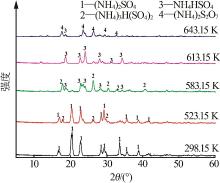
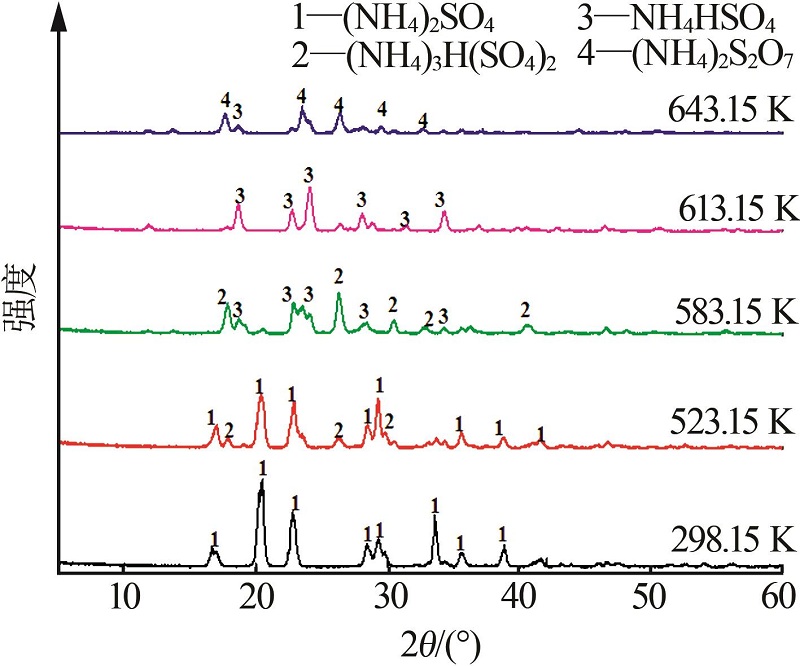
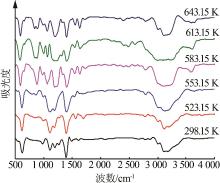
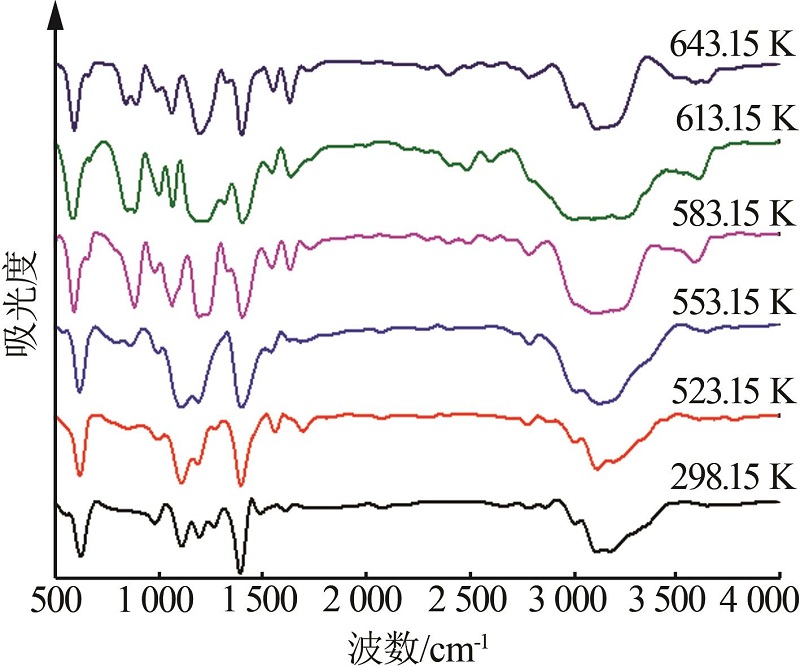
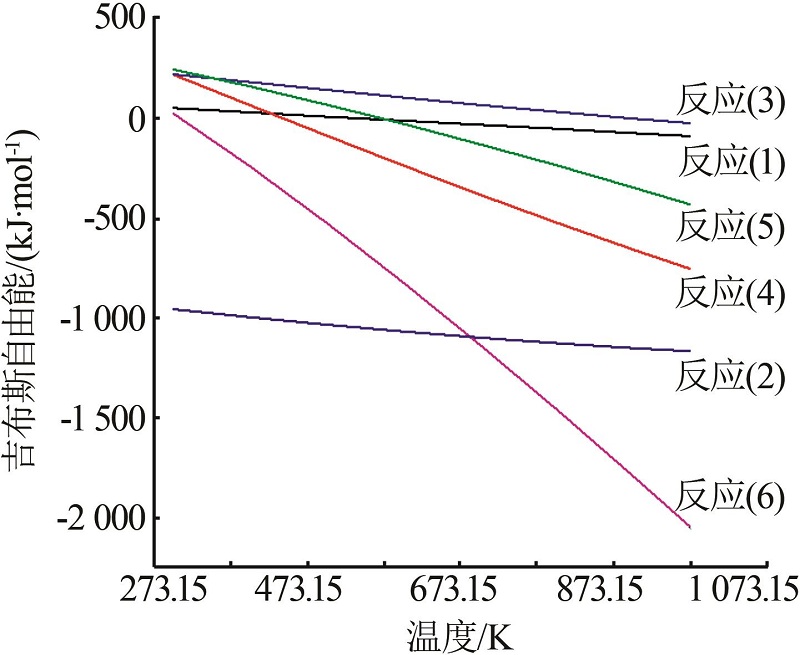
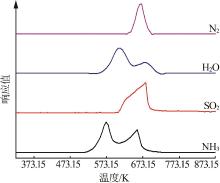
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (10): 86-92.doi: 10.19964/j.issn.1006-4990.2022-0105
• Research & Development • Previous Articles Next Articles
Study on decomposition process and thermal decomposition kinetics of ammonium sulfate
WU Di( ), LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun
), LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun
- School of Materials Science and Engineering;Shenyang University of Technology,Shenyang 110870,China
-
Received:2022-03-08Online:2023-10-10Published:2023-10-16
CLC Number:
Cite this article
WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun. Study on decomposition process and thermal decomposition kinetics of ammonium sulfate[J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92.
share this article
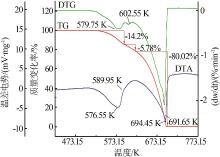
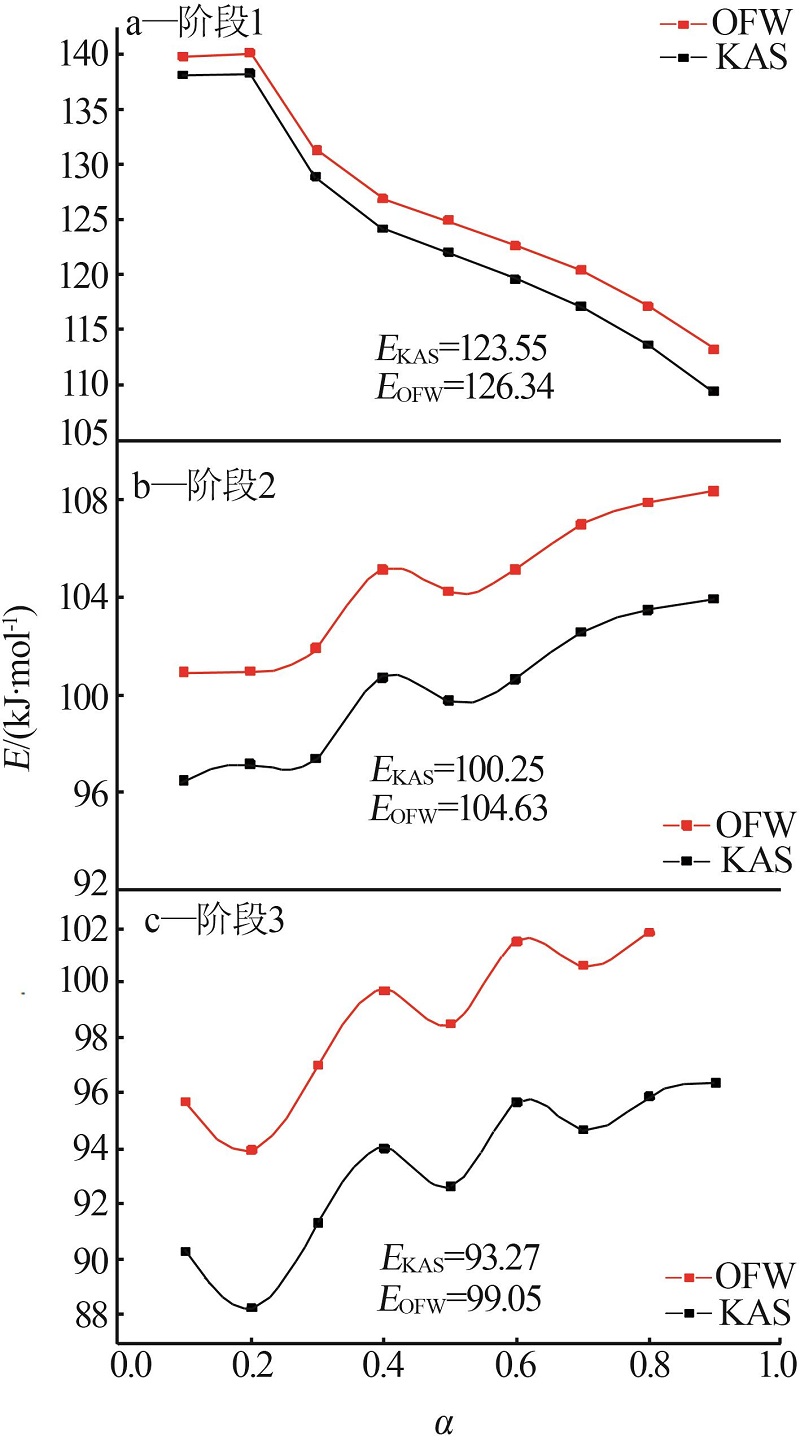
Table 2
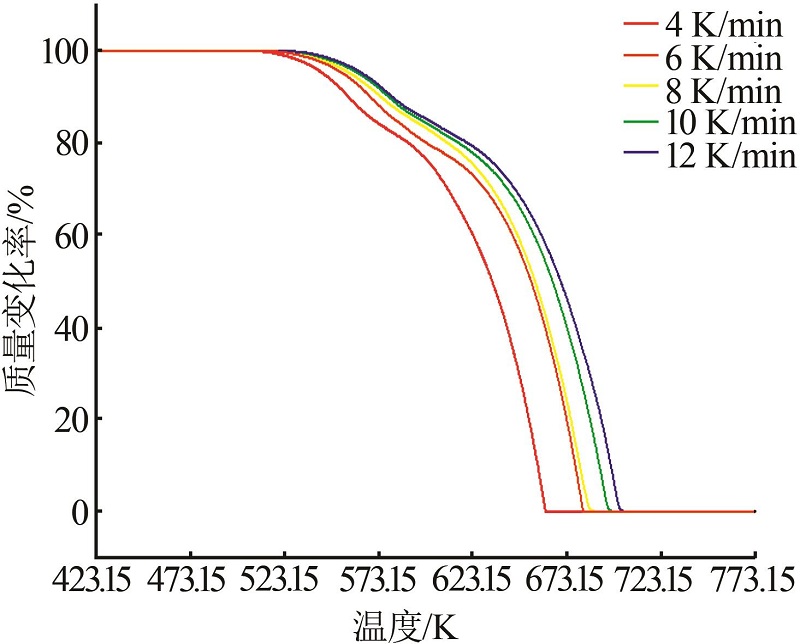
α-T data of ammonium sulfate at different heating rates"
| 阶段 | 升温速率/ (K·min-1) | 温度/K | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α=0.1 | α=0.2 | α=0.3 | α=0.4 | α=0.5 | α=0.6 | α=0.7 | α=0.8 | α=0.9 | ||
| 1 | 4 | 527.074 | 536.61 | 541.416 | 547.311 | 551.469 | 555.173 | 558.648 | 562.221 | 566.363 |
| 6 | 536.434 | 546.635 | 553.46 | 559.256 | 563.611 | 567.533 | 571.397 | 575.303 | 579.806 | |
