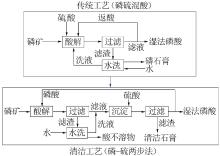
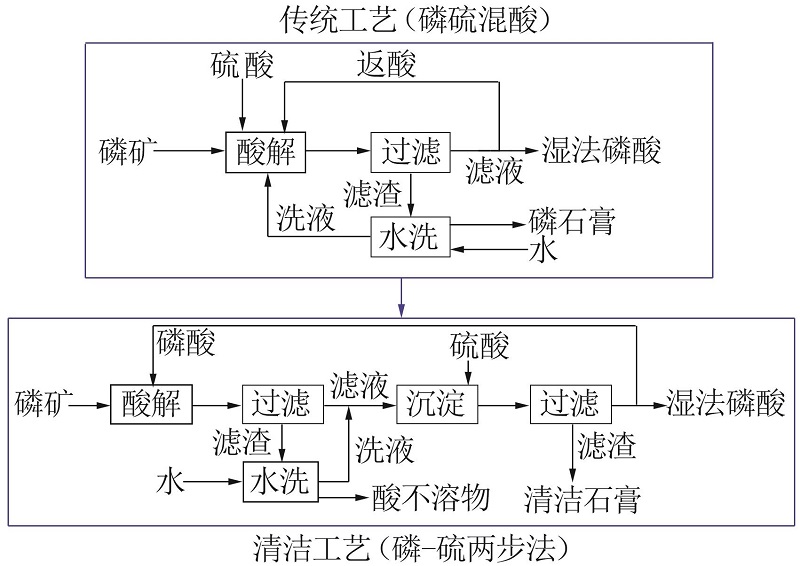
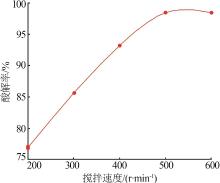
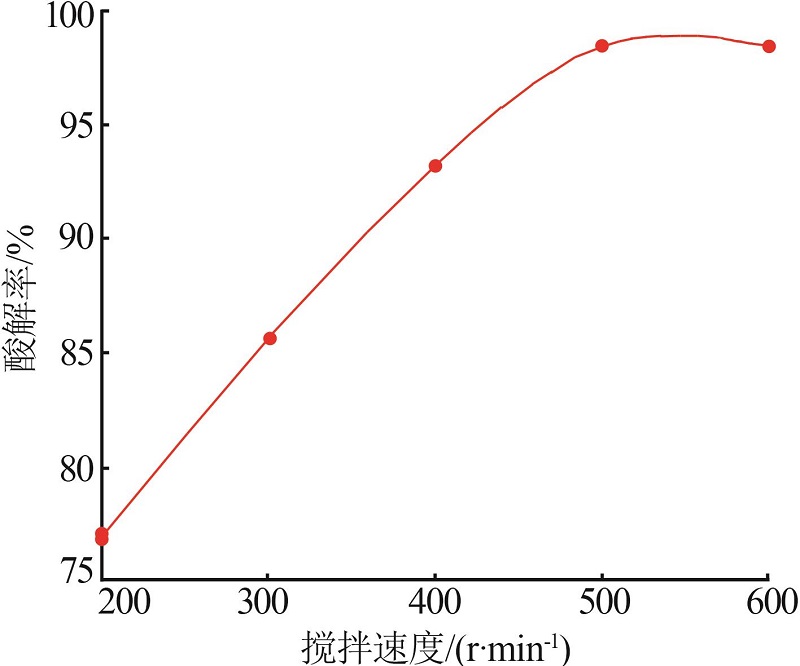
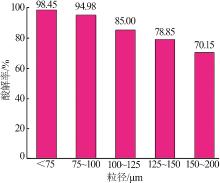
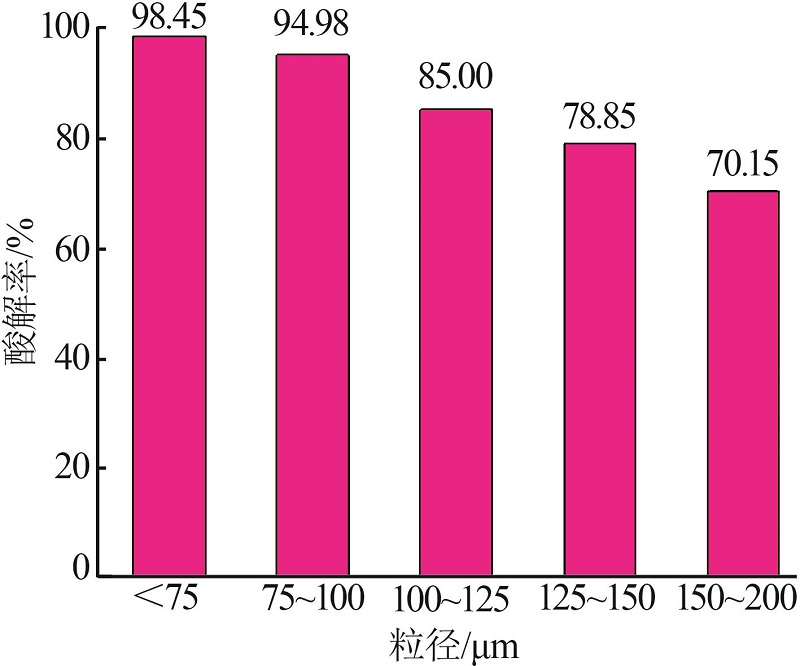
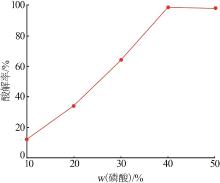
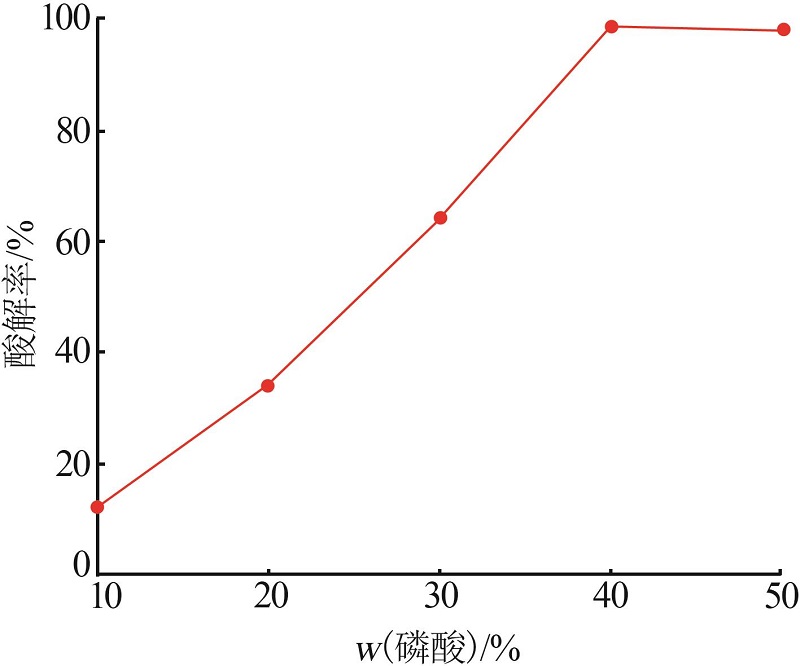
