear fuel reprocessing[J].Nuclear Technology,1982,57(2):192-202. |
| 12 | GONDA K,OKA K, HAYASHI K.Nonsoluble fission product residues,crud,and fine chips of zircaloy cladding in headend process of nuclear fuel reprocessing[J].Nuclear Technology,1984,65(1):102-108. |
| 13 | ADACHI T, OHNUKI M, YOSHIDA N,et al.Dissolution study of spent PWR fuel:Dissolution behavior and chemical properties of insoluble residues[J].Journal of Nuclear Materials,1990,174(1):60-71. |
| 14 | BURAKOV B E, POKHITONOV Y A, RYAZANTSEV V I,et al.Determination of the weight and composition of the precipitates formed in the course of dissolution of irradiated WWER oxide fuel[J].Radiochemistry,2010,52(4):403-407. |
| 15 | LIU F, YAN T H, LI B,et al.Dissolution behavior of irradiated fuels in nitric acid and characteristics of insoluble residue[J].Journal of Radioanalytical and Nuclear Chemistry,2020,326(1):337-341. |
| 16 | LIU Fang, YAN Taihong, LI Bin,et al.The formation and composition of secondary precipitate in course of spent fuel dissoluti-on[J].Journal of Nuclear Science and Technology,2021,58(3):315-321. |
| 17 | POINSSOT C, ROSTAING C, GREANDJEAN S,et al.Recycling the actinides,the cornerstone of any sustainable nuclear fuel cycles[J].Procedia Chemistry,2012,7:349-357. |
| 18 | NATARAJAN R,RAJ B.Technology development of fast reactor fuel reprocessing in India[J].Current Science,2015,108:30-38. |
| 19 | DESIGAN N, MAJI D, ANANTHASIVAN K,et al.Dissolution behaviour of simulated MOX nuclear fuel pellets in nitric acid medium[J].Progress in Nuclear Energy,2019,116:1-9. |
| 20 | PIERCE E M, ICENHOWER J P, SERNE R J,et al.Experimental determination of UO2(cr) dissolution kinetics:Effects of solution saturation state and pH[J].Journal of Nuclear Materials,2005,345(2/3):206-218. |
| 21 | AMME M, SVEDKAUSKAITE J, BORS W,et al.A kinetic study of UO2 dissolution and H2O2 stability in the presence of groundwater ions[J].Radiochimica Acta,2007,95(12):683-692. |
| 22 | HERRMANN B.Dissolution of unirradiated UO2-pellets in nitric acid[R].Germany:Kernforschungszentrum Karlsruhe GmbH,1984. |
| 23 | NISHIMURA K, CHIKAZAWA T, HASEGAWA S,et al.Effect of nitrous acid on dissolution of UO2 powders in nitric acid optimal conditions for dissolving UO2 [J].Journal of Nuclear Science and Technology,1995,32(2):157-159. |
| 24 | GLATZ J P, BOKELUND H, ZIERFUß S.Analysis of the off-gas from dissolution of nuclear oxideand carbide fuels in nitricacid[J].Radiochimica Acta,1990,51(1):17-22. |
| 25 | SAKURAI T, TAKAHASHI A, ISHIKAWA N,et al.The composition of NO x generated in the dissolution of uranium dioxide[J].Nuclear Technology,1988,83(1):24-30. |
| 26 | POGORELKO O N, USTINOV O A.Effect of urea on the dissolution of U,UO2,and U3O8 in concentrated HNO3 and on the release of nitrogen oxides[J].Radiochemistry,1993,35(2):182-186. |
| 27 | SHABBIR M, ROBINS R G.Kinetics of the dissolution of uranium dioxide in nitric acid.I[J].Journal of Applied Chemistry,1968,18(5):129-134. |
| 28 | SHABBIR M, ROBINS R G.Kinetics of the dissolution of uranium dioxide in nitric acid.II[J].Journal of Applied Chemistry,1969,19(2):52-56. |
| 29 | TAYLOR R F, SHARRATT E W, DE CHAZAL L E M,et al.Dissolution rates of uranium dioxide sintered pellets in nitric acid systems[J].Journal of Applied Chemistry,1963,13(1):32-40. |