| 8 | 539.245 | 549.356 | 556.176 | 561.854 | 566.718 | 571.328 | 575.467 | 579.908 | 584.906 | |
| 10 | 542.169 | 552.4 | 560.069 | 566.104 | 570.973 | 575.3 | 579.448 | 583.701 | 588.262 | |
| 12 | 545.182 | 555.169 | 562.435 | 568.201 | 572.901 | 577.243 | 581.326 | 585.999 | 591.967 | |
| 2 | 4 | 571.221 | 573.195 | 575.226 | 577.926 | 579.499 | 581.697 | 583.919 | 586.057 | 588.006 |
| 6 | 586.375 | 587.962 | 589.539 | 591.219 | 593.028 | 594.827 | 596.744 | 598.551 | 600.986 | |
| 8 | 592.578 | 594.517 | 596.464 | 598.429 | 600.327 | 602.248 | 603.649 | 605.421 | 607.225 | |
| 10 | 596.481 | 598.699 | 600.689 | 602.729 | 604.527 | 606.545 | 608.567 | 610.501 | 612.446 | |
| 12 | 600.525 | 602.713 | 604.619 | 606.705 | 608.786 | 611.074 | 613.12 | 615.337 | 617.551 | |
| 3 | 4 | 607.216 | 618.543 | 628.022 | 635.468 | 641.61 | 646.693 | 651.16 | 655.139 | 658.714 |
| 6 | 629.151 | 642.269 | 651.483 | 658.467 | 664.278 | 669.326 | 673.73 | 677.603 | 681.234 | |
| 8 | 629.781 | 642.085 | 650.9 | 657.726 | 663.193 | 668.549 | 673.045 | 677.251 | 681.123 | |
| 10 | 637.448 | 650.501 | 659.913 | 667.121 | 675.548 | 678.58 | 683.357 | 687.656 | 691.561 | |
| 12 | 641.613 | 654.517 | 664.004 | 671.574 | 678.076 | 684.014 | 689.832 | 693.736 | 697.537 | |
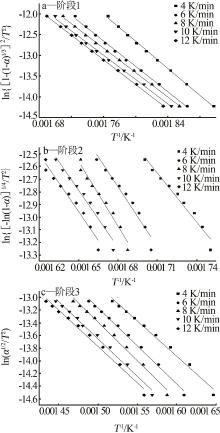
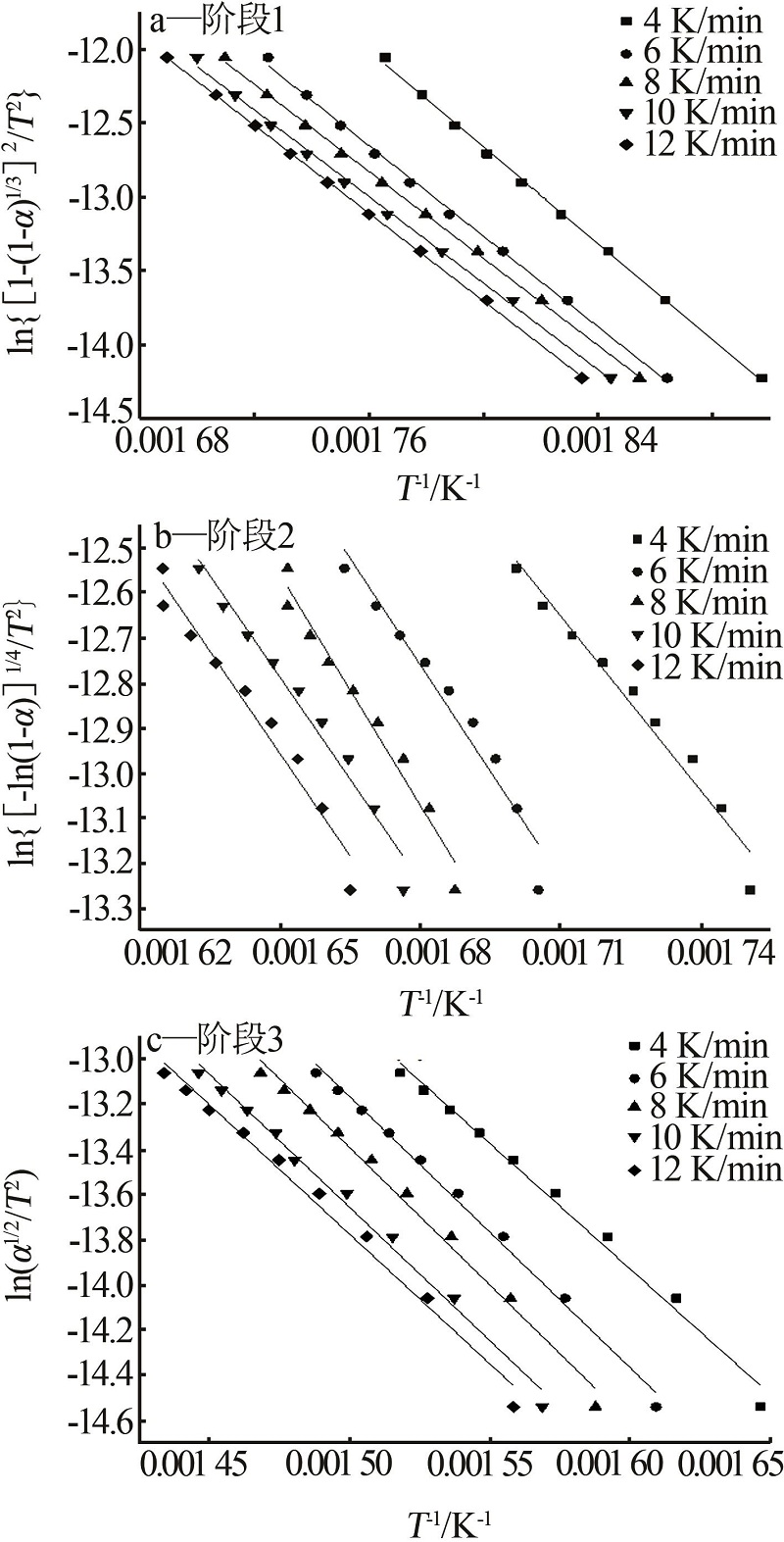
Table 3
Inference results by Coats-Redfern method"
| 机理函数 | 第一阶段 | 第二阶段 | 第三阶段 | |||||
|---|---|---|---|---|---|---|---|---|