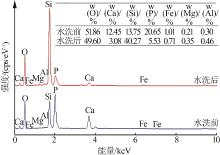
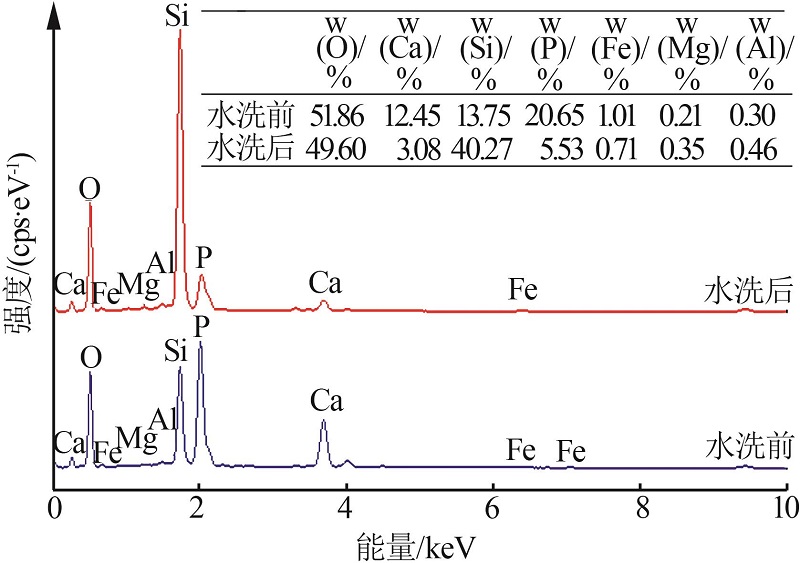
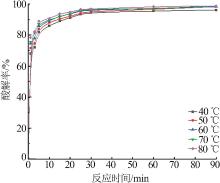
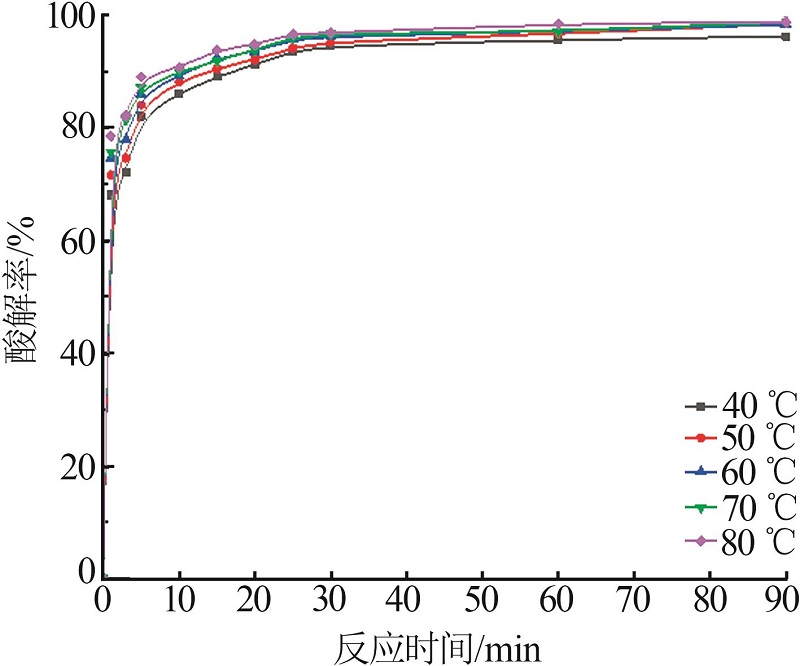
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (10): 96-101.doi: 10.19964/j.issn.1006-4990.2022-0103
• Research & Development • Previous Articles Next Articles