| 30 | SICSIC D.Modélisation thermodynamique et cinétique de la réduction de l'acide nitrique concentré[D].France:Université Pierre et Marie Curie,2011. |
| 31 | IKEDA Y, YASUIKE Y, TAKASHIMA Y,et al.17O NMR study on dissolution reaction of UO2 in nitric acid mechanism of electron transfer[J].Journal of Nuclear Science and Technology,1993,30(9):962-964. |
| 32 | CHARLIEr F, CANION D, MARC P,et al.Dissolution of uranium dioxide in nitric medium,towards a macroscopic model of reactors[C]//France:Joint 10th European Congress of Chemical Engineering & 3rd European Congress of Applied Biotechnology & 5th European Process Intensification Conferences,2015. |
| 33 | 刘方.UO2芯块溶解速率和真实乏燃料元件溶解及次级沉淀研究[D].北京:中国原子能科学研究院,2019. |
| LIU Fang.Study on dissolution rate of UO2 pellets and dissolution and secondary precipitation of real spent fuel component[D].Beijing:China Institute of Atomic Energy,2019. | |
| 34 | INOUE A, TSUJINO T.Dissolution rates of uranium oxide (U3O8) powders in nitric acid[J].Industrial & Engineering Chemistry Process Design and Development,1984,23(1):122-125. |
| 35 | DESIGAN N, AUGUSTINE E, MURALI R,et al.Dissolution kinetics of Indian PHWR natural UO2 fuel pellets in nitric acideffect of initial acidity and temperature[J].Progress in Nuclear Energy,2015,83:52-58. |
| 36 | IKEDA Y, YASUIKE Y, NISHIMURA K,et al.Kinetic study on dissolution of UO2 powders in nitric acid[J].Journal of Nuclear Materials,1995,224(3):266-272. |
| 37 | ZHAO Yunfeng, CHEN Jing.Studies on the dissolution kinetics of ceramic uranium dioxide particles in nitric acid by microwave heating[J].Journal of Nuclear Materials,2008,373(1/2/3):53-58. |
| 38 | ZHAO Yunfeng, CHEN Jing.Comparative studies on the dissolution of ceramic UO2 pellets in nitric acid by microwave and conventional heating[J].Radiochimica Acta,2008,96(8):467-471. |
| 39 | MARC P, MAGNALDO A, GODARD J,et al.A method for phenomenological and chemical kinetics study of autocatalytic reactive dissolution by optical microscopy:The case of uranium dioxide dissolution in nitric acid media[J].EPJ Nuclear Sciences & Technologies,2018,4.Doi:10.1051/epjn/2017026. |
| 40 | MARC P, MAGNALDO A, VAUDANO A,et al.Dissolution of uranium dioxide in nitric acid media:What do we know?[J].EPJ Nuclear Sciences & Technologies,2017,3.Doi:10.1051/epjn/2017005. |
| 41 | GARZON LOSIK G, LALLEMAN S, GIRAUD M,et al.Analysing the impact of autocatalysis on the dissolution kinetics of uranium and plutonium mixed oxide powders by optical microscopy[J].Hydrometallurgy,2023,216:106010. |
| 42 | FUKASAWA T, OZAWA Y, KAWAMURA F.Generation and decomposition behavior of nitrous acid during dissolution of UO2 pellets by nitric acid[J].Nuclear Technology,1991,94(1):108-113. |
| 43 | DESIGAN N, BHATT N P, PANDEY N K,et al.Mechanism of dissolution of nuclear fuel in nitric acid relevant to nuclear fuel reprocessing[J].Journal of Radioanalytical and Nuclear Chemistry,2017,312(1):141-149. |
| 44 | HOMMA S, KOGA J, MATSUMOTO S,et al.Dissolution rate equation of UO2 pellet[J].Journal of Nuclear Science and Technology,1993,30(9):959-961. |
| 45 | URIARTE A L, RAINEY R H.Dissolution of high-density UO2,PuO2,and UO2-PuO2 pellets in inorganic acids[R].American:Oak Ridge National Laboratory,1965. |
| 46 | BENSMAIN B, CHEGROUCHE S, BARKAT M,et al.The dissolution of uranium oxides:Thermodynamic and kinetic investigations[J].Hydrometallurgy,2016,160:73-78. |
