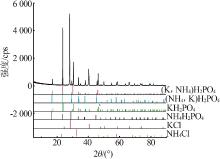
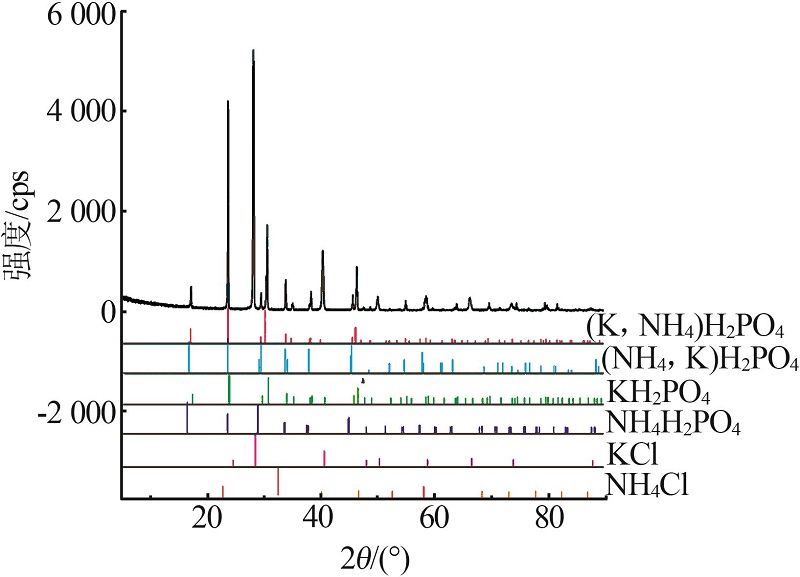
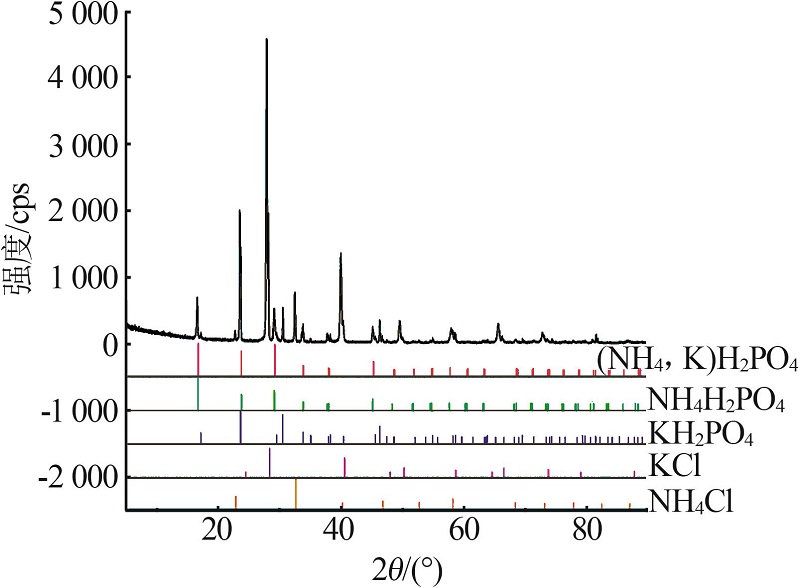
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (10): 102-108.doi: 10.19964/j.issn.1006-4990.2022-0050
• Research & Development • Previous Articles Next Articles
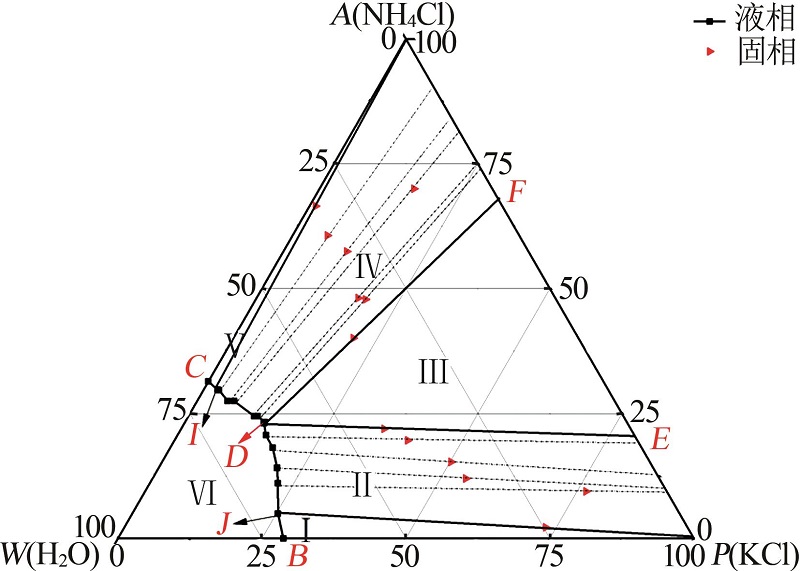
Study on phase equilibria of reciprocal quaternary system of K+,NH4+//Cl-,H2PO4--H2O at 313.15 K
FAN Xiaojuan( ),ZHU Jing,DENG Wenqing,CHEN Yan,LI Tianxiang(
),ZHU Jing,DENG Wenqing,CHEN Yan,LI Tianxiang( )
)
- School of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
-
Received:2022-01-25Online:2022-10-10Published:2022-11-03 -
Contact:LI Tianxiang E-mail:1733744949@qq.com;txli@gzu.edu.cn
CLC Number:
Cite this article
FAN Xiaojuan,ZHU Jing,DENG Wenqing,CHEN Yan,LI Tianxiang. Study on phase equilibria of reciprocal quaternary system of K+,NH4+//Cl-,H2PO4--H2O at 313.15 K[J]. Inorganic Chemicals Industry, 2022, 54(10): 102-108.
share this article
Table 1
Solubility data of ternary system of KCl-NH4Cl-H2O at 313.15 K"
| 编号 | 液相 | 湿渣 | 平衡 固相 | |||||
|---|---|---|---|---|---|---|---|---|
w (KCl) | w (NH4Cl) | w (H2O) | w (KCl) | w (NH4Cl) | w (H2O) | |||
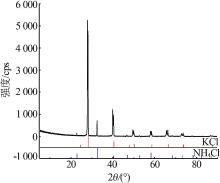
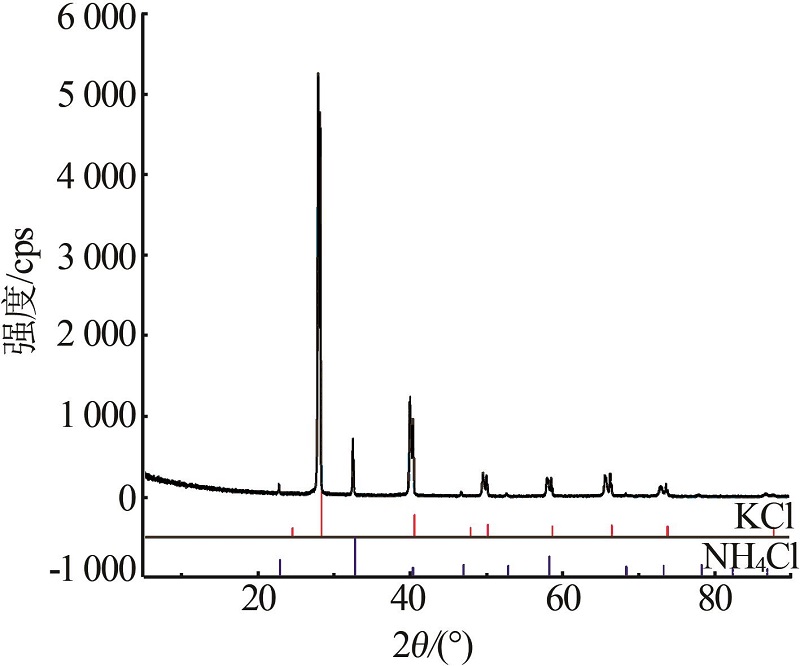
| 1(B) | 28.72 | 0.00 | 71.28 | — | — | — | KCl | |
| 2 | 25.31 | 4.95 | 69.74 | 73.14 | 2.22 | 24.64 | (K,NH4)Cl | |
| 3 | 22.26 | 11.04 | 66.70 | 76.56 | 9.43 | 14.01 | (K,NH4)Cl | |
| 4 | 20.58 | 14.12 | 65.30 | 54.41 | 12.00 | 33.59 | (K,NH4)Cl | |
| 5 | 17.81 | 18.14 | 64.05 | 50.18 | 15.32 | 34.50 | (K,NH4)Cl | |
| 6 | 15.37 | 20.67 | 63.96 | 40.53 | 19.62 | 39.85 | (K,NH4)Cl | |
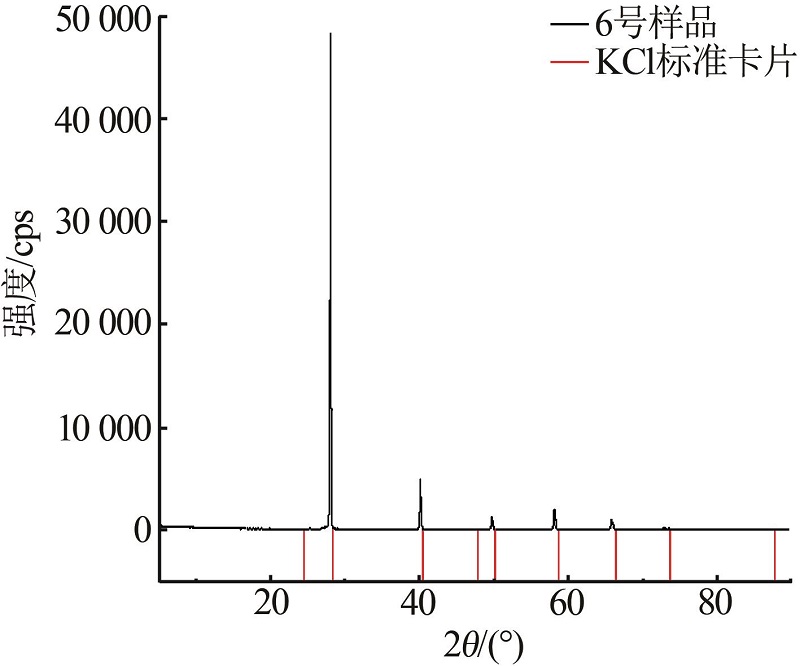