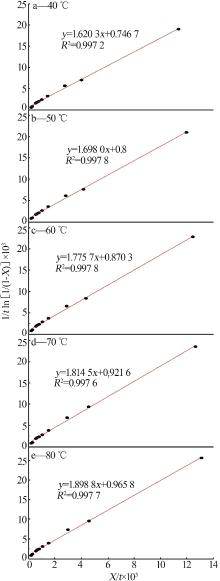
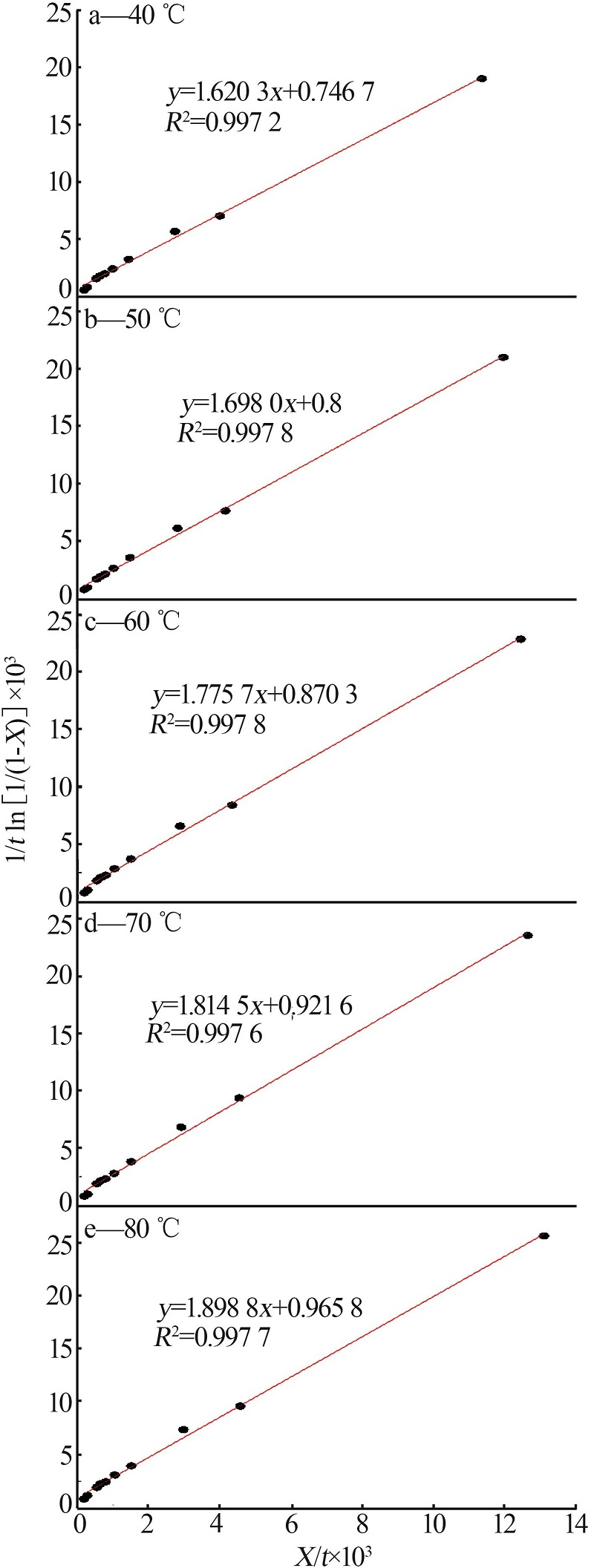
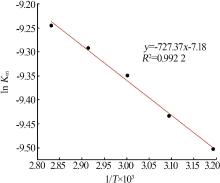
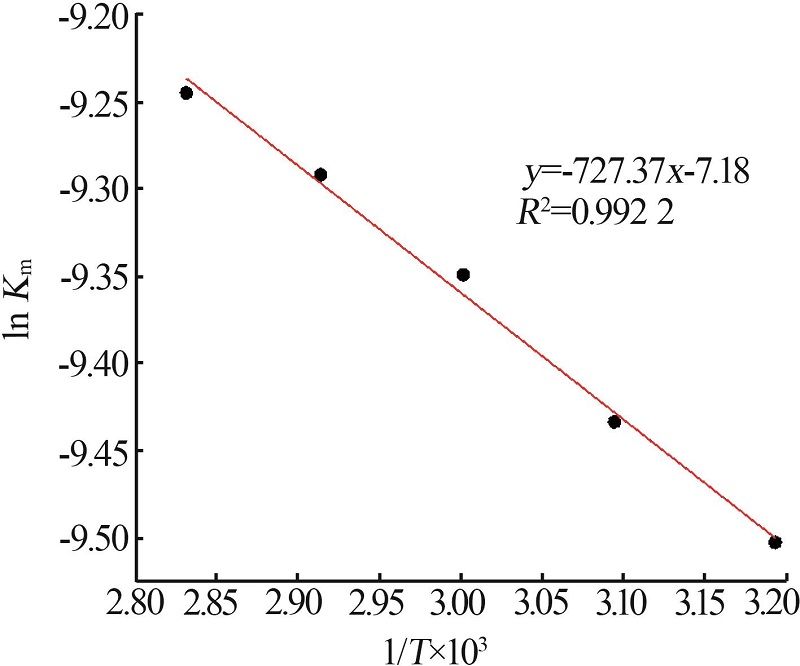
Leaching behavior of phosphate ore in phosphoric acid
ZHENG Hanxiao( ),LÜ Li(
),LÜ Li( ),TANG Shengwei,HE Yanjun,ZHANG Tao
),TANG Shengwei,HE Yanjun,ZHANG Tao
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2022-03-07Online:2022-10-10Published:2022-11-03 -
Contact:Lü Li E-mail:18860381132@163.com;lily@scu.edu.cn
CLC Number:
Cite this article
ZHENG Hanxiao,LÜ Li,TANG Shengwei,HE Yanjun,ZHANG Tao. Leaching behavior of phosphate ore in phosphoric acid[J]. Inorganic Chemicals Industry, 2022, 54(10): 96-101.
