Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (9): 67-74.doi: 10.19964/j.issn.1006-4990.2023-0530
• Research & Development • Previous Articles Next Articles
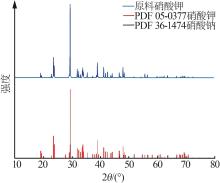
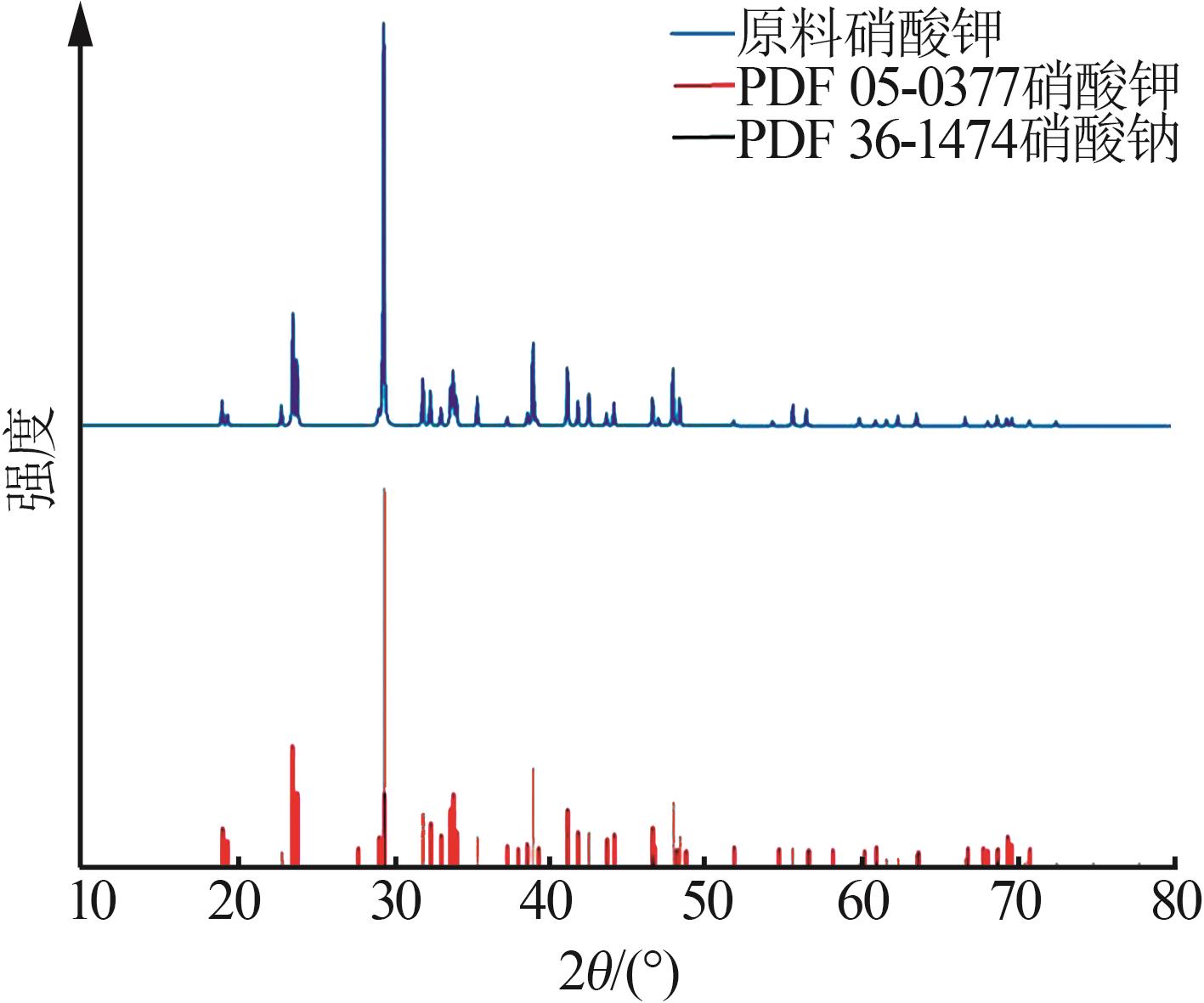
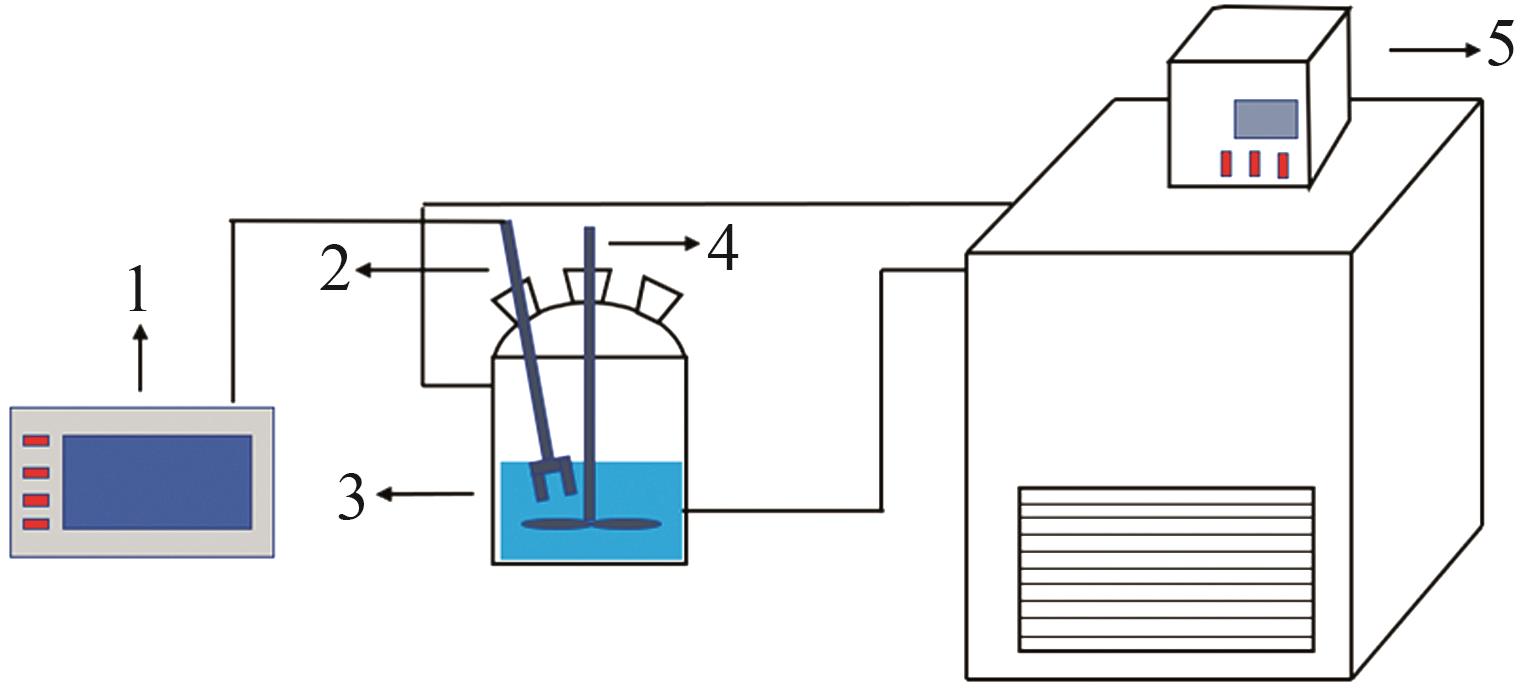
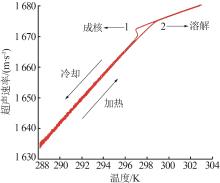
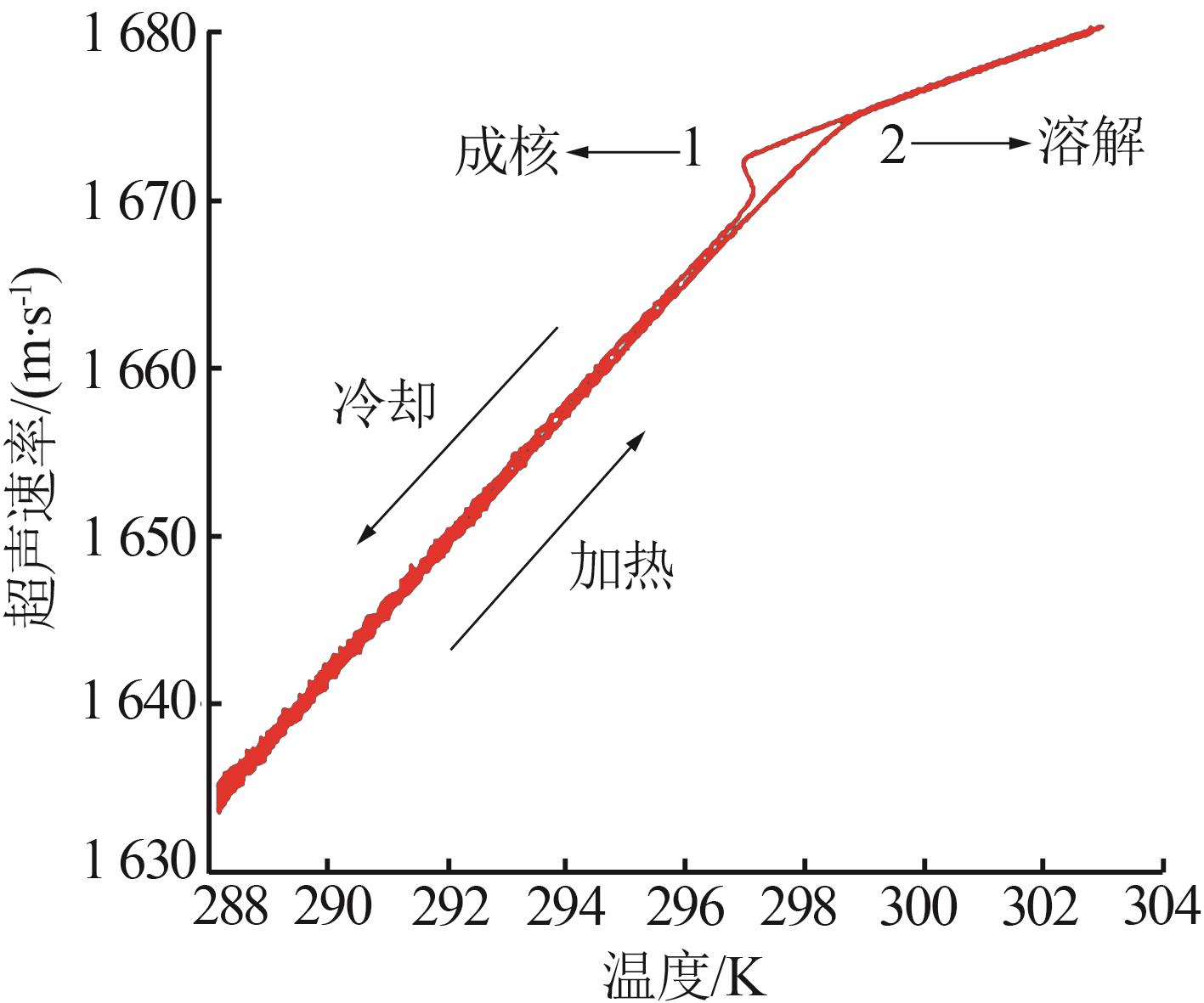
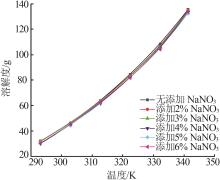
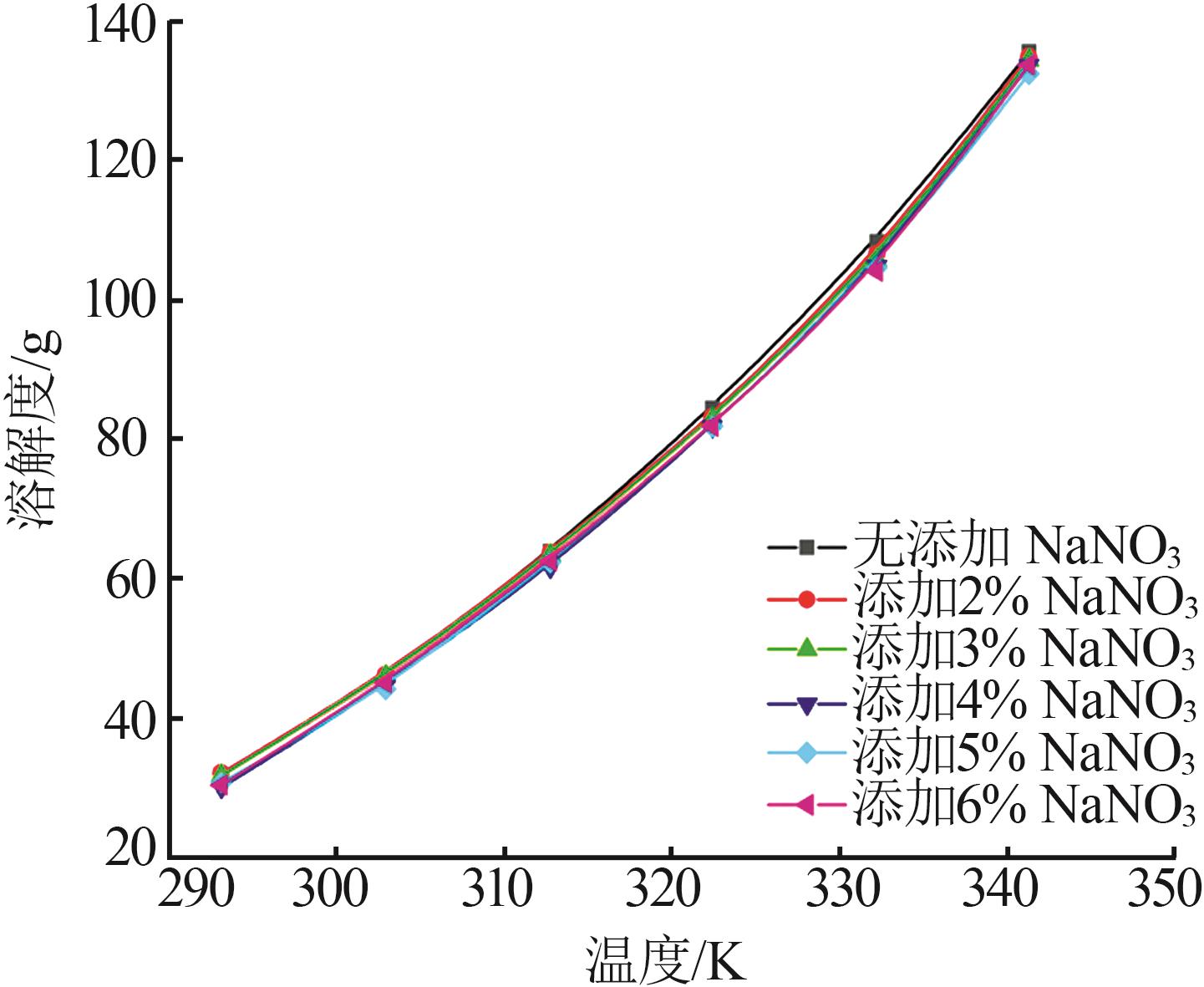
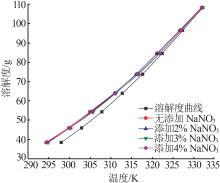
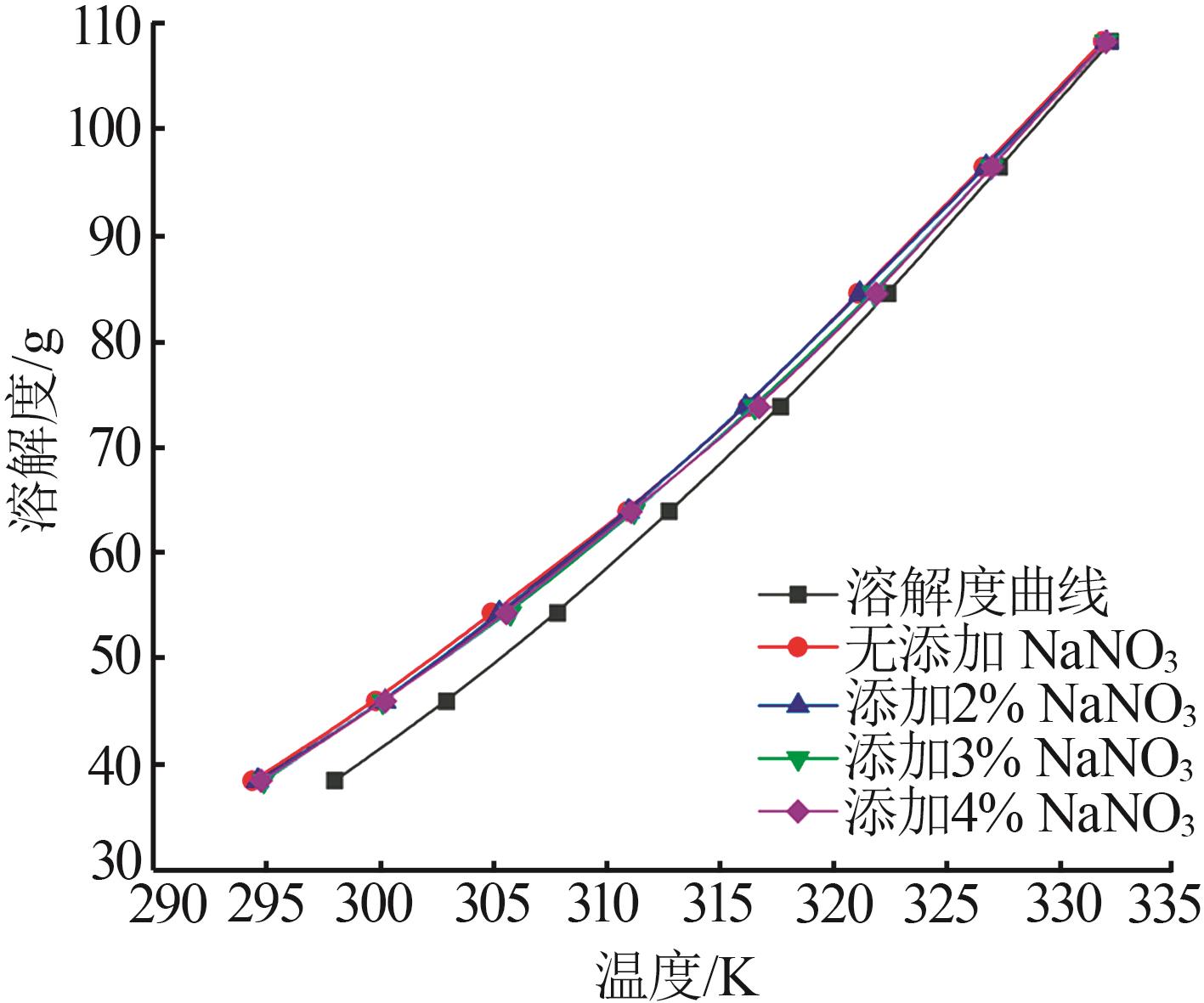
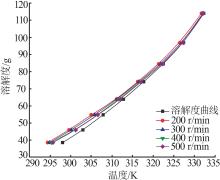
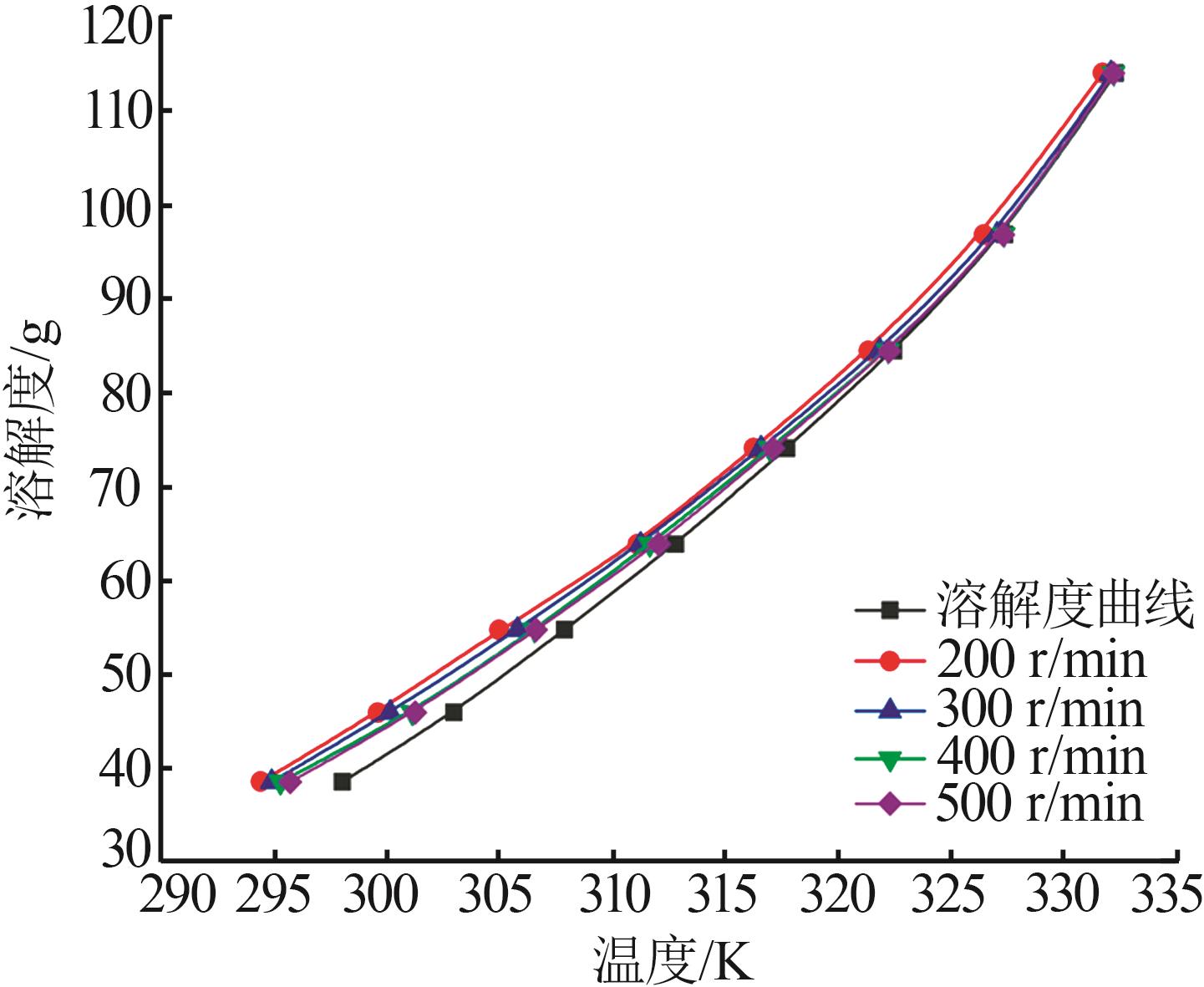
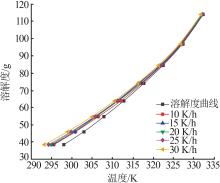
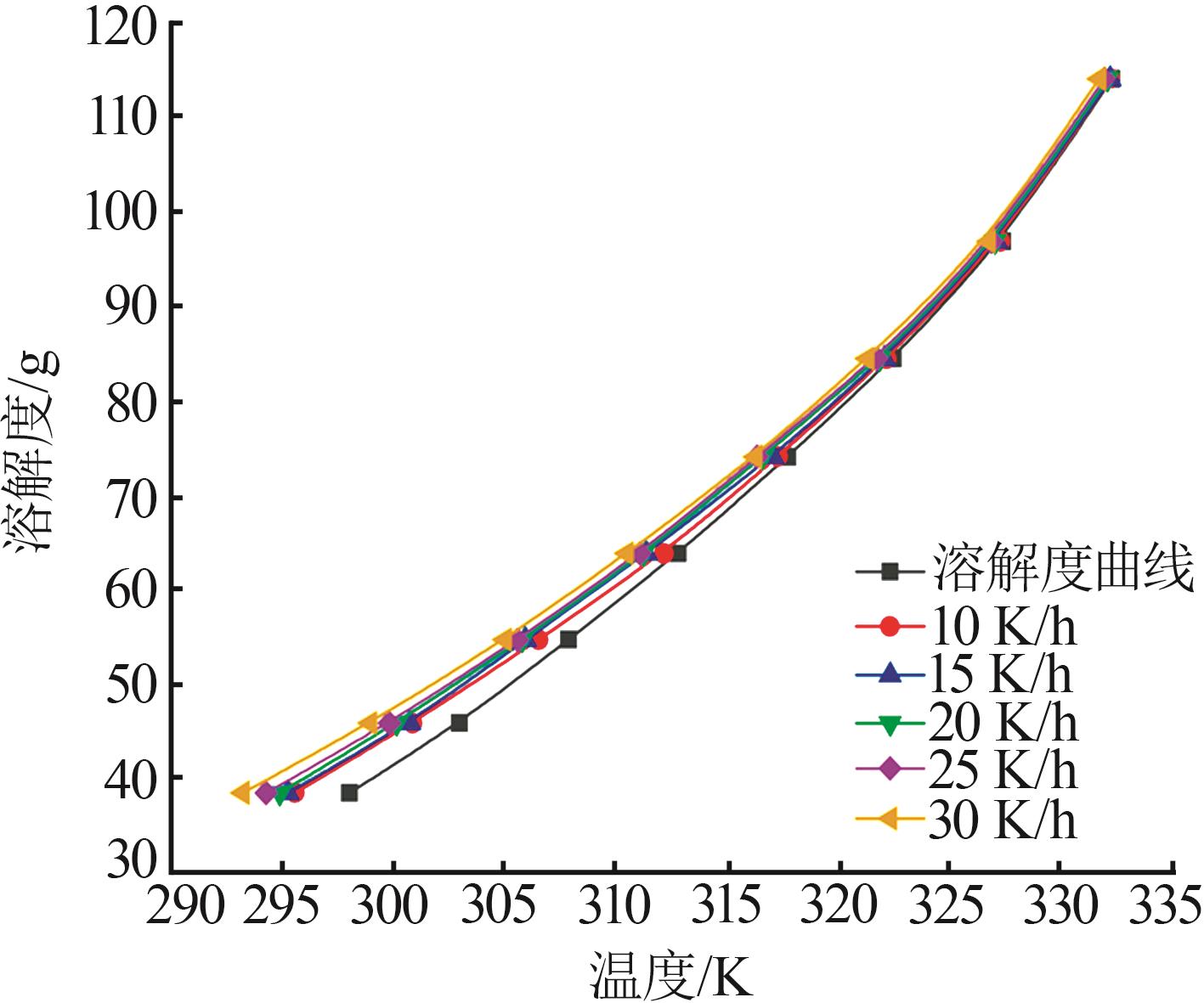
Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3
ZOU Yang1,2( ), LU Zhiyan1(
), LU Zhiyan1( ), HU Zhilin1, SUN Ze1,3
), HU Zhilin1, SUN Ze1,3
- 1.School of Resources and Environmental Engineering,East China University of Science and Technology,Shanghai 200237,China
2.Shanghai Boiler Works Co. ,Ltd. ,Shanghai 200245,China
3.Qinghai Minzu University,Xining 810007,China
-
Received:2023-11-09Online:2024-09-10Published:2024-09-26 -
Contact:LU Zhiyan E-mail:147952419zou@163.com;zylu@ecust.edu.cn
CLC Number:
Cite this article
ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3[J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74.
share this article
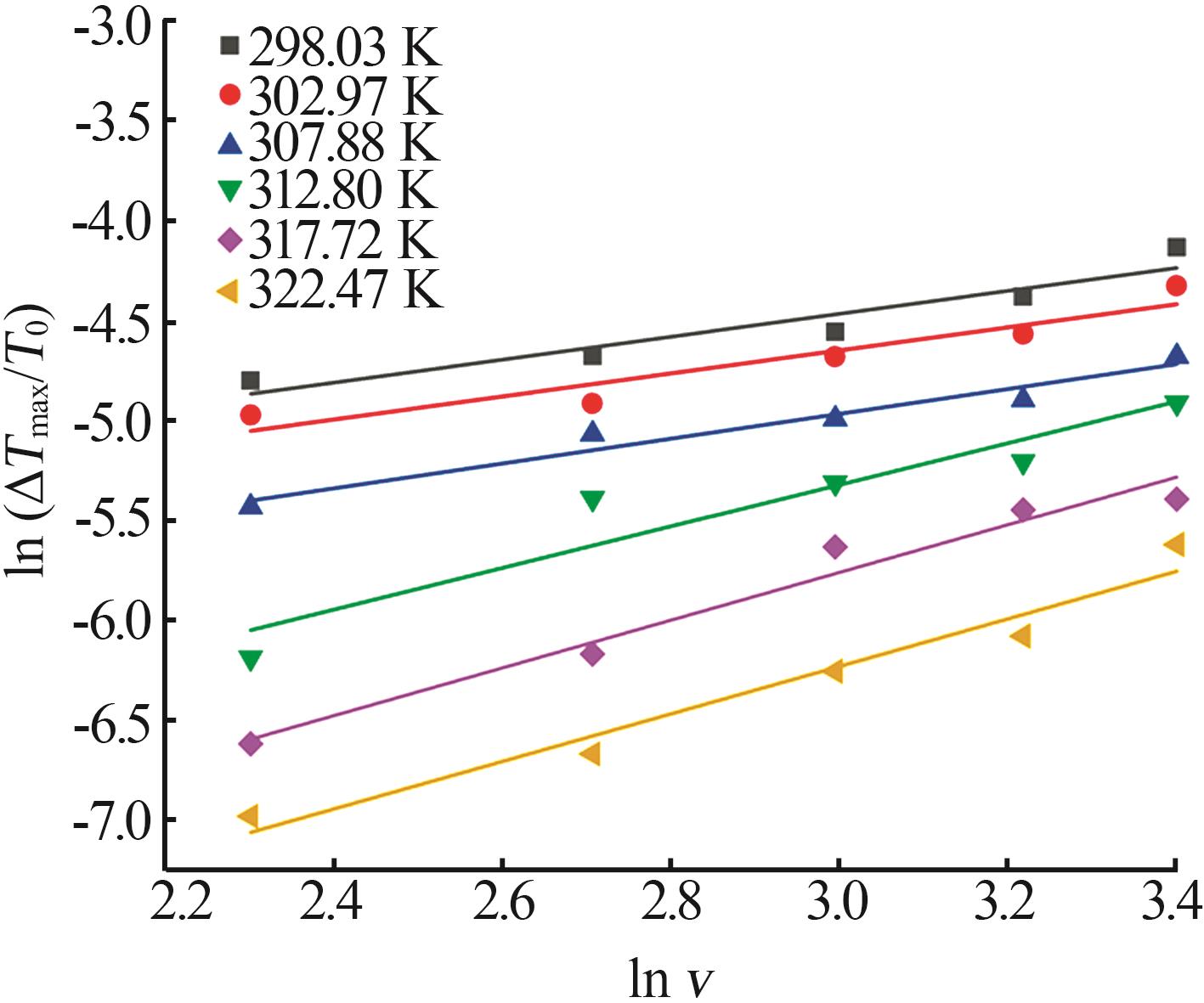
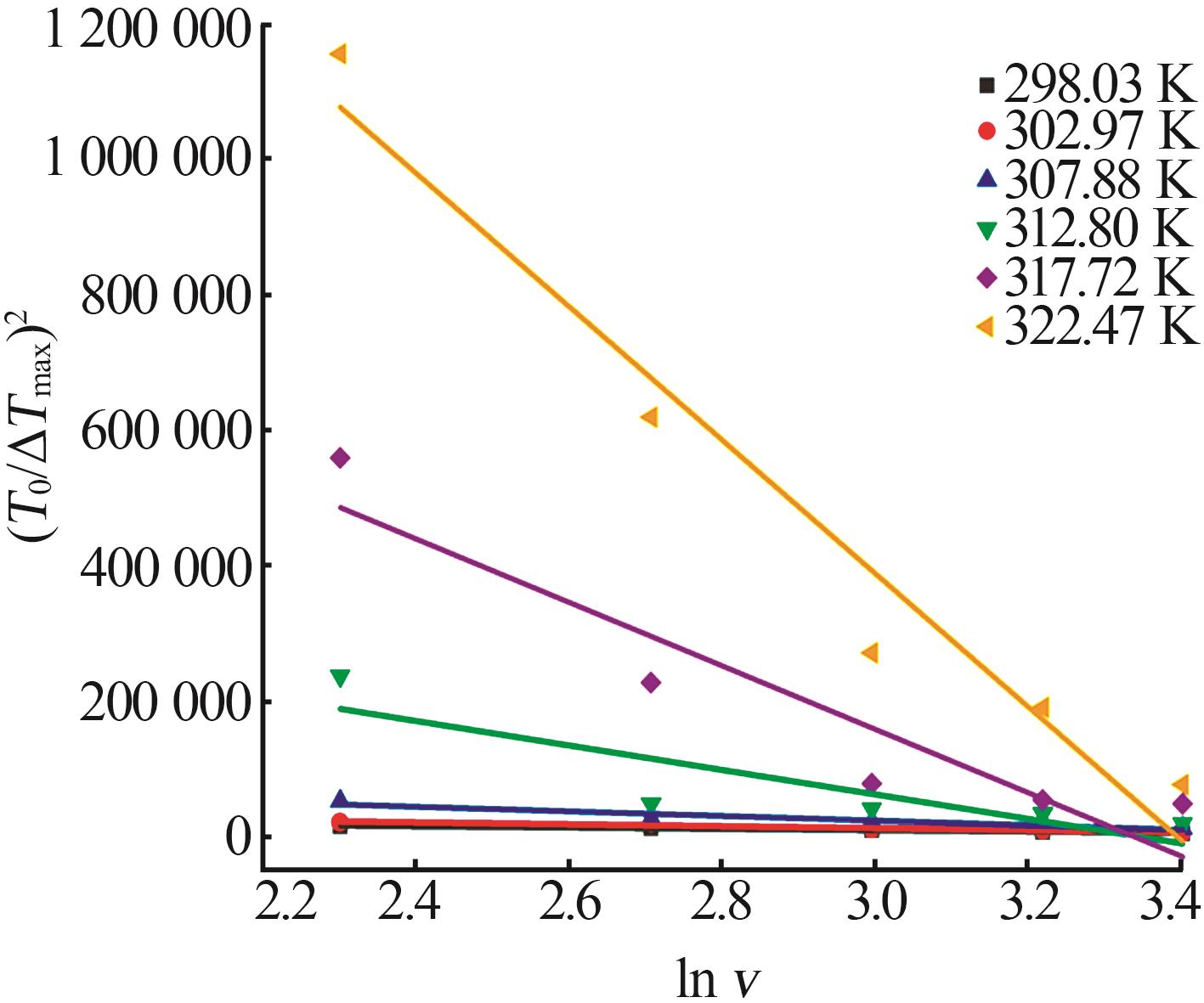
Table 7
Kinetic parameters estimated using classical 3D nucleation theory equation at different saturation temperatures"
| T0/K | F1 | F | F1/F | A/f | BTlim3×10-5 | R2 |
|---|---|---|---|---|---|---|
| 298.03 | 9 716.64 | 37 494.04 | 0.26 | 1.42 | 2.10 | 0.980 8 |
| 302.97 | 14 192.66 | 54 643.05 | 0.26 | 1.36 | 1.46 | 0.937 2 |
| 307.88 | 34 040.48 | 125 163.90 | 0.27 | 1.10 | 0.62 | 0.860 7 |
| 312.80 | 181 076.62 | 604 924.40 | 0.30 | 0.76 | 0.12 | 0.665 2 |
| 317.72 | 469 463.03 | 1 566 360.00 | 0.30 | 0.73 | 0.05 | 0.838 1 |
| 322.47 | 987 117.47 | 3 349 680.00 | 0.29 | 0.75 | 0.02 | 0.944 7 |
| 1 | 熊增华,王兴富,王石军,等.我国硝酸钾产业发展现状与展望[J].化工矿物与加工,2021,50(5):49-53. |
| XIONG Zenghua, WANG Xingfu, WANG Shijun,et al.Current status and prospect of potassium nitrate development in China[J].Industrial Minerals & Processing,2021,50(5):49-53. | |
| 2 | 李涛,高书宝,郝晓翠,等.硝酸钾制备技术研究进展[J].盐科学与化工,2023,52(6):7-11. |
| LI Tao, GAO Shubao, HAO Xiaocui,et al.Research progress in preparation technology of potassium nitrate[J].Journal of Salt Science and Chemical Industry,2023,52(6):7-11. | |