| 7 | 13.83 | 23.05 | 63.11 | 35.36 | 21.03 | 43.61 | (K,NH4)Cl | |
| 8(D) | 13.85 | 23.30 | 62.85 | 20.80 | 40.00 | 39.20 | (K,NH4)Cl+ (NH4,K)Cl | |
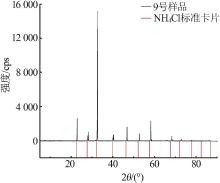
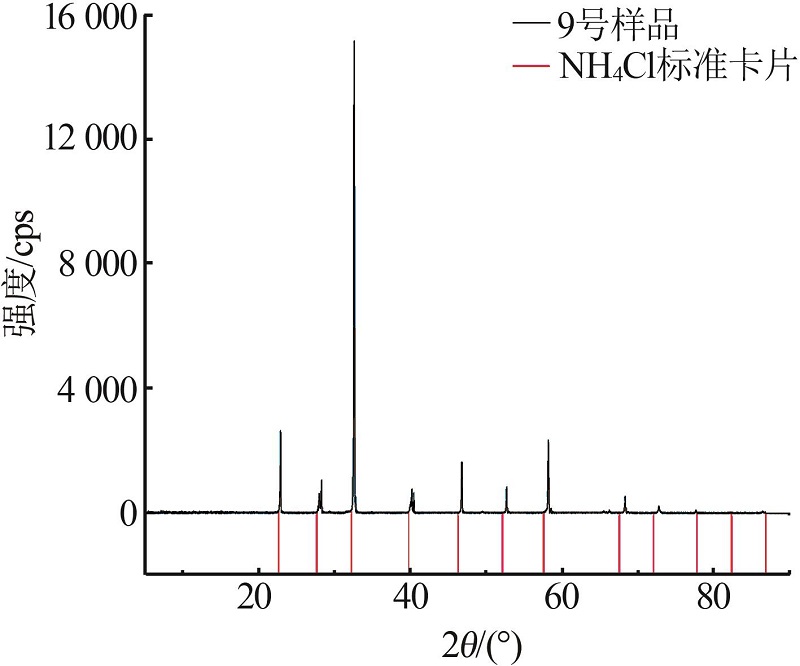
| 9 | 12.01 | 24.48 | 63.51 | 19.00 | 47.70 | 33.30 | (NH4,K)Cl | |
| 10 | 11.44 | 24.48 | 64.09 | 17.56 | 48.00 | 34.44 | (NH4,K)Cl | |
| 11 | 6.44 | 27.47 | 66.09 | 16.39 | 70.00 | 13.61 | (NH4,K)Cl | |
| 12 | 5.29 | 27.53 | 67.18 | 11.00 | 57.43 | 31.57 | (NH4,K)Cl | |
| 13 | 2.73 | 29.67 | 67.60 | 6.00 | 60.08 | 33.92 | (NH4,K)Cl | |
| 14 | 2.38 | 29.80 | 67.82 | 1.03 | 65.16 | 33.81 | (NH4,K)Cl | |
| 15(C) | 0.00 | 31.41 | 68.59 | — | — | — | NH4Cl | |
Table 2
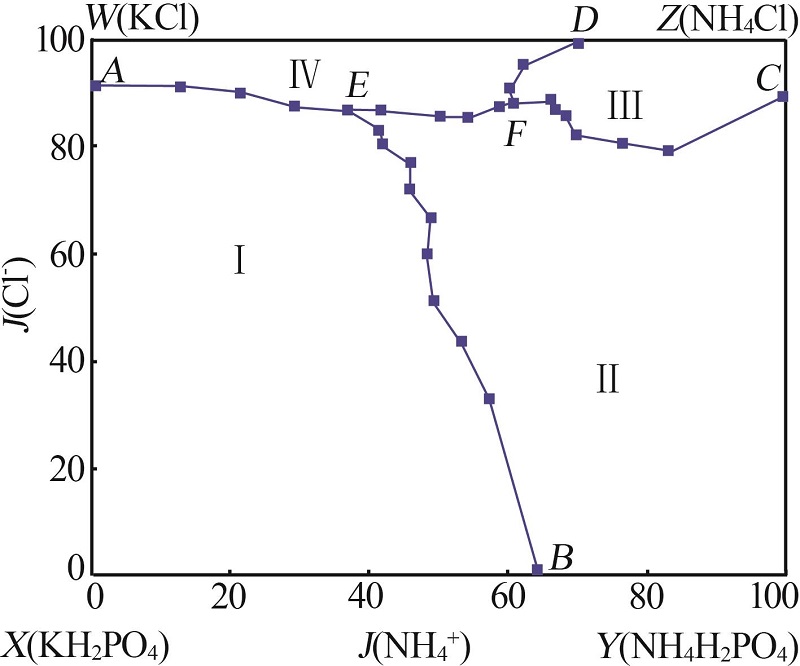
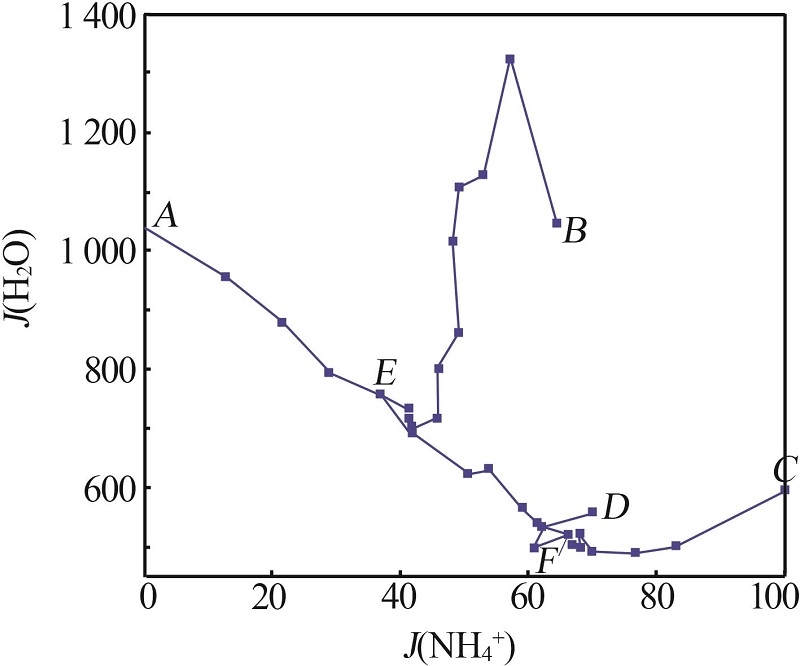
Solubility data of the reciprocal quaternary system of K+,NH4+//Cl-,H2PO4- -H2O at 313.15 K"
| 编号 | 液相质量分数/% | 耶涅克指数J/% | 平衡固相 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2PO4- | Cl- | NH4+ | K+ | H2O | NH4+ | Cl- | H2O | |||
| 1(A) | 3.19 | 12.04 | 0.00 | 14.53 | 70.24 | 0.00 | 91.19 | 1 043.95 | KCl+KH2PO4 | |
| 2 | 3.41 | 12.80 | 0.95 | 14.22 | 68.62 | 12.70 | 91.15 | 958.94 | KNl+KNP | |
| 3 | 4.07 | 13.59 | 1.66 | 13.14 | 67.54 | 21.51 | 90.15 | 879.24 | KNl+KNP | |
| 4 | 5.37 | 14.20 | 2.39 | 12.54 | 65.50 | 29.23 | 87.89 | 795.22 | KNl+KNP | |
| 5(E) | 6.01 | 14.52 | 3.13 | 11.63 | 64.71 | 36.82 | 86.89 | 760.00 | KNl+KNP+NKP | |
| 6 | 6.37 | 15.48 | 3.78 | 11.45 | 62.91 | 41.72 | 86.95 | 693.29 | KNl+KNP+NKl+NKP | |
| 7 | 7.49 | 16.37 | 4.91 | 10.39 | 60.84 | 50.58 | 85.70 | 625.05 | KNl+KNP+NKl+NKP | |
| 8 | 7.56 | 16.31 | 5.16 | 9.55 | 61.43 | 53.96 | 85.55 | 632.38 | KNl+KNP+NKl+NKP | |