| E/(kJ·mol-1) | R2 | E/(kJ·mol-1) | R2 | E/(kJ·mol-1) | R2 | |||
| α2 | 237.411 | 0.94 | 749.528 | 0.89 | 278.720 | 0.99 | ||
| α+(1-α)ln(1-α) | 277.369 | 0.99 | 834.992 | 0.92 | 308.109 | 0.99 | ||
| [1-(1-α)1/3]2 | 125.218 | 0.99 | 22.54 | 0.98 | 23.095 | 0.99 | ||
| 1-2/3α-(1-α)2/3 | 288.660 | 0.99 | 871.099 | 0.93 | 320.438 | 0.99 | ||
| [-ln(1-α)]1/4 | 35.472 | 0.99 | 110.214 | 0.99 | 38.650 | 0.98 | ||
| [-ln(1-α)]1/3 | 50.399 | 0.99 | 168.997 | 0.96 | 55.168 | 0.98 | ||
| [-ln(1-α)]1/2 | 80.25 | 0.99 | 258.461 | 0.96 | 88.203 | 0.98 | ||
| [-ln(1-α)]2/3 | 110.108 | 0.99 | 347.925 | 0.96 | 121.238 | 0.98 | ||
| -ln(1-α) | 170.081 | 0.99 | 526.884 | 0.97 | 187.308 | 0.98 | ||
| α1/2 | 55.580 | 0.97 | 179.873 | 0.89 | 97.706 | 0.99 | ||
| 1-(1-α)1/3 | 151.229 | 0.99 | 467.396 | 0.94 | 176.256 | 0.99 | ||
| 1-(1-α)1/2 | 142.761 | 0.99 | 440.415 | 0.93 | 158.099 | 0.99 | ||
| (1-α)-1 | 108.929 | 0.76 | 327.614 | 0.87 | 116.467 | 0.69 | ||
| (1-α)-2 | 27.048 | 0.77 | 120.788 | 0.89 | 243.835 | 0.71 | ||
| 1 | 韦聚才,石霖,吴旭.膜电极电解器电解脱硫废水制备硫酸铵副产氢[J].电化学,2022,28(5):4-12. |
| WEI Jucai, SHI Lin, WU Xu.Simultaneous hydrogen and (NH4)2SO4 productions from desulfurization wastewater electrolysis using MEA electrolyser[J].Journal of Electrochemistry,2022,28(5):4-12. | |
| 2 | ALAM T, SURYANTO P, KASTONO D,et al.Interactions of biochar briquette with ammonium sulfate fertilizer for controlled nitrogen loss in soybean intercopping with melaleuca cajuputi[J].Legume Research,2021,44:339-343. |