| 3 | 田昊东,徐驰,许少坤,等.玻璃化学强化技术研究进展[J].硅酸盐通报,2022,41(7):2502-2510. |
| TIAN Haodong, XU Chi, XU Shaokun,et al.Research progress on chemical strengthening technology of glass[J].Bulletin of the Chinese Ceramic Society,2022,41(7):2502-2510. | |
| 4 | 司敏杰,郭卫,田芳,等.超薄盖板玻璃二步法离子强化工艺研究进展[J].玻璃,2021,48(2):13-17. |
| SI Minjie, GUO Wei, TIAN Fang,et al.Research progress of two-step ion strengthening process for ultra-thin cover glass[J].Glass,2021,48(2):13-17. | |
| 5 | HASSANI H, SGLAVO V M.Effect of Na contamination on the chemical strengthening of soda-lime silicate float glass by ion-exchange in molten potassium nitrate[J].Journal of Non-Crystalline Solids,2019,515:143-148. |
| 6 | 陈学文,张华,章磊,等.从混合盐中分离硝酸钠和硝酸钾的结晶与双效MVR系统:中国,219314589U[P].2023-07-07. |
| 7 | WANG Shaona, FENG Man, DU Hao,et al.Determination of metastable zone width,induction time and primary nucleation kinetics for cooling crystallization of sodium orthovanadate from NaOH solution[J].Journal of Crystal Growth,2020,545:125721. |
| 8 | 刘欣玉,孙杰,罗义芬,等.ADN的溶解度、结晶介稳区及诱导期的测定[J].含能材料,2019,27(9):766-772. |
| LIU Xinyu, SUN Jie, LUO Yifen,et al.Measurement of solubility,metastable zone and induction period of ADN[J].Chinese Journal of Energetic Materials,2019,27(9):766-772. | |