| 9 | 6.92 | 17.89 | 6.42 | 9.67 | 59.10 | 58.99 | 87.64 | 568.05 | KNl+KNP+NKl+NKP | |
| 10 | 6.97 | 18.64 | 6.81 | 9.29 | 58.29 | 61.37 | 88.00 | 540.02 | KNl+KNP+NKl+NKP | |
| 11(F) | 6.99 | 19.34 | 7.38 | 8.15 | 58.14 | 66.26 | 88.36 | 521.30 | KNl+NKl+NKP | |
| 12(B) | 31.16 | 0.00 | 3.73 | 4.47 | 60.64 | 64.40 | 0.00 | 1 047.74 | KNP+NKP | |
| 13 | 18.93 | 3.43 | 3.01 | 4.89 | 69.73 | 57.14 | 33.23 | 1 323.88 | KNP+NKP | |
| 14 | 18.08 | 5.15 | 3.18 | 6.09 | 67.51 | 53.06 | 43.87 | 1 128.26 | KNP+NKP | |
| 15 | 16.04 | 6.19 | 3.03 | 6.73 | 68.01 | 49.36 | 51.41 | 1 108.97 | KNP+NKP | |
| 16 | 14.30 | 7.77 | 3.19 | 7.42 | 67.32 | 48.21 | 59.85 | 1 017.53 | KNP+NKP | |
| 17 | 13.40 | 9.85 | 3.69 | 8.26 | 64.79 | 49.20 | 66.85 | 862.75 | KNP+NKP | |
| 18 | 11.96 | 11.25 | 3.64 | 9.33 | 63.82 | 45.85 | 72.08 | 802.28 | KNP+NKP | |
| 19 | 10.60 | 13.10 | 3.96 | 10.14 | 62.20 | 45.83 | 77.22 | 719.60 | KNP+NKP | |
| 20 | 9.14 | 14.00 | 3.68 | 11.03 | 62.14 | 41.96 | 80.77 | 703.66 | KNP+NKP | |
| 21 | 8.18 | 14.17 | 3.66 | 11.25 | 62.74 | 41.33 | 82.61 | 717.86 | KNP+NKP | |
| 22 | 7.97 | 14.01 | 3.64 | 11.16 | 63.21 | 41.44 | 82.82 | 733.08 | KNP+NKP | |
| 23(E) | 6.01 | 14.52 | 3.13 | 11.63 | 64.71 | 36.82 | 86.89 | 760.00 | KNl+KNP+NKP | |
| 24(C) | 6.21 | 18.86 | 10.75 | 0.00 | 64.18 | 100.00 | 89.29 | 596.20 | NH4Cl+NH4H2PO4 | |
| 25 | 12.47 | 17.52 | 9.34 | 4.08 | 56.59 | 83.24 | 79.40 | 503.13 | NKl+NKP | |
| 26 | 11.86 | 17.99 | 8.70 | 5.72 | 55.73 | 76.72 | 80.63 | 490.16 | NKl+NKP | |
| 27 | 10.76 | 18.20 | 7.90 | 7.43 | 55.71 | 69.75 | 82.27 | 494.24 | NKl+NKP | |
| 28 | 8.32 | 18.65 | 7.50 | 7.64 | 57.88 | 68.03 | 86.02 | 523.85 | NKl+NKP | |