| 3 | YIN Shu, ALDAHRI T, ROHANI S,et al.Insights into the roasting kinetics and mechanism of blast furnace slag with ammonium sulfate for CO2 mineralization[J].Industrial & Engineering Chemistry Research,2019,58:14026-14036. |
| 4 | BATTISTA F, MASALA C, ZAMBONI A,et al.Valorisation of agricultural digestate for the ammonium sulfate recovery and soil improvers production[J].Waste and Biomass Valorization,2021,12(12):6903-6916. |
| 5 | SONG Xingfu, ZHAO Jingcai, LI Yunzhao,et al.Thermal decomposition mechanism of ammonium sulfate catalyzed by ferric oxi-de[J].Frontiers of Chemical Science and Engineering,2013,7(2):210-217. |
| 6 | KIYOURA R, URANO K.Mechanism,kinetics,and equilibrium of thermal decomposition of ammonium sulfate[J].Industrial & Engineering Chemistry Process Design and Development,1970,9(4):489-494. |
| 7 | KUMMER J T.Thermal decomposition of ammonium nitrate[J].Journal of the American Chemical Society,1947,69(10).Doi:10.1021/ja01202a507. |
| 8 | DIXON P.Formation of sulphamic acid during the thermal decomposition of ammonium sulphate[J].Nature,1944,154(3918):706. |
| 9 | ZHU Zhenping, NIU Hongxian, LIU Zhenyu,et al.Decomposition and reactivity of NH4HSO4 on V2O5/AC catalysts used for NO reduction with ammonia[J].Journal of Catalysis,2000,195(2):268-278. |
| 10 | LI Jie, LI Yandong, DUAN Huamei,et al.Experimental and kinetic study of magnesium extraction and leaching from laterite nickel ore by roasting with ammonium sulfate[J].Russian Journal of Non-Ferrous Metals,2018,59(6):596-604. |
