Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (2): 79-84.doi: 10.19964/j.issn.1006-4990.2022-0232
• Research & Development • Previous Articles Next Articles
Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction
ZHANG Xing( ),XU Jie(
),XU Jie( ),WANG Zibing,HOU Peng,HE Long,LIU Huan
),WANG Zibing,HOU Peng,HE Long,LIU Huan
- School of Metallurgy and Energy,North China University of Science and Technology,Tangshan 063210,China
-
Received:2022-04-21Online:2023-02-10Published:2023-02-16 -
Contact:XU Jie E-mail:1010549980@qq.com;xujie_xujie@126.com
CLC Number:
Cite this article
ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction[J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84.
share this article
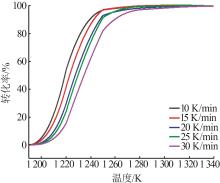
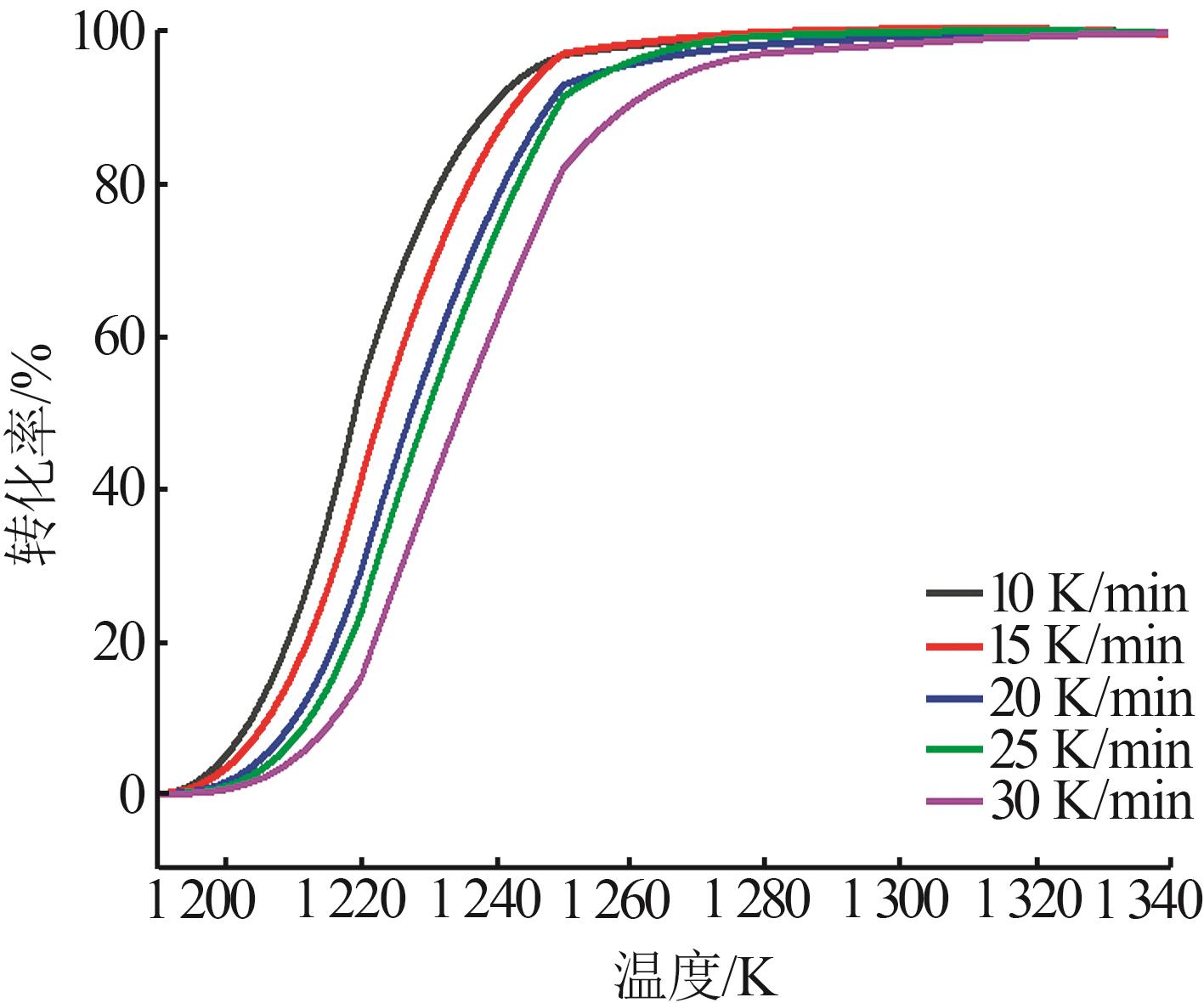
Table 2
Reaction temperature corresponding to each conversion at different heating rates"
| 转化率/% | 反应温度/K | ||||
|---|---|---|---|---|---|
| 10 K·min-1 | 15 K·min-1 | 20 K·min-1 | 25 K·min-1 | 30 K·min-1 | |
| 20 | 1 211.7 | 1 213.9 | 1 217.0 | 1 218.8 | 1 222.1 |
| 25 | 1 212.9 | 1 215.3 | 1 218.6 | 1 220.5 | 1 224.2 |
| 30 | 1 214.2 | 1 216.7 | 1 220.3 | 1 222.2 | 1 226.2 |
| 35 | 1 215.4 | 1 218.2 | 1 221.9 | 1 223.9 | 1 228.2 |
| 40 | 1 216.6 | 1 219.6 | 1 223.5 | 1 225.6 | 1 230.2 |
| 45 | 1 217.8 | 1 221.1 | 1 225.2 | 1 227.3 | 1 232.2 |
| 50 | 1 219.1 | 1 222.6 | 1 226.9 | 1 229.1 | 1 234.2 |
| 55 | 1 220.4 | 1 224.1 | 1 228.6 | 1 230.9 | 1 236.3 |
| 60 | 1 221.8 | 1 225.7 | 1 230.5 | 1 232.8 | 1 238.4 |
| 65 | 1 223.4 | 1 227.4 | 1 232.4 | 1 234.8 | 1 240.6 |
| 70 | 1 225.0 | 1 229.2 | 1 234.4 | 1 236.9 | 1 243.1 |
| 75 | 1 226.8 | 1 231.2 | 1 236.7 | 1 239.3 | 1 245.7 |
