Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (2): 61-66.doi: 10.19964/j.issn.1006-4990.2022-0273
• Research & Development • Previous Articles Next Articles
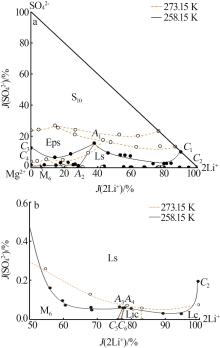
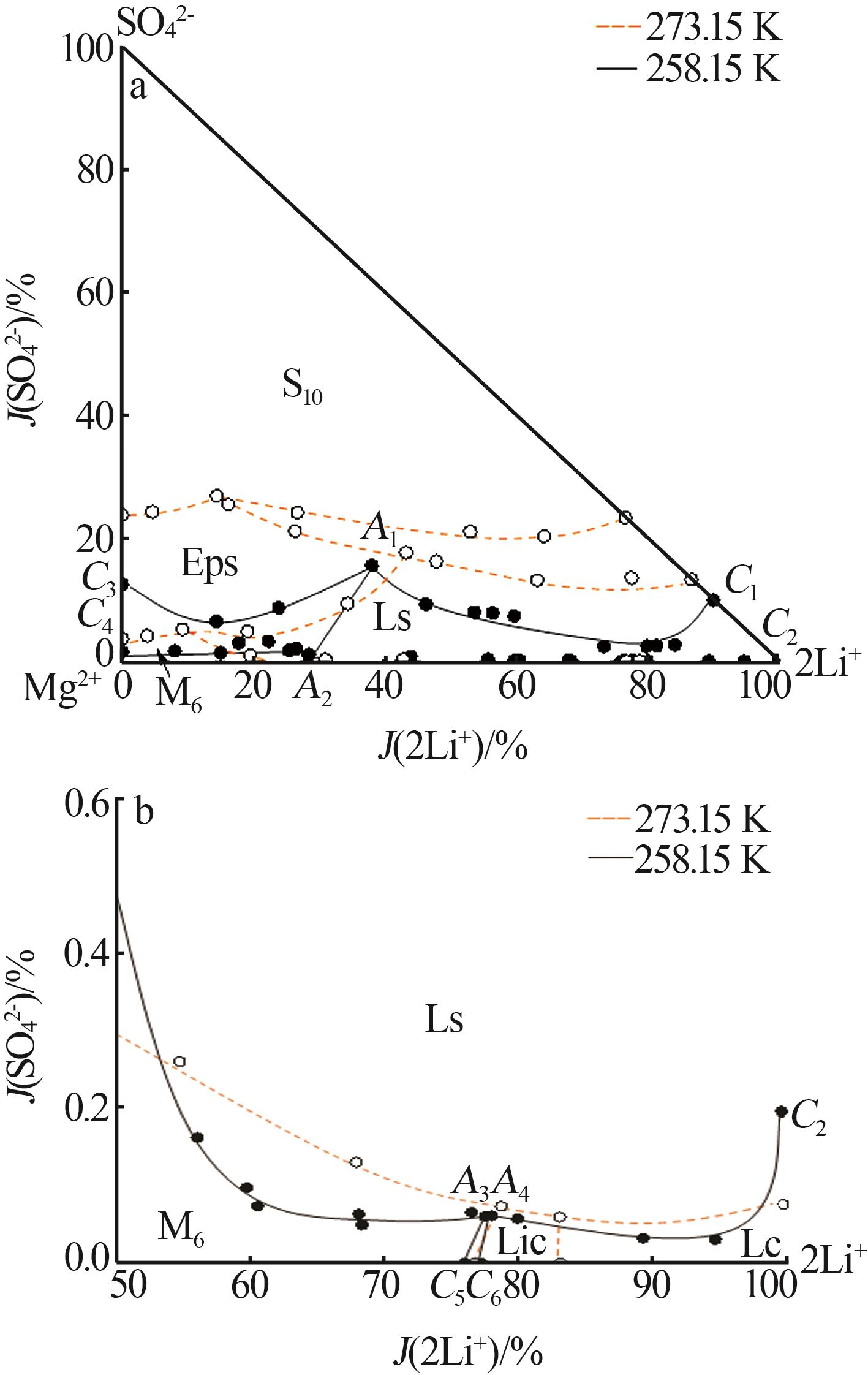
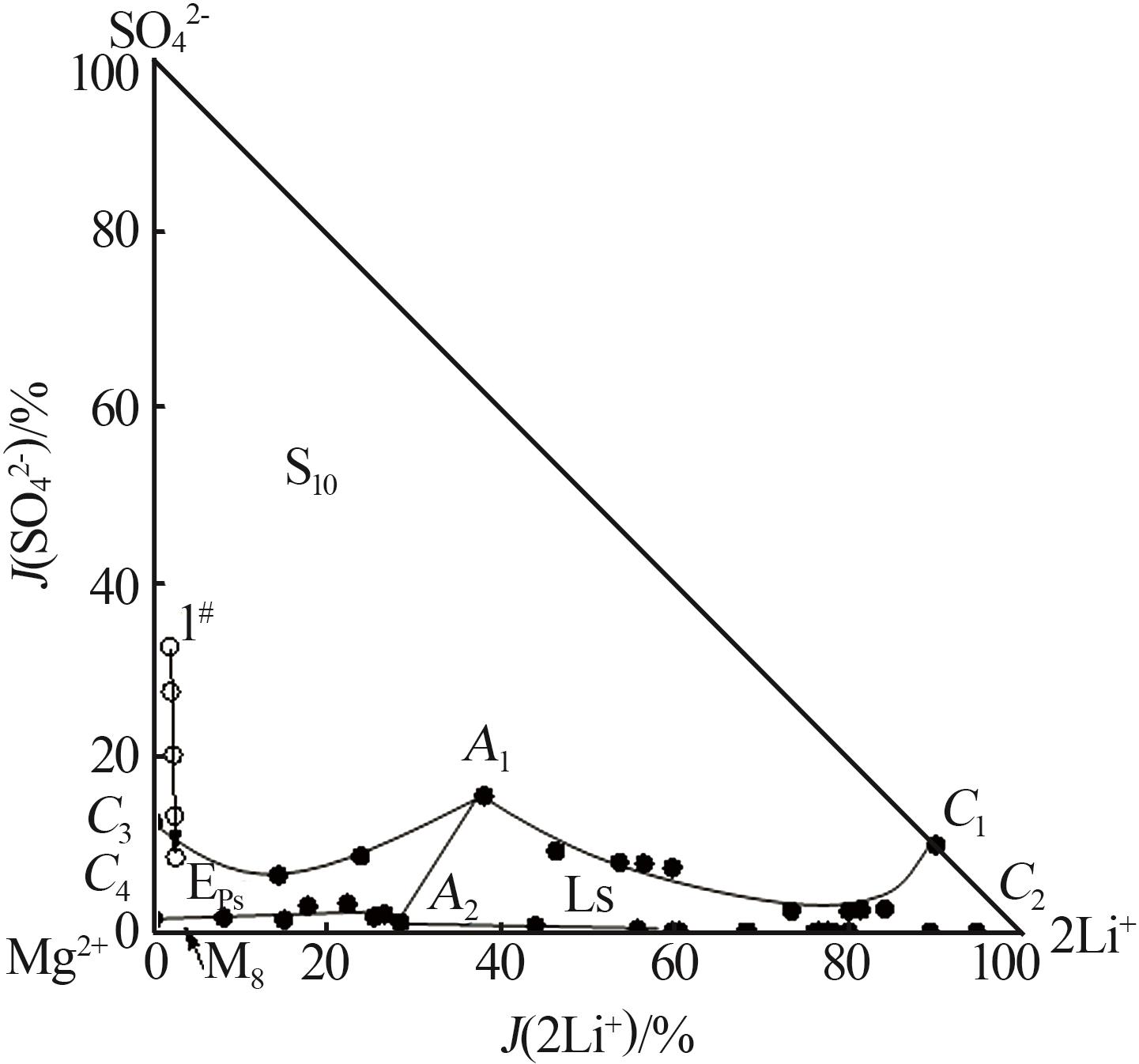
Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K
YAN Fangning( ),GUO Jinchun,HUANG Xueli(
),GUO Jinchun,HUANG Xueli( ),ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing
),ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing
- College of Chemical Engineering,Xinjiang University,Key Laboratory of Cleaner Transition of Coal & Chemicals Engineering of Xinjiang Uyghur Autonomous Region,Urumqi 830017,China
-
Received:2022-05-07Online:2023-02-10Published:2023-02-16 -
Contact:HUANG Xueli E-mail:1324648810@qq.com;xuelih@163.com
CLC Number:
Cite this article
YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K[J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66.