| 9 | 罗西,常海,刘红妮,等.PYX在DMSO中的结晶热力学[J].火炸药学报,2022,45(2):205-211. |
| LUO Xi, Hai CHANG, LIU Hongni,et al.Crystallization thermodynamics of PYX in DMSO[J].Chinese Journal of Explosives & Propellants,2022,45(2):205-211. | |
| 10 | 胡超群,朱亮,沙作良,等.杂质离子对共晶冷冻结晶过程中冰晶结晶特性的影响[J].无机盐工业,2017,49(5):25-29. |
| HU Chaoqun, ZHU Liang, SHA Zuoliang,et al.Effect of impurity ions on crystallization properties of ice crystals in eutectic freeze crystallization process[J].Inorganic Chemicals Industry,2017,49(5):25-29. | |
| 11 | CAO Yuechao, YAO Tuo, ZHANG Guimin,et al.Nucleation behavior of isosorbide 5-mononitrate revealed from metastable zone widths by combining nucleation theory model and molecular simulation[J].Journal of Molecular Liquids,2022,363:119846. |
| 12 | NÝVLT J.Kinetics of nucleation in solutions[J].Journal of Crystal Growth,1968,3/4:377-383. |
| 13 | SANGWAL K.A novel self-consistent Nývlt-like equation for metastable zone width determined by the polythermal method[J].Crystal Research and Technology,2009,44(3):231-247. |
| 14 | SANGWAL K.Novel approach to analyze metastable zone width determined by the polythermal method:Physical interpretation of various parameters[J].Crystal Growth & Design,2009,9(2):942-950. |