| 80 | 1 228.8 | 1 233.4 | 1 239.2 | 1 241.9 | 1 248.5 |
| 85 | 1 231.4 | 1 236.1 | 1 242.3 | 1 244.8 | 1 252.0 |
| 90 | 1 234.7 | 1 239.4 | 1 245.2 | 1 249.6 | 1 256.4 |
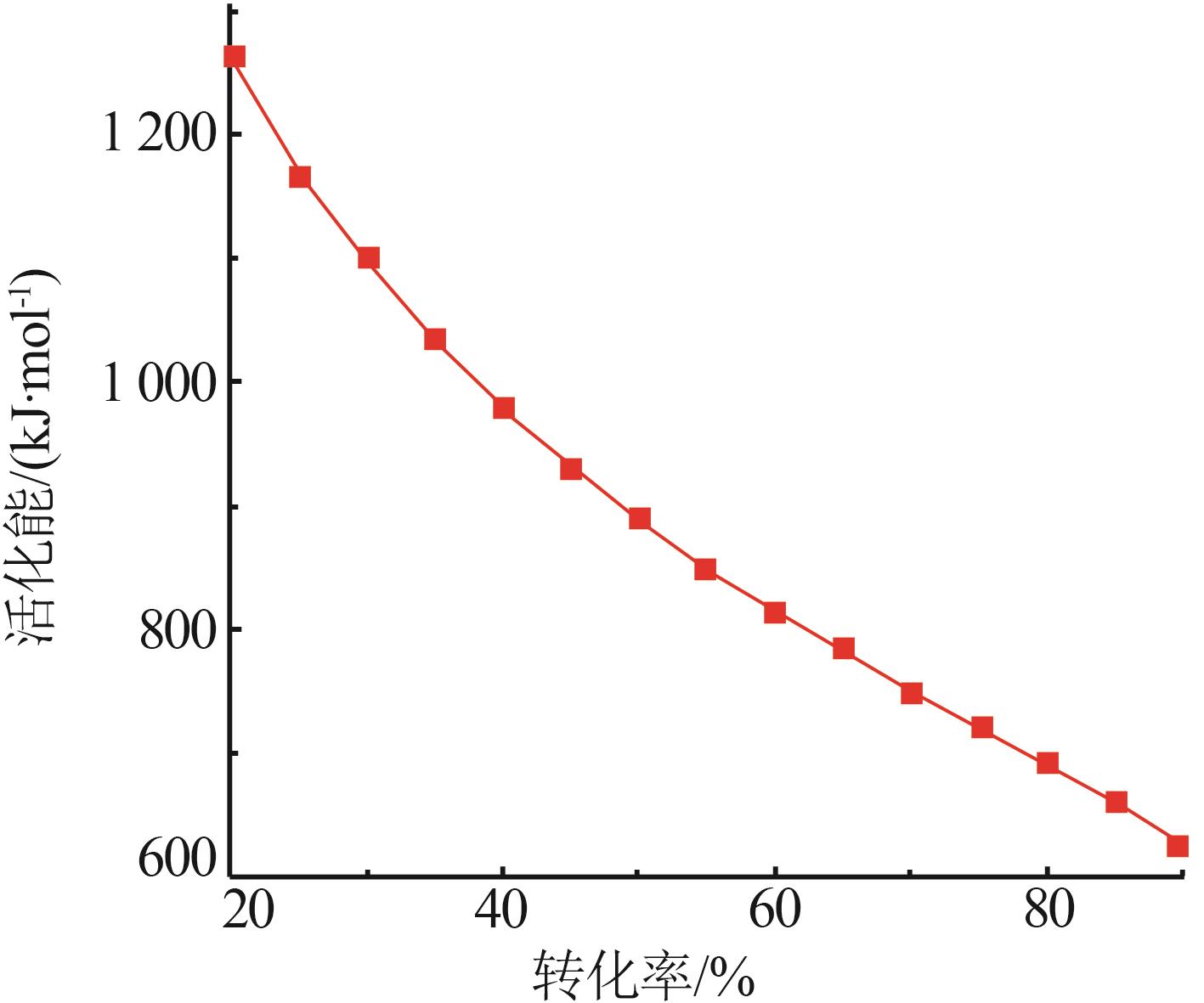
Table 5
Apparent activation energies and correlation coefficients corresponding to linear optimalmechanism functions at different heating rates"
| 10 K/min | 15 K/min | 20 K/min | 25 K/min | 30 K/min | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(kJ·mol-1) | R2 | E/ (kJ·mol-1) | R2 | E/ (kJ·mol-1) | R2 | E/ (kJ·mol-1) | R2 | E/ (kJ·mol-1) | R2 | E/ (kJ·mol-1) | R2 | ||||||
| 410.6 | 0.996 2 | 287.7 | 0.961 2 | 257.2 | 0.970 4 | 228.9 | 0.973 6 | 215.5 | 0.967 3 | 191.5 | 0.974 8 | ||||||
| 554.3 | 0.996 2 | 390.3 | 0.962 5 | 349.8 | 0.971 5 | 311.9 | 0.974 7 | 294.1 | 0.968 8 | 262.2 | 0.976 1 | ||||||
| 669.1 | 0.996 2 | 472.4 | 0.963 1 | 423.8 | 0.972 0 | 378.4 | 0.975 3 | 357.0 | 0.969 5 | 318.8 | 0.976 7 | ||||||
| 841.3 | 0.996 2 | 595.6 | 0.963 8 | 534.8 | 0.972 6 | 478.1 | 0.975 8 | 451.4 | 0.970 2 | 403.6 | 0.977 3 | ||||||
| 1 128 | 0.996 2 | 800.9 | 0.964 3 | 719.8 | 0.973 1 | 644.3 | 0.976 3 | 608.6 | 0.970 8 | 544.9 | 0.977 8 | ||||||
| 1 272 | 0.996 2 | 903.5 | 0.964 5 | 812.3 | 0.973 2 | 727.4 | 0.976 5 | 687.3 | 0.971 1 | 615.6 | 0.978 0 | ||||||
| 1 703 | 0.996 2 | 1 211 | 0.964 9 | 1 090 | 0.973 6 | 976.6 | 0.976 8 | 923.2 | 0.971 5 | 827.7 | 0.978 4 | ||||||
| 2 565 | 0.996 2 | 1 827 | 0.965 3 | 1 645 | 0.973 9 | 1 475 | 0.977 1 | 1 395 | 0.971 9 | 1 252 | 0.978 7 | ||||||
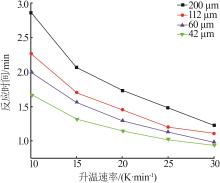
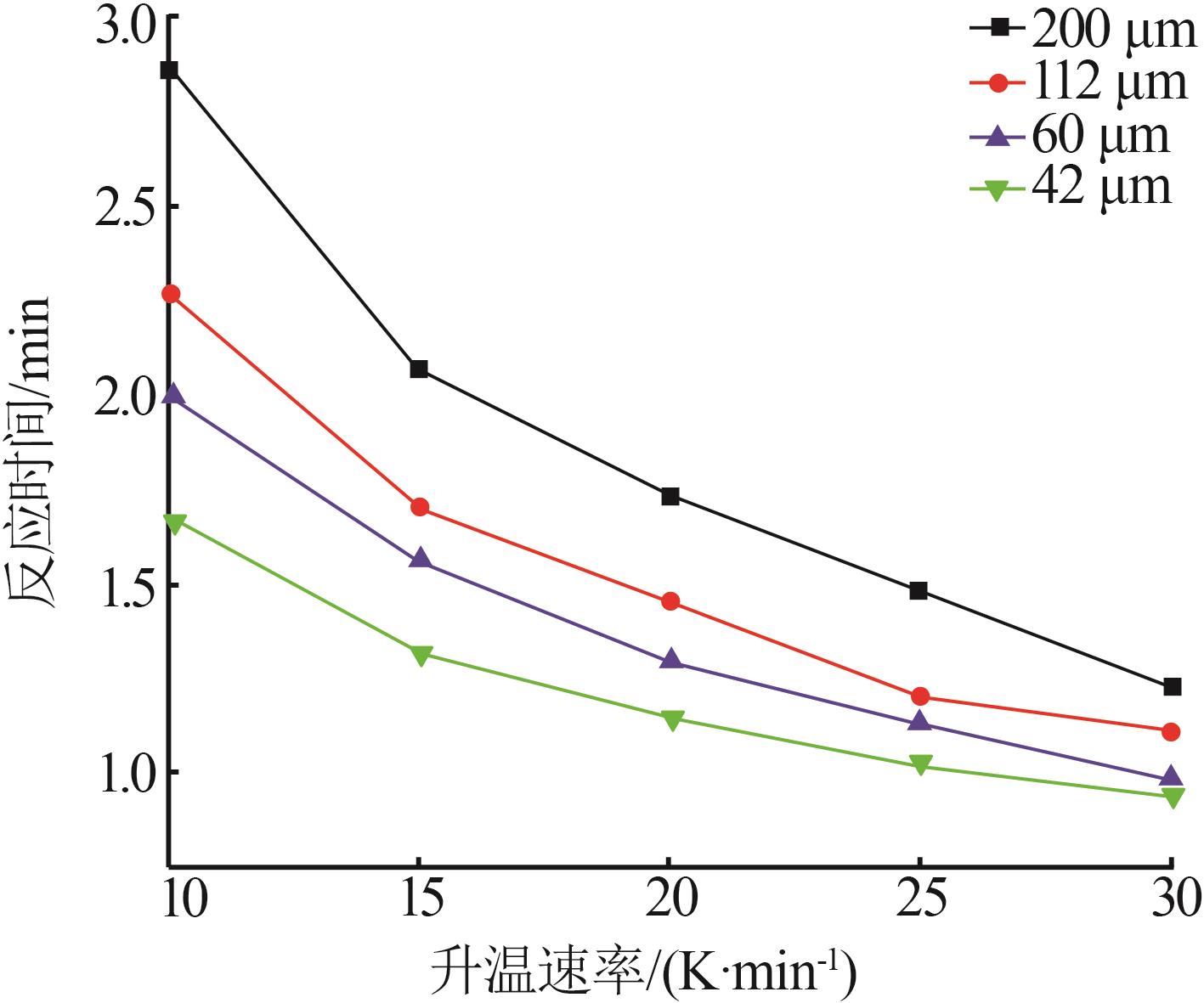
Table 7