| 11 | WU Yan, XIN Haixia, XU Yujun,et al.Preparation of ultrafine α-Al2O3 powder from fly ash by ammonium sulfate roasting technology[J].Journal of Physics:Conference Series,2019,1347(1).Doi:10.1088/1742-6596/1347/1/012111. |
| 12 | ZHANG Xianghui, HE Chuan, WANG Ling,et al.Non-isothermal kinetic analysis of thermal dehydration of La2(CO3)3·3.4H2O in air[J].Transactions of Nonferrous Metals Society of China,2014,24(10):3378-3385. |
| 13 | LIAN Jingbao, LIU Fan, ZHANG Jing,et al.Template-free hydrothermal synthesis of Gd2O2SO4:Eu3+ hollow spheres based on urea-ammonium sulfate (UAS) system[J].Optik,2016,127(20):8621-8628. |
| 14 | KIEFER W.Recent advances in linear and nonlinear Raman spectroscopy I[J].Journal of Raman Spectroscopy,2007,38(12):1538-1553. |
| 15 | ANTO P L, ANTO R J, VARGHESE H T,et al.FT-IR,FT-Raman and SERS spectra of anilinium sulfate[J].Journal of Raman Spectroscopy,2009,40(12):1810-1815. |
| 16 | ZAIRI I, GARCÍA-GRANDA S, LITAIEM H.Optical,thermal,vibrational and structural investigation to characterize phase transitions in the ammonium sulfate tellurate[J].Journal of Molecular Structure,2021,1242.Doi:10.1016/J.MOLSTRUC.2021.130816. |
| 17 | JAYARAMAN K, KÖK M V, GÖKALP I.Combustion mechanism and model free kinetics of different origin coal samples:Thermal analysis approach[J].Energy,2020,204.Doi:10.1016/j.energy.2020.117905. |
| 18 | ZHANG Wei, WANG Chang'an, LI Guangyu,et al.TG analysis and kinetic study of organic constituents in wastewater from coal-gasification process[J].Asia-Pacific Journal of Chemical Engineering,2017,12(3):406-414. |