share this article
| [1] | LV Li, ZHENG Dongyao, TANG Shengwei, et al.Phosphate ore particles dissolution kinetics in hydrochloric acid based on a structure-related segmented model[J].Powder Technology, 2021, 392: 141-149. |
| [2] | 廖杭, 吕莉, 唐盛伟, 等. 盐酸法制磷酸中氯化钙废水的除氟工艺[J].工业水处理, 2021, 41(11): 89-93. |
| LIAO Hang, Li LÜ, TANG Shengwei, et al.Defluorination process of calcium chloride wastewater from phosphoric acid production by hydrochloric acid method[J].Industrial Water Treatment, 2021, 41(11): 89-93. | |
| [3] | 郑东钥, 唐盛伟, 吕莉.盐酸分解高硅混合型胶磷矿工艺研究[J].无机盐工业, 2021, 53(2): 81-83, 104. |
| ZHENG Dongyao, TANG Shengwei, Li LÜ.Study on process of hydrochloric acid leaching high silicon mixed phosphate ore[J].Inorganic Chemicals Industry, 2021, 53(2): 81-83, 104. | |
| [4] | 何燕君, 郑寒笑, 吕莉, 等. 低品位混合型磷矿的碳热还原工艺[J].无机盐工业, 2021, 53(11): 95-99. |
| HE Yanjun, ZHENG Hanxiao, Li LÜ, et al.Carbothermal reduction process of low-grade mixed phosphate ore[J].Inorganic Che-Industry micals, 2021, 53(11): 95-99. | |
| [5] | ZHANG P.Comprehensive recovery and sustainable development of phosphate resources[J].Procedia Engineering, 2014, 83: 37-51. |
| [6] | LASSIS M, MIZANE A, DADDA N, et al.Dissolution of Djebel Onk phosphate ore using sulfuric acid[J].Environmental Nanotechnology,Monitoring & Management, 2015, 4: 12-16. |
| [7] | ANTAR K, JEMAL M.Kinetics and thermodynamics of the attack of a phosphate ore by acid solutions at different temperatures[J].Thermochimica Acta, 2008, 474(1/2): 32-35. |
| [8] | CANUT M M C, JACOMINO V M F, BRÅTVEIT K, et al.Microstructural analyses of phosphogypsum generated by Brazilian fertilizer industries[J].Materials Characterization, 2008, 59(4): 365-373. |
| [9] | TAYIBI H, CHOURA M, LÓPEZ F A, et al.Environmental impact and management of phosphogypsum[J].Journal of Environmental Management, 2009, 90(8): 2377-2386. |
| [10] | AL-FARISS T F, ARAFAT Y, EL-ALEEM F A ABD, et al.Investigating sodium sulphate as a phosphate depressant in acidic media[J].Separation and Purification Technology, 2014, 124: 163-169. |
| [11] | 曾亚平, 党亚固, 费德君.制备高品质磷石膏的湿法磷酸新工艺的开发[J].化工进展, 2015, 34(S1): 167-172. |
| ZENG Yaping, DANG Yagu, FEI Dejun.Development of the new wet phosphoric process of preparing high-quality phosphogyps-um[J].Chemical Industry and Engineering Progress, 2015, 34(S1): 167-172. | |
| [12] | SERDYUK V V, PANOV V P, TERESHCHENKO L Y, et al.Mechanism of the dissolution of apatite by phosphoric acid in the presence of electrolytes[J].Journal of Applied Chemistry of the Ussr, 1982, 55(10): 2004-2008. |
| [13] | HUFFMAN E O, CATE W E, DEMING M E, et al.Solubility of phosphates,rates of solution of calcium phosphates in phosphoric acid solutions[J].Journal of Agricultural and Food Chemistry, 1957, 5(4): 266-275. |
| [14] | SIKDAR S K,ORE F, MOORE J H.Crystallization of calcium sulfate hemihydrate in reagent-grade phosphoric acid[J].Industrial Mineral Technology, 1980, 9: 133-137. |
| [15] | ECONOMOU E D, VAIMAAKIS T C, PAPAMICHAEL E M.The kinctics of dissolution of the carbonate minerals of phosphate ores using dilute acetic acid solutions:The case of pH range from 3.96 to 6.40[J].Journal of Colloid and Interface Science, 2002, 24(1): 133-141. |