Reaction time from 20% to 90% conversion oflimestone with different average particle sizesat different heating rates"
| 平均粒径/μm | t/min | |||
|---|---|---|---|---|
| 10 | 200 | 1 212 | 1 240 | 2.862 |
| 112 | 1 212 | 1 235 | 2.273 | |
| 60 | 1 210 | 1 230 | 2.000 | |
| 42 | 1 211 | 1 228 | 1.671 | |
| 15 | 200 | 1 214 | 1 245 | 2.069 |
| 112 | 1 214 | 1 239 | 1.700 | |
| 60 | 1 214 | 1 238 | 1.567 | |
| 42 | 1 212 | 1 232 | 1.320 | |
| 20 | 200 | 1 217 | 1 252 | 1.730 |
| 112 | 1 217 | 1 245 | 1.450 | |
| 60 | 1 216 | 1 242 | 1.300 | |
| 42 | 1 217 | 1 240 | 1.150 | |
| 25 | 200 | 1 220 | 1 260 | 1.483 |
| 112 | 1 219 | 1 250 | 1.200 | |
| 60 | 1 218 | 1 246 | 1.134 | |
| 42 | 1 220 | 1 246 | 1.028 | |
| 30 | 200 | 1 222 | 1 268 | 1.224 |
| 112 | 1 222 | 1 256 | 1.111 | |
| 60 | 1 222 | 1 251 | 0.989 | |
| 42 | 1 222 | 1 251 | 0.932 |
| 1 | 张程.全球能源危机与碳减排征途[J].检察风云,2021(21):68-69. |
| ZHANG Cheng.The global energy crisis and the journey of carbon reduction[J].Procuratorial Wind and Cloud,2021(21):68-69. | |
| 2 | 张生春.积极推进工业领域碳减排[J].中国发展观察,2021(21):16-18,41. |
| ZHANG Shengchun.Actively promote carbon emission reduction in the industrial sector[J].China Development Observation,2021(21):16-18,41. | |
| 3 | 刘淑娟,赵旭东,王娜,等.建材工业能源消耗与二氧化碳减排形势研究:以水泥及石灰行业为代表[J].居业,2021,13(8):137-138. |
| LIU Shujuan, ZHAO Xudong, WANG Na,et al.Research on energy consumption and carbon dioxide emission reduction of building materials industry:Represented by cement and lime industry[J].Create Living,2021,13(8):137-138. | |
| 4 | 李素珍,赵楠,张哲.中国城市尺度工业过程二氧化碳排放[C]//中国环境科学学会2021年科学技术年会——环境工程技术创新与应用分会场论文集(一).天津,2021:261-264. |
| 5 | 贾楠楠,李晓.碳达峰、碳中和背景下我国冶金石灰产业发展[J].耐火与石灰,2022,47(1):1-4. |