| 15 | 彭思瑶,施云海.硫酸铵介稳区宽度的研究及成核动力学计算[J].无机盐工业,2018,50(1):41-45. |
| PENG Siyao, SHI Yunhai.Study on metastable zone width and nucleation kinetics of ammonium sulfate crystallization proce-ss[J].Inorganic Chemicals Industry,2018,50(1):41-45. | |
| 16 | BIAN Chao, CHEN Hang, SONG Xingfu,et al.Metastable zone width and the primary nucleation kinetics for cooling crystallization of NaNO3 from NaCl-NaNO3-H2O system[J].Journal of Crystal Growth,2019,518:5-13. |
| 17 | 张文豪,杨立斌,沙作良,等.七水氯化镧结晶介稳区的测定和初级成核分析[J].无机盐工业,2019,51(7):43-47. |
| ZHANG Wenhao, YANG Libin, SHA Zuoliang,et al.Determination of crystalline metastable zone and primary nucleation analysis of lanthanum chloride heptahydrate[J].Inorganic Chemicals Industry,2019,51(7):43-47. | |
| 18 | OMAR W, ULRICH J.Application of ultrasonics in the on-line determination of supersaturation[J].Crystal Research and Technology,1999,34(3):379-389. |
| 19 | NEMDILI L, KOUTCHOUKALI O, MAMERI F,et al.Crystallization study of potassium sulfate-water system,metastable zone width and induction time measurements using ultrasonic,turbidity and 3D-ORM techniques[J].Journal of Crystal Growth,2018,500:44-51. |
| 20 | JIN Miaomiao, FROHBERG P, SUN Yuzhu,et al.Study on metastable zone width and crystal growth of a ternary system:Case study MgCl2·6H2O·1,4-dioxane[J].Chemical Engineering Science,2015,133:181-189. |