| 29 | 8.66 | 19.18 | 7.58 | 7.63 | 56.95 | 68.28 | 85.87 | 500.38 | NKl+NKP | |
| 30 | 7.95 | 19.28 | 7.55 | 8.11 | 57.11 | 66.86 | 86.93 | 505.32 | NKl+NKP | |
| 31(F) | 6.99 | 19.34 | 7.38 | 8.15 | 58.14 | 66.26 | 88.36 | 521.30 | KNl+NKl+NKP | |
| 32(D) | 0.00 | 22.03 | 7.86 | 7.25 | 62.86 | 70.15 | 100.00 | 559.91 | KNl+KNP | |
| 33 | 2.66 | 21.10 | 6.99 | 9.18 | 60.05 | 62.27 | 95.60 | 533.78 | KNl+KNP | |
| 34 | 5.46 | 20.52 | 6.91 | 9.87 | 57.24 | 60.28 | 91.16 | 498.85 | KNl+KNP | |
| 35 | 7.03 | 19.86 | 6.78 | 9.65 | 56.68 | 60.36 | 88.57 | 496.00 | KNl+KNP | |
| 36 | 7.41 | 19.54 | 6.94 | 9.56 | 56.55 | 61.13 | 87.85 | 498.99 | KNl+KNP | |
| 37 | 7.23 | 19.71 | 6.95 | 9.64 | 56.47 | 60.96 | 88.20 | 495.82 | KNl+KNP | |
| 38(F) | 6.99 | 19.34 | 7.38 | 8.15 | 58.14 | 66.26 | 88.36 | 521.30 | KNl+NKl+NKP | |
| [1] | GUO Lei, HU Yangdong, WANG Yan, et al.A quasi-ternary wet residue method applied to solid-liquid equilibrium systems[J].Fluid Phase Equilibria, 2018, 456: 161-167. |
| [2] | 岳焕芳, 程明, 王俊英, 等. 水溶肥应用现状和发展前景[J].蔬菜, 2017(2): 28-31. |
| YUE Huanfang, CHENG Ming, WANG Junying, et al.Application status and development prospect of water soluble fertilizer[J].Vegetables, 2017(2): 28-31. | |
| [3] | 梁红江.AZF工艺生产粒状水溶肥的技术开发[D].秦皇岛:燕山大学, 2018. |
| LIANG Hongjiang.Development of AZF technology for the production of granular water soluble fertilzer[D].Qinhuangdao:Yanshan University, 2018. | |
| [4] | 蒋成君, 程桂林.共结晶分离技术研究进展[J].化工进展, 2020, 39(1): 311-319. |
| JIANG Chengjun, CHENG Guilin.Progress in co-crystallization as a separation technology[J].Chemical Industry and Engineering Pro-gress, 2020, 39(1): 311-319. | |
| [5] | Patole T, Deshpande A.Co-crystallization-a technique for solubility enhancement[J].IJPSR, 2014, 5(9): 3566-3576. |