| JIA Nannan, LI Xiao.Development of metallurgical lime industry in China under the background of carbon peak and carbon neutralization[J].Refractories & Lime,2022,47(1):1-4. | |
| 6 | 郭汉杰,尹志明,王宏伟.冶金活性石灰烧制过程最佳工艺制度[J].北京科技大学学报,2008,30(2):148-151. |
| GUO Hanjie, YIN Zhiming, WANG Hongwei.Optimum schedule in calcination process of metallurgical active lime[J].Journal of Uni-versity of Science and Technology Beijing,2008,30(2):148-151. | |
| 7 | 张冬梅.浅析建筑材料石灰的性能及应用[J].四川水泥,2021(7):105-106. |
| ZHANG Dongmei.Analysis on the properties and application of building material lime[J].Sichuan Cement,2021(7):105-106. | |
| 8 | 王乃光,阿娜尔,刘启旺,等.有机酸盐强化石灰石湿法烟气脱硫试验研究[J].中国电机工程学报,2008,28(17):61-65. |
| WANG Naiguang, Naer A, LIU Qiwang,et al.Experimental investigation on intensifying effect of organic alkali on wet flue gas desulfurization with limestone[J].Proceedings of the CSEE,2008,28(17):61-65. | |
| 9 | 潘梦雅,李玉娇,陆伟星,等.生石灰成分中氧化钙含量对脱硫脱硝效率的影响[J].广州化工,2020,48(4):23-26. |
| PAN Mengya, LI Yujiao, LU Weixing,et al.Effect of calcium oxide content in lime composition on desulfurization and denitrification efficiency[J].Guangzhou Chemical Industry,2020,48(4):23-26. | |
| 10 | 张东方.石灰-石膏脱硫工艺运行的关键技术指标和管理要点[J].砖瓦,2020(4):20-24. |
| ZHANG Dongfang.Key technical indicators and management of lime-gypsum desulfurization process[J].Brick-Tile,2020(4):20-24. | |
| 11 | 苏晖.石灰石膏法烟气脱硫技术在大气污染治理中的应用[J].环境与发展,2019,31(7):86-87. |
| SU Hui.Application of lime gypsum flue gas desulfurization technology in air pollution control[J].Environment and Development, 2019,31(7):86-87. | |
| 12 | AR İ, DOĞU G.Calcination kinetics of high purity limestones[J].Chemical Engineering Journal,2001,83(2):131-137. |