| 19 | 赵鹏达,倪珊梅,宋小文,等.可见光诱导8-氨基喹啉衍生物二氟烷基化反应[J].精细化工,2020,37(8):1678-1683. |
| ZHAO Pengda, NI Shanmei, SONG Xiaowen,et al.Visible light induced difluoroalkylation of 8-aminoquinoline derivatives[J].Fine Chemicals,2020,37(8):1678-1683. | |
| 20 | SBIRRAZZUOLI N.Determination of pre-exponential factor and reaction mechanism in a model-free way[J].Thermochimica Acta,2020,691.Doi:10.1016/j.tca.2020.178707. |
| 21 | 阎杰,谢军,宋丽华,等.煤与焦油、秸秆混合压球共热解的动力学[J].煤炭学报,2018,43(7):2052-2060. |
| YAN Jie, XIE Jun, SONG Lihua,et al.Kinetics of co-pyrolysis of coal,tar and straw mixed briquette[J].Journal of China Coal Society,2018,43(7):2052-2060. | |
| 22 | SINGH S, PATIL T, TEKADE S P,et al.Studies on individual pyrolysis and co-pyrolysis of corn cob and polyethylene:Thermal degradation behavior,possible synergism,kinetics,and thermodynamic analysis[J].Science of the Total Environment,2021,783.Doi:10.1016/j.scitotenv.2021.147004. |
| 23 | ASHRAF A, SATTAR H, MUNIR S.A comparative applicability study of model-fitting and model-free kinetic analysis approaches to non-isothermal pyrolysis of coal and agricultural residues[J].Fuel,2019,240:326-333. |
| 24 | HU Jinghui, GONG Li, HE Jiyu,et al.Studies on multistep thermal decomposition behavior of polytriazole polyethylene oxide-tetrahydrofuran elastomer[J].Polymers for Advanced Technologies,2020,31(4):749-758. |
| 25 | 周绿山.硫酸铵与硫酸氢铵混合物催化热分解过程研究[D].昆明:昆明理工大学,2014. |