| [16] | SIETSE V D S, MESZAROS Y, MARCHEE W G J, et al.The digestion of phosphate ore in phosphoric acid[J].Industrial & Engineering Chemistry Research, 1987, 26(12): 2501-2505. |
| [17] | 严永华, 刘期崇, 夏代宽, 等. 磷酸分解磷矿石的动力学[J].高校化学工程学报, 1998, 12(3): 265-270. |
| YAN Yonghua, LIU Qichong, XIA Daikuan, et al.Reaction kinetics of phosphate ore in phosphoric acid[J].Journal of Chemical Engineering of Chinese Universities, 1998, 12(3): 265-270. | |
| [18] | 周海, 杨三可, 解田.磷酸分解磷矿的动力学及影响因素研究[J].应用化工, 2021, 50(6): 1472-1477. |
| ZHOU Hai, YANG Sanke, XIE Tian.Study on the dissolution kinetics of phosphate ore in phosphoric acid and its influencing factors[J].Applied Chemical Industry, 2021, 50(6): 1472-1477. | |
| [19] | 谢晨光, 唐盛伟, 王辛龙, 等. Ca(H2PO4)2-H3PO4-H2O体系的相平衡过程研究[J].无机盐工业, 2019, 51(2): 26-29. |
| XIE Chenguang, TANG Shengwei, WANG Xinlong, et al.Study on phase equilibrium of Ca(H2PO4)2-H3PO4-H2O system[J].Inorganic Chemicals Industry, 2019, 51(2): 26-29. |
| [1] | ZENG Yijun, JIANG Ziwen, JIAN Chengzong, QUAN Xuejun. Study on deep extraction of chromium from calcium-free roasting slag of chromite ore [J]. Inorganic Chemicals Industry, 2025, 57(1): 90-96. |
| [2] | MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru. Kinetic study of lithium extraction from solution with iron-based ionic liquid system [J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66. |
| [3] | FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui. Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid [J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43. |
| [4] | ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. |
| [5] | CHENG Ziyang, CHEN Guofu. Early hydration kinetics research of nano-SiO2 and cement composite cementitious materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 80-87. |
| [6] | ZHANG Yu, ZHAO Guiyan, TIAN Yongchang, QIU Xiaokui, SUN Jiali, XU Lixin. Reaction kinetics of ethylenediamine hydrochloride with calcium hydroxide [J]. Inorganic Chemicals Industry, 2024, 56(5): 64-69. |
| [7] | WANG Jianrui, ZHANG Song, ZHANG Jie. Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63. |
| [8] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [9] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [10] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [11] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [12] | ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction [J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84. |
| [13] | DING Ning, ZHANG Jian, PING Qingwei, SHENG Xueru, LI Na. Study on adsorption and release properties of matrine by magnesium-modified diatomite [J]. Inorganic Chemicals Industry, 2023, 55(11): 37-46. |
| [14] | LI Xiyan, ZHANG Hong, LIU Xuejing, YANG Hao, XU Shuai, LI Jiaxin, XIE Jiaqi, XU Guangwen. Study on decomposition characteristic and kinetics of magnesite in inhibitory atmosphere [J]. Inorganic Chemicals Industry, 2023, 55(10): 50-55. |
| [15] | PENG Jiaoyu, TAN Yuqin, YANG Keli, DONG Yaping, ZHANG Bo, LI Wu. Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake [J]. Inorganic Chemicals Industry, 2023, 55(10): 56-62. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||