| 21 | TITIZ-SARGUT S, ULRICH J.Application of a protected ultrasound sensor for the determination of the width of the metastable zone[J].Chemical Engineering and Processing:Process Intensification,2003,42(11):841-846. |
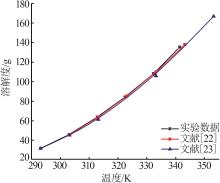
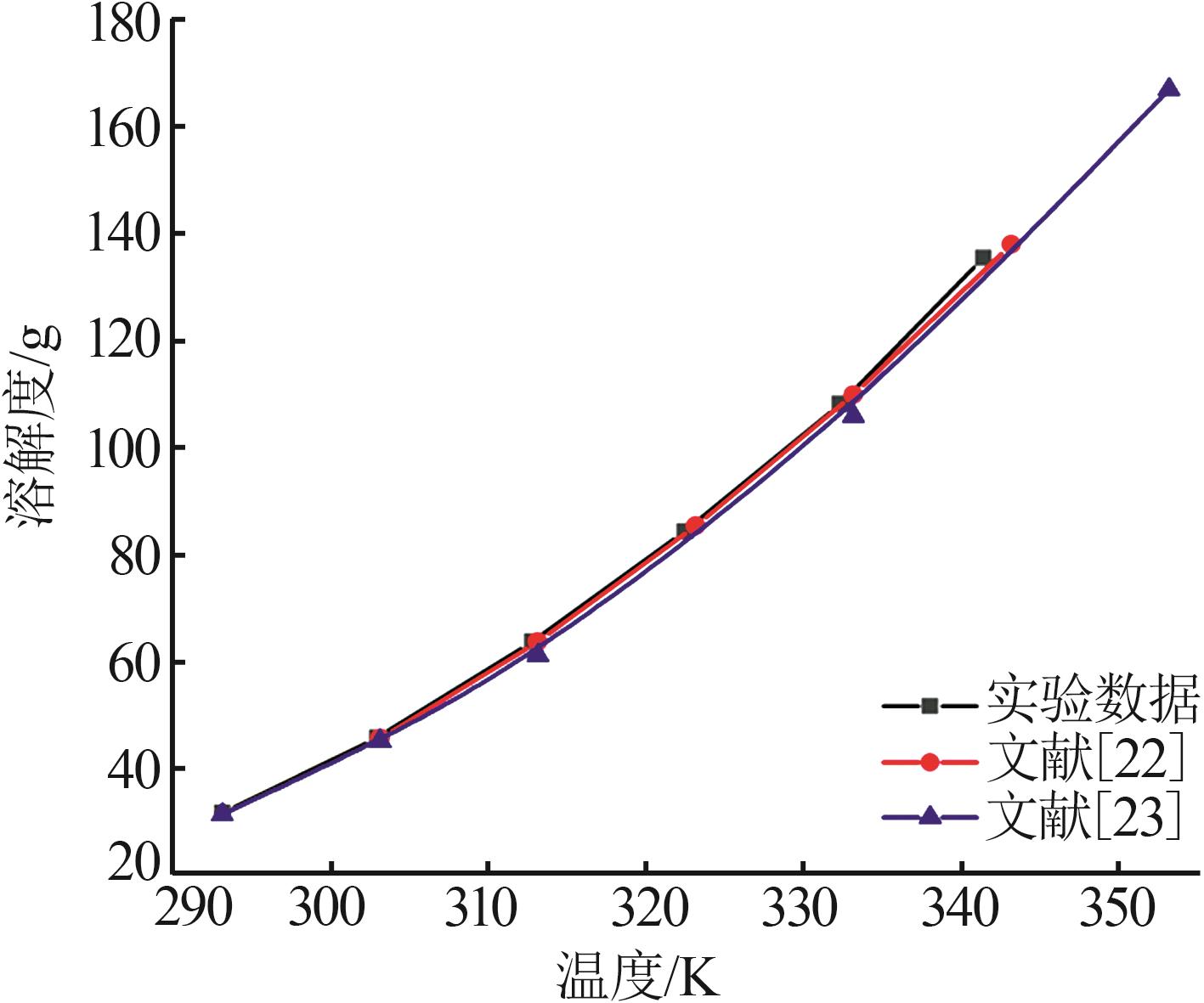
| 22 | 刘光启.化学化工物性数据手册-无机卷[M].北京:化学工业出版社,2002. |
| 23 | DEAN J A.Lange's Handbook of Chemistry [M].魏俊发等,译.北京:科学出版社,2003. |
| 24 | CHEN Zhirong, ZHOU Rongfan, YIN Hong,et al.Study on the nucleation kinetics of DL-methionine based on the metastable zone width of unseeded batch crystallization[J].Journal of Crystal Growth,2023,601:126941. |
| 25 | KASHCHIEV D, BORISSOVA A, HAMMOND R B,et al.Effect of cooling rate on the critical undercooling for crystallization[J].Journal of Crystal Growth,2010,312(5):698-704. |
| [1] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [2] | FAN Le, LI Hao, LIU Guochang, XU Shoujiang, WANG Haitao, LI Guocai, CHANG Na. Preparation of acid-base by bipolar membrane electrodialysis using NaNO3 as resource [J]. Inorganic Chemicals Industry, 2024, 56(7): 104-111. |