| [1] | HU Dian, GUO Ze, ZHANG Hanquan, LU Manman. Research on effects of roasting process and typical impurities on reduction and decomposition process of phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(7): 88-95. |
| [2] | QI Xingzhao, WANG Feng, WU Jie, ZHANG Qi, TANG Zhongfeng. Study on pyrolysis behavior and mechanism of calcium carbide slag/magnesium carbonate system [J]. Inorganic Chemicals Industry, 2024, 56(10): 118-126. |
| [3] | WEI Tianshun, JI Lijun, SHENG Yong, CHEN Kui, WU Yanyang, WU Bin. Study on fractional crystallization process of ammonium sulfate and sodium sulfate in high salt wastewater [J]. Inorganic Chemicals Industry, 2024, 56(1): 102-106. |
| [4] | WANG Song, WANG Jiawei, GOU Bibo, YANG Pan, HE Yue, YANG Chunyuan, WANG Haifeng. Study on distribution law of magnesium in leaching solution of rhodochrosite by complex salt crystallization method [J]. Inorganic Chemicals Industry, 2023, 55(4): 65-71. |
| [5] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [6] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [7] | PAN Sicheng,XU Hongbin,ZHANG Hongling,DONG Yuming,ZHANG Hongjun,LOU Taiping. Study on preparation of metatitanic acid by hydrolysis of leaching solution of roasted mixture of red mud and ammonium sulfate [J]. Inorganic Chemicals Industry, 2023, 55(2): 85-91. |
| [8] | YANG Han,DENG Fuli,DING Yao,LONG Bingwen. Experimental study on comprehensive utilization of citric acid soluble phosphorus sludge [J]. Inorganic Chemicals Industry, 2022, 54(12): 87-91. |
| [9] | QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin. Preparation and characterization of anhydrous calcium sulfate whisker [J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115. |