| [6] | 胡雪, 朱静, 王睿哲, 等. (NH2)2CO-NH4H2PO4-H2O三元系10 ℃相平衡研究[J].无机盐工业, 2019, 51(5): 41-44. |
| HU Xue, ZHU Jing, WANG Ruizhe, et al.Research on phase equili-brium of ternary system of(NH2)2CO-NH4H2PO4-H2O at 10 ℃[J].Inorganic Chemicals Industry,2019, 51(5): 41-44. | |
| [7] | 黄林川, 李天祥, 杨家敏, 等. 三元体系KH2PO4-CO(NH2)2-H2O在283.15 K的固液相平衡测定与关联[J].化学工业与工程, 2021, 38(3): 64-69. |
| HUANG Linchuan, LI Tianxiang, YANG Jiamin, et al.Determination and correlation of solid-liquid equilibrium of ternary system KH2PO4-CO(NH2)2-H2O at 283.15 K[J].Chemical Industry and Engineering, 2021, 38(3): 64-69. | |
| [8] | 杨家敏, 朱静, 胡雪, 等. 283.15 K下三元KCl-NH4Cl-H2O和KH2PO4-NH4H2PO4-H2O体系固-液相平衡测定与关联[J].无机盐工业, 2021, 53(1): 30-35. |
| YANG Jiamin, ZHU Jing, HU Xue, et al.Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K[J].Inorganic Chemicals Industry, 2021, 53(1): 30-35. | |
| [9] | 吴强, 胡雪, 朱静, 等. KH2PO4-KCl-H2O、NH4H2PO4-NH4Cl-H2O三元体系283.15 K相平衡研究[J].无机盐工业, 2020, 52(11): 24-28. |
| WU Qiang, HU Xue, ZHU Jing, et al.Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K[J].Inorganic Chemicals Industry, 2020, 52(11): 24-28. | |
| [10] | 王肖丽, 朱静, 吴强, 等. NH4 +,K+//H2PO4 -,CO(NH2)2-H2O四元体系298.15 K相平衡研究[J].化学工程, 2021, 49(8): 39-44. |
| WANG Xiaoli, ZHU Jing, WU Qiang, et al.Phase equilibrium of quaternary system NH4 +,K+//H2PO4 -,CO(NH2)2-H2O at 298.15 K[J].Chemical Engineering(China), 2021, 49(8): 39-44. | |
| [11] | SHEN Wei, WANG Yilan, HE Tingting, et al.Study on the phase equilibrium of the quaternary systems Na+ (K+)// H2PO4 -,Cl-,SO4 2--H2O at 313.15 K[J].Fluid Phase Equilibria, 2015, 403: 85-94. |
| [12] | 何婷婷.Na+,NH4 +//Cl-,H2PO4 -,SO4 2--H2O子体系稳定相平衡研究[D].银川:宁夏大学, 2018. |