| 13 | 张保生,刘建忠,周俊虎,等.粒度对石灰石分解动力学影响的热重实验研究[J].中国电机工程学报,2010,30(2):50-55. |
| ZHANG Baosheng, LIU Jianzhong, ZHOU Junhu,et al.Experimental study on the impaction of particle size to limestone decomposition kinetics by thermogravimetry[J].Proceedings of the CSEE,2010,30(2):50-55. | |
| 14 | 王理猷,薛正良,陈凯峰,等.大粒径石灰石高温分解动力学[J].重庆大学学报,2020,43(8):32-46. |
| WANG Liyou, XUE Zhengliang, CHEN Kaifeng,et al.High-temperature decomposition kinetics of large particle-size limesto-ne[J].Journal of Chongqing University,2020,43(8):32-46. | |
| 15 | 陈海,张世红,杨海平,等.大粒径石灰石热分解动力学研究[J].无机盐工业,2013,45(9):11-14. |
| CHEN Hai, ZHANG Shihong, YANG Haiping,et al.Study on thermal decomposition kinetics of limestone with large particle size[J].Inorganic Chemicals Industry,2013,45(9):11-14. | |
| 16 | 李佳容,朱建国,朱书骏,等.快速加热条件下碳酸钙分解动力学[J].中国粉体技术,2018,24(6):1-7. |
| LI Jiarong, ZHU Jianguo, ZHU Shujun,et al.Kinetics of calcium carbonate decomposition under rapid heating condition[J].China Powder Science and Technology,2018,24(6):1-7. | |
| 17 | 曹静,乔秀臣,柳成亮,等.石灰石在二氧化碳与空气混合气氛下的分解动力学[J].无机盐工业,2016,48(12):32-36. |
| CAO Jing, QIAO Xiuchen, LIU Chengliang,et al.A study on kinetics of limestone decomposition in air and CO2 atmospher-es[J].Inorganic Chemicals Industry,2016,48(12):32-36. | |
| 18 | 张文仙,刘联胜,曹和军,等.二氧化碳浓度对石灰石分解反应动力学的影响[J].无机盐工业,2020,52(3):59-63. |
| ZHANG Wenxian, LIU Liansheng, CAO Hejun,et al.Effect on kinetics of limestone decomposition under different CO2 atmospheres[J].Inorganic Chemicals Industry,2020,52(3):59-63. | |
| 19 | 胡荣祖,高胜利,赵凤起.热分析动力学[M].北京:科学出版社,2008:151-155,119-120. |
| 20 | 郭汉杰.活性石灰生产理论与工艺[M].北京:化学工业出版社,2014:14-24. |