| [10] | Hu Min,Gong Hanzhang,Wu Huadong,Guo Jia,Zhang Linfeng,Zhou Yuxin. Preparation of battery-grade lithium carbonate from lithium-containing industrial waste [J]. Inorganic Chemicals Industry, 2020, 52(3): 80-84. |
| [11] | Liu Yuelong,Wang Linlin,Liu Gousheng. Extraction of rubidium and cerium salts from lithium tail liquid of medium and low grade lithium clay by ammonium sulfate process [J]. Inorganic Chemicals Industry, 2020, 52(11): 60-63. |
| [12] | Liu Gousheng,Wang Linlin,Liu Yuelong. Preparation of lithium carbonate from medium-and low-grade lithium clay by ammonium sulfate process [J]. Inorganic Chemicals Industry, 2020, 52(10): 125-129. |
| [13] | Su Xiaoli,Qin Fengting,Cai Tiancong,Ma Chunyu,Tang Changqing. Preparation of nano-sized zinc oxide by thermal decomposition with different carbonate precipitates [J]. Inorganic Chemicals Industry, 2019, 51(9): 36-39. |
| [14] | Cheng Jialin1,Chi Yongqing1,Jia Panfeng2,Lü Yazhen1,Wang Yuanyang1. A new process for producing potassium sulfate by conversion method of ammonium chloride purified by inducing ammonia [J]. Inorganic Chemicals Industry, 2019, 51(10): 56-59. |
| [15] | Tian Sen1,2,3,Ye Xuemei1,2,3,Feng Haitao1,2,Dong Yaping1,2,Li Wu1,2,Zhang Bo1,2,Li Bo1,2. Effect of temperature on preparation of chromium oxide by thermal decomposition of ammonium dichromate [J]. Inorganic Chemicals Industry, 2019, 51(10): 60-63. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||