| 47 | FUKASAWA T, OZAWA Y.Relationship between dissolution rate of uranium dioxide pellets in nitric acid solutions and their porosity[J].Journal of Radioanalytical and Nuclear Chemistry,1986,106(6):345-356. |
| 48 | MINEO H, ISOGAI H, MORITA Y,et al.An investigation into dissolution rate of spent nuclear fuel in aqueous reprocessing[J].Journal of Nuclear Science and Technology,2004,41(2):126-134. |
| 49 | PERUSKI K M, SPANO T L, VICK M C,et al.Elucidating the composition and structure of uranium oxide powders produced via NO2 voloxidation[J].ACS Omega,2024,9(9):10979-10991. |
| 50 | SALMI T, GRÉNMAN H, WÄRNÅ J,et al.Revisiting shrinking particle and product layer models for fluid-solid reactions from ideal surfaces to real surfaces[J].Chemical Engineering and Processing:Process Intensification,2011,50(10):1076-1084. |
| 51 | CHEN Changqing, ZHU Tun.The kinetics of cobalt(Ⅱ) extraction with ehehpa in heptane from acetate system using an improved Lewis cell technique[J].Solvent Extraction and Ion Exchange,1994,12(5):1013-1032. |
| 52 | SHABB IR M, ROBINS R G.The effect of crystallographic orientation on the dissolution of uranium dioxide in nitric acid[J].Journal of Nuclear Materials,1968,25(2):236-237. |
| 53 | CORDARA T, SZENKNECT S, CLAPAREDE L,et al.Kinetics of dissolution of UO2 in nitric acid solutions:A multiparametric study of the non-catalysed reaction[J].Journal of Nuclear Materials,2017,496:251-264. |
| 54 | IKEDA Y, YASUIKE Y, NISHIMURA K,et al.Dissolution behavior of pulverized irradiated fuels in nitric acid solutions[J].Journal of Nuclear Science and Technology,1999,36(4):358-363. |
| 55 | ARAI Y, MAEDA A, SHIOZAWA K I,et al.Chemical forms of solid fission products in the irradiated uranium:Plutonium mixed nitride fuel[J].Journal of Nuclear Materials,1994,210(1/2):161-166. |
| 56 | KOGA J, HONMA S, KANEHIRA O,et al.Fuel dissolution rate and its mechanism[C]// Japan:Proceedings of the third international conference on nuclear fuel reprocessing and waste management,1991. |
| 57 | INOUE A.Mechanism of the oxidative dissolution of UO2 in HNO3 solution[J].Journal of Nuclear Materials,1986,138(1):152-154. |
| 58 | LINDMAN N, SIMONSSON D.On the application of the shrinking core model to liquid-solid reactions[J].Chemical Engineering Science,1979,34(1):31-35. |
| 59 | GAO J Y.Studying dissolution with a model integrating solid-liquid interface kinetics and diffusion kinetics[J].Analytical Chemistry,2012,84(24):10671-10678. |
| 60 | CASTELL M R, DUDAREV S L, MUGGELBERG C,et al.Surface structure and bonding in the strongly correlated metal oxides NiO and UO2 [J].Journal of Vacuum Science & Technology A:Vacuum,Surfaces,and Films,1998,16(3):1055-1058. |
| 61 | MUGGELBERG C, CASTELL M R, BRIGGS G A D,et al.An STM study of the UO2(001) surface[J].Applied Surface Science,1999,142(1/2/3/4):124-128. |
| 62 | BERTOLOTTO S, SZENKNECT S, LALLEMAN S,et al.Effect of surface orientation on dissolution rate and surface dynamics of UO2 single crystals in nitric acid[J].Corrosion Science,2020,176:109020. |
| 63 | SALMI T, GRÉNMAN H, WÄRNÅ J,et al.New modelling approach to liquid-solid reaction kinetics:From ideal particles to real particles[J].Chemical Engineering Research and Design,2013,91(10):1876-1889. |
| 64 | AUGUSTINE E, DESIGAN N, PANDEY N K,et al.Analysis of kinetic data for the dissolution of UO2 fuel pellets in nitricacid[J].Journal of Radioanalytical and Nuclear Chemistry,2020,324(1):211-218. |