| [1] | HUANG Tianyin, SUN Ling, ZHAO Qinzheng, CHEN Xin, SONG Xiaojie, WU Bingdang. Study on performance and mechanism of titanium salt coagulant for treatment of oily wastewater [J]. Inorganic Chemicals Industry, 2025, 57(2): 68-75. |
| [2] | WANG Jie, ZHAO Xubo, TANG Yong, QIN Lingyi, CHEN Xiaopeng, LIAO Dankui, TONG Zhangfa, WANG Linlin. Preparation and structural characterization of high specific surface area calcium hydroxide by wet digestion of quicklime [J]. Inorganic Chemicals Industry, 2024, 56(12): 104-112. |
| [3] | YANG Fengling,ZHAI Min,REN Lei,ZHANG Yuanyuan,CHENG Fangqin,DONG Hongyu. Influencing factors of crystallization products in wet desulfurization of carbide slag [J]. Inorganic Chemicals Industry, 2023, 55(2): 92-98. |
| [4] | LIU Yue, ZHENG Qiang, XING Jiabin, XING Run, MA Yali, JIA Songyan, LI Xue. Study on process and application of high-performance calcium hydroxide prepared by dry digestion from limestone [J]. Inorganic Chemicals Industry, 2023, 55(10): 42-49. |
| [5] | CHEN Xiaoqing, ZHOU Jian′an, WANG Yi, HAN Juan, WANG Bao, PEI Peiyan. Study on decomposition characteristic of limestone powder in high temperature flue gas of converter [J]. Inorganic Chemicals Industry, 2023, 55(10): 70-77. |
| [6] | YANG Yongyu,TIAN Peng,ZHOU Ruohui,XU Qianjin,LIU Kunji,NING Guiling. Effect of gibbsite activation on preparation of boehmite by hydrothermal method [J]. Inorganic Chemicals Industry, 2022, 54(9): 55-62. |
| [7] | XU Qingying,YANG Dingyi,LÜ Wei,LI Xiang,QIAN Yunfeng,DU Baocong. Effect of grinding time on properties of phosphogypsum based cementitious materials [J]. Inorganic Chemicals Industry, 2022, 54(5): 101-108. |