| HE Tingting.Research on stable equilibria in quinary system Na+,NH4 +//Cl-,H2PO4 -,SO4 2--H2O and its sub-system[D].Yinchuan:Ningxia University, 2018. | |
| [13] |
ZHANG Yujia, YU Mei, LIU Ju, et al.Solid-liquid equilibrium,structural features and separation process of ammonium potassium dihydrogen phosphate solid solution[J].Chemical Physics, 2021,544.Doi:10.1016/j.chemphys.2021.111109 .
doi: 10.1016/j.chemphys.2021.111109 |
| [14] | 胡雪.常压下(NH2)2CO-NH4H2PO4-KCl-H2O体系在 283.15 K下的相平衡研究[D].贵阳:贵州大学,2019. |
| HU Xue.Determination and correlation of phase equilibrium system of(NH2)2CO-NH4H2PO4-KCl-H2O at 283.15 K[D].Guiyang:Guizhou University,2019. | |
| [15] | 吴强.KCl-NH4H2PO4-(NH2)2CO-H2O体系在 298.15 K下固-液相平衡研究[D].贵阳:贵州大学,2020. |
| WU Qiang.Study on the solid-liquid equilibrium of KCl-NH4H2PO4-(NH2)2CO-H2O system at 298.15 K[D].Guiyang:Guizhou University,2020. | |
| [16] | 邓天龙, 周桓, 陈侠.水盐体系相图及应用[M].北京:化学工业出版社, 2013. |
| DENG Tianlong, ZHOU Heng, CHEN Xia.Phase diagram of water salt system and its application[M].Beijing:Chemical Industry Press, 2013. | |
| [17] | 肖玲.甲醛法测定铵盐含氮量[J].化工技术与开发, 2003, 32(5): 25-26. |
| XIAO Ling.Determination of nitrogen in ammonium salt by formol titration method[J].Technology & Development of Chemical Industry, 2003, 32(5): 25-26. | |
| [18] | 王清华.钼锑抗分光光度法测定海水中活性磷酸盐的浓度[J].广西质量监督导报, 2008(8): 118-119. |
| WANG Qinghua.Determination of active phosphate concentration in seawater by molybdenum antimony anti spectrophotome-try[J].Guangxi Quality Supervision Guide Periodical, 2008(8): 118-119. | |
| [19] | 周何华, 邱燕飞, 杜玉辉, 等. 铁铵矾指示剂法测定氯化物[J].工业水处理, 2004, 24(3): 47-50. |