| [3] | WANG Yifu,JIN Yang,LI Jun. Measurement method and effect of metastable zone of microscale flow system [J]. Inorganic Chemicals Industry, 2022, 54(2): 45-49. |
| [4] | YANG Junfang,ZHOU Huan,ZHA Zhengjiong,WANG Yongcheng. Preparation process and product characterization of various copper oxychloride with respective crystal form from etching waste solution [J]. Inorganic Chemicals Industry, 2021, 53(11): 107-113. |
| [5] | Wang Yanfei,Jiao Jian,Jiang Shuwan,Xu Shijie. Study on nucleation kinetics of sodium sulfate-water system based on inverse solubility [J]. Inorganic Chemicals Industry, 2021, 53(10): 41-46. |
| [6] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [7] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [8] | Su Lei,Zhang Haijun,Wang Jin,Yu Xuefeng,Dong Changji,Sun Ze,Song Xingfu,Yu Jianguo. Study on preparation of molten salt grade potassium nitrate by reactive extraction method [J]. Inorganic Chemicals Industry, 2020, 52(5): 35-39. |
| [9] | Shi Zhonglu,Du Peiying,Yu Xuefeng,Niu Lihui,Wang Wanqing. Study on direct preparation of potassium nitrate from nitric acid and potassium chloride [J]. Inorganic Chemicals Industry, 2019, 51(8): 37-39. |
| [10] | Zhang Wenhao,Yang Libin,Sha Zuoliang,Wang Yanfei,Zhu Liang,Zhao Xiaoyu. Determination of crystalline metastable zone and primary nucleationanalysis of lanthanum chloride heptahydrate [J]. Inorganic Chemicals Industry, 2019, 51(7): 43-47. |
| [11] | XIONG Xing, ZHANG Bi-Jiang, XU De-Jun, ZHANG Zhi-Ye, RUAN Yong-Gang, YANG Lin, ZHOU Xue-Ke, WANG Xin-Long. Study on effects of cooling rate on ammonium dihydrogen phosphate′s metastable zone [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 48-. |
| [12] | ZHANG Feng-Zhen, WANG Hai, LIU Xing-Yong, TANG Xiu-Hua, YANG Hu. Study on metastable zone width of glauber salt′s vacuum evaporation cooling crystallization [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 27-. |
| [13] | DUAN Zheng-Kang, XIE Fan, ZHANG Tao, LI Sheng, YAN Jian-Hua. Summary of production process of potassium nitrate and existing problems and solutions in complex decomposition cycle method [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 4-. |
| [14] | ZHANG Gang. Analysis on hazard class of potassium nitrate for agricultural use [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 55-. |
| [15] | XIAO Jing-Bo, HU Cai-Hua. Study on synthesis of boric acid and sodium nitrate by using borax mine in Tibet under assistance of nitric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 38-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||