| [8] | HU Zhongyang,SITU Yue,HUANG Hong,ZOU Jiantao. Spherical silica synthesized from cheap silicon and its particle size control [J]. Inorganic Chemicals Industry, 2022, 54(5): 79-83. |
| [9] | LU Zheng,CHEN Kunfeng,XUE Dongfeng. Study on large-scale preparation and electrochemical properties of high thermal stabilized α-Fe2O3 [J]. Inorganic Chemicals Industry, 2022, 54(3): 45-50. |
| [10] | Wang Bin,Deng Xiaochuan,Shi Yifei,Dong Chaochao,Fan Faying,Zhu Chaoliang,Fan Jie,Ma Wanxia,Zuo Fangtao. Optimizing preparation process of lithium carbonate reaction crystallization by Taguchi experimental design method [J]. Inorganic Chemicals Industry, 2021, 53(8): 60-65. |
| [11] | Qiu Zhanjiang,Qin Xuezheng,Qin Xuehong,Yuan Shaoqiang,Yang Yuehui. Study on fractal characteristics of particle size distribution of zircon sand in ball milling [J]. Inorganic Chemicals Industry, 2020, 52(5): 56-58. |
| [12] | Jing Ting. Study on extrudability of silicone sealant by using modified nano-sized calcium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(4): 57-60. |
| [13] | Zhang Wenxian,Liu Liansheng,Cao Hejun,Wu Binke,Cheng Zhenpeng. Effect on kinetics of limestone decomposition under different CO2 atmospheres [J]. Inorganic Chemicals Industry, 2020, 52(3): 59-63. |
| [14] | Chen Wen,Long Xiang,Li Haiyan,Yang Hui,Jia Guifa,Jin Yaqiu. Determination of particle size of gas phase oxided titanium dioxide by laser particle size analyzer [J]. Inorganic Chemicals Industry, 2020, 52(2): 69-71. |
| [15] | Chang Yuefan,Zhang Huijie,Wang Shanshan,Xue Yongqiang. Controllable preparation of nano-calcium carbonate with different particle sizes [J]. Inorganic Chemicals Industry, 2020, 52(12): 29-33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||