| ZHOU Hehua, QIU Yanfei, DU Yuhui, et al.Determination of chloride by ferric ammonium alum indicator method[J].Industrial Water Treatment, 2004, 24(3): 47-50. | |
| [20] | 任宝山, 李志广.25 ℃ NH4Cl-KCl- H2O三元体系相平衡的研究[J].河北工业大学学报, 2000, 29(6): 1-3. |
| REN Baoshan, LI Zhiguang.The study on ternary system phase equilibrium of NH4Cl-KCl-H2O at 25 ℃[J].Journal of Hebei University of Technology, 2000, 29(6): 1-3. | |
| [21] | 陆江林, 程银芳, 黄婷, 等. 301.15 K时KCl-NH4Cl-H2O三元水盐体系相平衡的研究[J].湖北师范学院学报:自然科学版, 2010, 30(4): 95-98. |
| LU Jianglin, CHENG Yinfang, HUANG Ting, et al.The study on ternary system phase equilibrium of KCl-NH4Cl-H2O at 30115 K[J].Journal of Hubei Normal University:Natural Science, 2010, 30(4): 95-98. | |
| [22] | 赵长伟, 马沛生, 郭瓦力, 等. KCl-NH4Cl-H2O三元水盐体系溶解度的研究[J].化学工业与工程, 2003, 20(3): 145-149. |
| ZHAO Changwei, MA Peisheng, GUO Wali, et al.Study on the solubility of the three-component system KCl-NH4Cl-H2O[J].Chemical Industry and Engineering, 2003, 20(3): 145-149. | |
| [23] | ZHAO Bin, GENG Guoyue, CHEN Jianxin, et al.The ternary system phase equilibrium of KCl-NH4Cl-H2O at 80 ℃[J].Advanced Materials Research, 2013, 834-836: 519-522. |
| [1] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [2] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [3] | YANG Bo, MA Zhen, ZENG Ying, YAN Xiongzhong, LI Qi, HOU Yuansheng, YU Xudong. Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K [J]. Inorganic Chemicals Industry, 2024, 56(11): 116-122. |
| [4] | GAN Shunpeng, DING Ding, SUN Chenggao, JIANG Shipeng, GUAN Junfang. Study on cold decomposition crystallization process of underground primary high-sodium carnallite ore [J]. Inorganic Chemicals Industry, 2024, 56(1): 67-72. |