| [1] | TAN Shanyi, WEN Huizi, HE Shuyu, ZHANG Liwen, CHEN Shaohua, XI Benjun. Study on leaching behavior and kinetics of phosphorus from phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(2): 105-112. |
| [2] | YUAN Jinghua, WU Junhu, YANG XiuShan, XU Dehua, ZHANG Zhiye. Research on process of production ammonium dihydrogen phosphate by ammonia purification of nitro-phosphoric acid [J]. Inorganic Chemicals Industry, 2023, 55(9): 50-56. |
| [3] | WANG Haosen, REN Bingchen, XU Dehua, YANG Xiushan, ZHANG Zhiye. Leaching process and kinetics of phosphorus-potassium ore decomposition by nitric acid [J]. Inorganic Chemicals Industry, 2023, 55(5): 45-51. |
| [4] | MA Dianpu, PU Youfu, LI Jun, CHEN Lishi, LIU Hengyu, QIN Deqing, FU Zewei, PENG Jubo. Preparation of high purity ultrafine tin dioxide particles by liquid oxidation-spray drying method [J]. Inorganic Chemicals Industry, 2023, 55(4): 54-59. |
| [5] | XIAO Yong,YANG Xiushan,XU Dehua,WANG Xinlong,ZHANG Zhiye. Study on treatment of medium and low grade high magnesium collophanite by nitric acid method [J]. Inorganic Chemicals Industry, 2022, 54(1): 71-76. |
| [6] | Su Shu,Xu Dehua,Li Chaorong,Yang Xiushan,Wang Xinlong,Zhang Zhiye. Study on defluorination of phosphoric acid by nitric acid process [J]. Inorganic Chemicals Industry, 2021, 53(9): 24-29. |
| [7] | Yang Ping,Yang Lin,Feng Yang,Lin Qian,Cao Jianxin. Migration and distribution of iodine in process of decomposing phosphate ore by nitric acid [J]. Inorganic Chemicals Industry, 2020, 52(4): 53-56. |
| [8] | WEN Yan-Bing, ZHANG Qin, GU Chun-Guang, LU Yu-Lian, ZHANG Lan-Xi, HAN Yu. Research progress on pretreatment technology of medium-and low-grade phosphate rock [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 7-. |
| [9] | LUO Jin-Bo, WANG Hai-Feng, WANG Jia-Wei, ZHAO Ping-Yuan, ZHOU Ling-Ling. Experimental study on impurity removing from manganese anode slime by nitric acid pickling [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 63-. |
| [10] | XIAO Jing-Bo, HU Cai-Hua. Study on synthesis of boric acid and sodium nitrate by using borax mine in Tibet under assistance of nitric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 38-. |
| [11] | ZHU Jun, ZHONG Jian-Chu, WANG Hong-Zhi. Study on preparation of boric acid by leaching ulexite with nitric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(3): 36-. |
| [12] | JIANG Xiang-Fei, LI Jun, LIU Xue-Feng, LUO Jian-Hong, JIN Yang, YAO Wei-Dong. Study on purification of yellow phosphorus by nitric acid oxidation combined with zone melting [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(3): 32-. |
| [13] | ZHANG Fu-Yuan, ZHUO Jian-Jin, ZHANG Yu-Ming, PENG Guo-Min. Research on acid leaching process of lead from leaching residue with precipitation transformation method [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(11): 47-. |
| [14] | KANG Xin-Ying, SU Yi, CHA Zuo-Tong. Study on separating characteristics of iron and calcium in the acid leaching process of yellow phosphorus slag [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(8): 43-. |
| [15] | CAO Yu-Qing, LI Jun, LUO Jian-Hong, YANG Zhao-Peng. Study on crystallization kinetics of calcium nitrate tetrahydrate [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(12): 22-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||