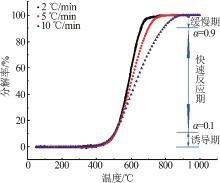
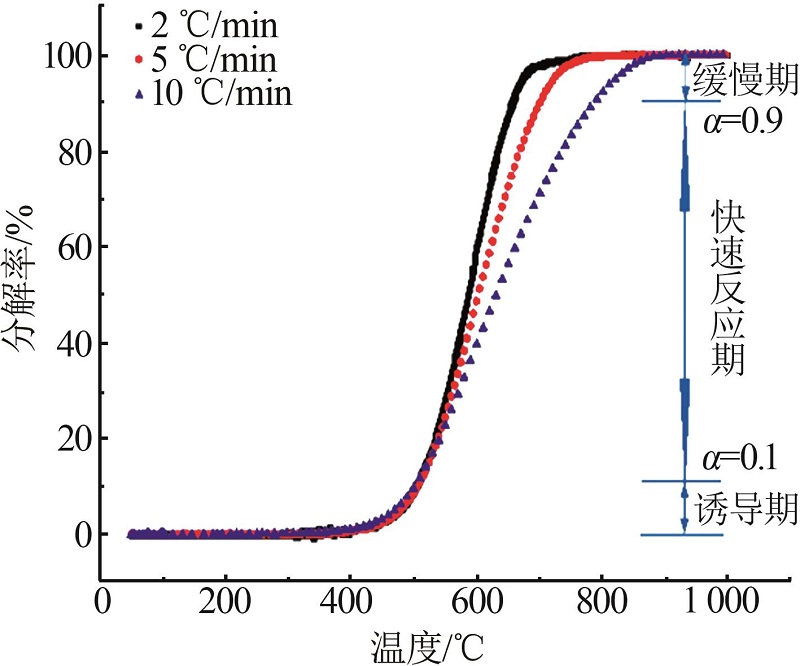
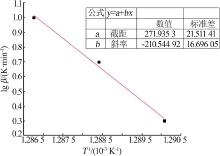
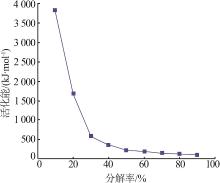
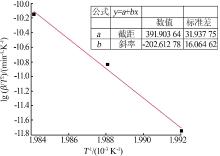
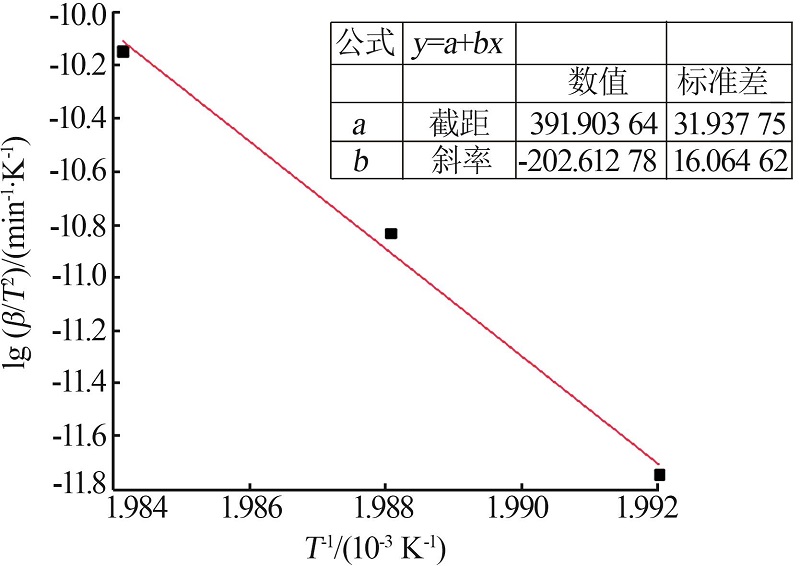
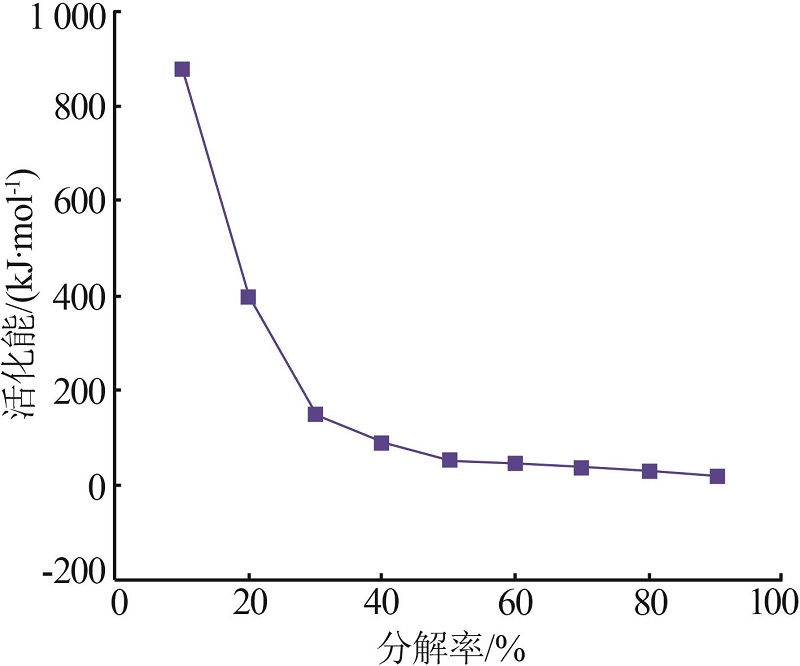
| [1] |
ZENG Yijun, JIANG Ziwen, JIAN Chengzong, QUAN Xuejun.
Study on deep extraction of chromium from calcium-free roasting slag of chromite ore
[J]. Inorganic Chemicals Industry, 2025, 57(1): 90-96.
|
| [2] |
MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru.
Kinetic study of lithium extraction from solution with iron-based ionic liquid system
[J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66.
|
| [3] |
FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui.
Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid
[J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43.
|
| [4] |
ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze.
Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3
[J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74.
|
| [5] |
CHENG Ziyang, CHEN Guofu.
Early hydration kinetics research of nano-SiO2 and cement composite cementitious materials
[J]. Inorganic Chemicals Industry, 2024, 56(7): 80-87.
|
| [6] |
ZHANG Yu, ZHAO Guiyan, TIAN Yongchang, QIU Xiaokui, SUN Jiali, XU Lixin.
Reaction kinetics of ethylenediamine hydrochloride with calcium hydroxide
[J]. Inorganic Chemicals Industry, 2024, 56(5): 64-69.
|
| [7] |
ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping.
Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash
[J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134.
|
| [8] |
FAN Fangfang, TONG Zhongkai, ZUO Weiyuan.
Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar
[J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115.
|
| [9] |
ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang.
Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings
[J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54.
|
| [10] |
ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan.
Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction
[J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84.
|
| [11] |
DING Ning, ZHANG Jian, PING Qingwei, SHENG Xueru, LI Na.
Study on adsorption and release properties of matrine by magnesium-modified diatomite
[J]. Inorganic Chemicals Industry, 2023, 55(11): 37-46.
|
| [12] |
LI Xiyan, ZHANG Hong, LIU Xuejing, YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen.
Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere
[J]. Inorganic Chemicals Industry, 2023, 55(10): 50-55.
|
| [13] |
PENG Jiaoyu, TAN Yuqin, YANG Keli, DONG Yaping, ZHANG Bo, LI Wu.
Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake
[J]. Inorganic Chemicals Industry, 2023, 55(10): 56-62.
|
| [14] |
WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun.
Study on decomposition process and thermal decomposition kinetics of ammonium sulfate
[J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92.
|
| [15] |
ZHU Yue,QIU Shengbo,LIU Chenglin,YU Jianguo.
Study on enhancement of spodumene phase reconstruction process by mechanical activation
[J]. Inorganic Chemicals Industry, 2023, 55(1): 81-86.
|
 ), LI Zhixun1, FENG Runtang1,2,3, ZHANG Jie1, ZHENG Quanfu1,2,3, SHI Xuwu2, DU Yongbin3
), LI Zhixun1, FENG Runtang1,2,3, ZHANG Jie1, ZHENG Quanfu1,2,3, SHI Xuwu2, DU Yongbin3