| [5] | WANG Linjian, CHANG Liming, CHEN Hang, SONG Xingfu. Effect of air flow and temperature on DPNB froth properties in KCl flotation [J]. Inorganic Chemicals Industry, 2023, 55(5): 39-44. |
| [6] | GONG Xuemin, ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian. Study on crystallization process of Na2CO3 hydrate in seawater system and its application [J]. Inorganic Chemicals Industry, 2023, 55(3): 55-59. |
| [7] | YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K [J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66. |
| [8] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [9] | WU Sida,LIN Rushan,ZHANG Lei,JIA Yanhong. Research progress on purification process for active crack elements in waste salt by dry post-treatment [J]. Inorganic Chemicals Industry, 2022, 54(4): 81-87. |
| [10] | LU Ziyu,LIU Zhaogang,WU Jinxiu,HU YanHong,LIU Xingyu. Study on phase equilibrium of ternary system of Na2CO3-NaF-H2O at 298.15 K and 318.15 K [J]. Inorganic Chemicals Industry, 2022, 54(3): 66-70. |
| [11] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [12] | Liu Zhiguo,Zhang Na,Chen Chuan,Xu Xianzhen. Study on thermodynamic model of vapor-liquid equilibrium of electrolyte system [J]. Inorganic Chemicals Industry, 2021, 53(8): 55-59. |
| [13] | Chen Xulong,Dong Weibing,Wan Junfeng,Wang Dongdong,Wang Gang. Study on the crystallization process and agglomeration mechanism of spherical KCl [J]. Inorganic Chemicals Industry, 2021, 53(7): 53-57. |
| [14] | Hou Yifei,Bai Ni,Sun Jian,Jiao Lina,Ju Dianchun. Effects of ZrO2 on the physicochemical properties of NaCl-KCl molten salt system for the purification of crude ZrCl4 [J]. Inorganic Chemicals Industry, 2021, 53(7): 63-67. |
| [15] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||