share this article
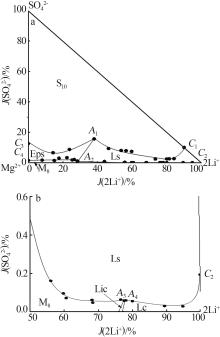
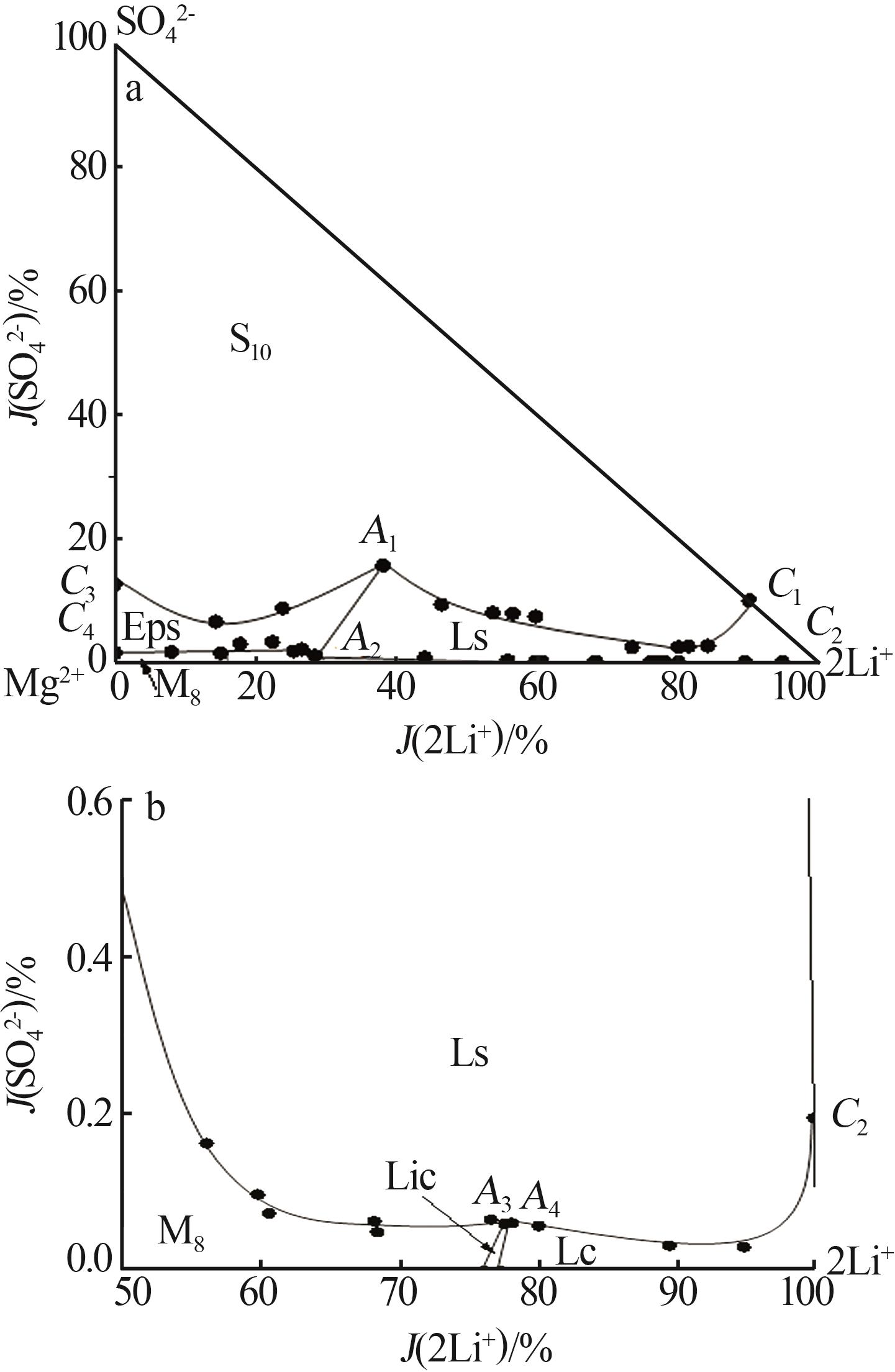
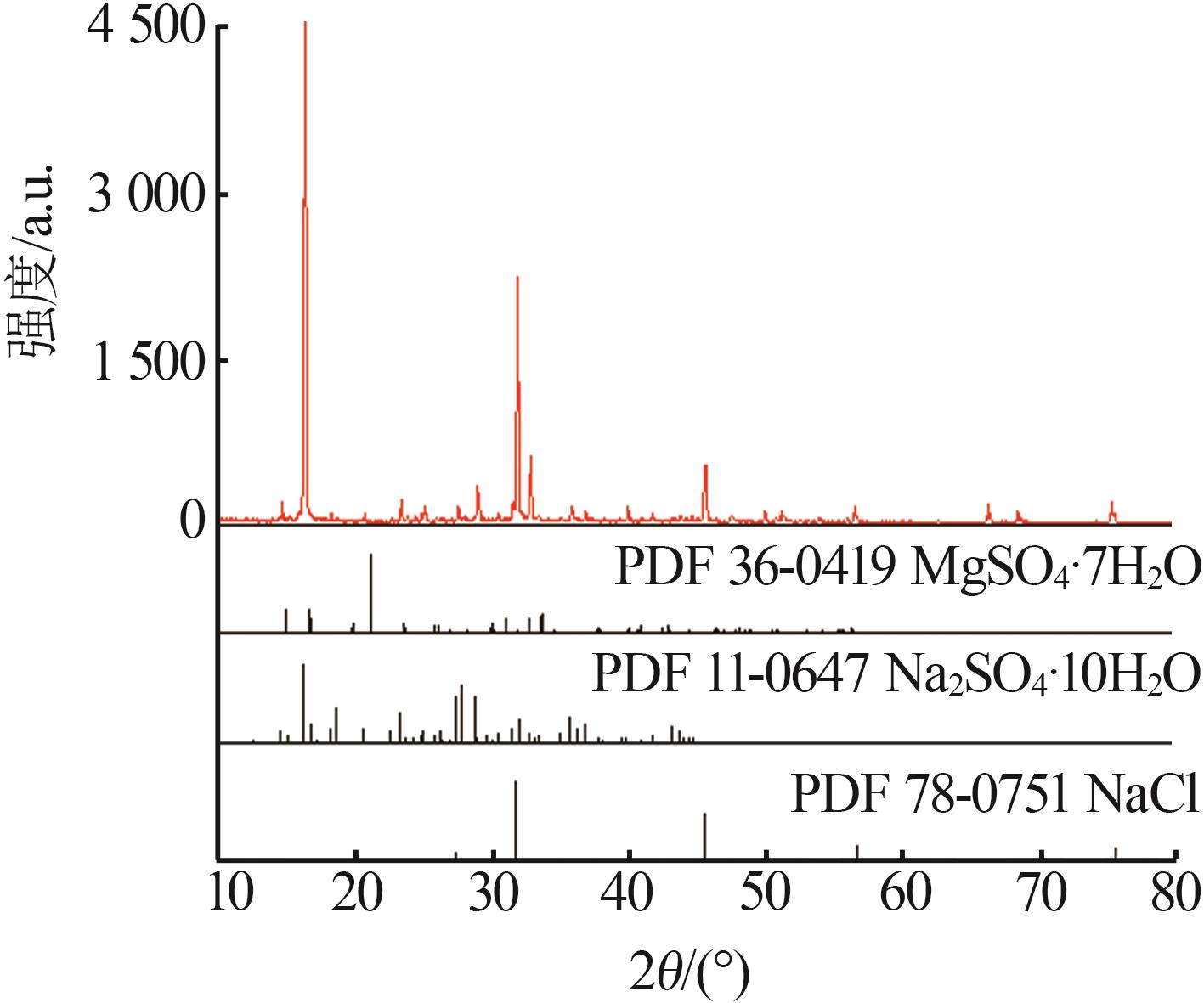
Table 1
Solubilities of Li+,Na+,Mg2+//SO42-,Cl--H2O system saturated with NaCl·2H2O at 258.15 K"
| 编号 | w(液相组成)/% | 相图指数/% | 平衡固相组成 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Cl- | SO42- | Na+ | 2Li+ | SO42- | 2Na+ | H2O | |||
| 1,C3[ | 0.000 | 4.450 | 15.45 | 2.521 | 2.808 | 0.00 | 12.54 | 29.17 | 2 101 | Hy+S10+Eps | |
| 2 | 0.443 | 4.284 | 16.79 | 1.391 | 1.982 | 14.33 | 6.506 | 19.36 | 1 873 | Hy+S10+Eps | |
| 3 | 0.863 | 4.280 | 18.30 | 2.181 | 1.960 | 23.82 | 8.699 | 16.33 | 1 541 | Hy+S10+Eps | |
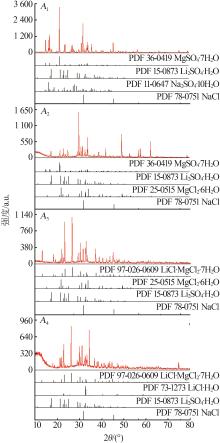
| 4,A1 | 1.244 | 2.656 | 16.50 | 3.531 | 3.245 | 38.03 | 15.60 | 29.94 | 1 715 | Hy+S10+Eps+Ls | |
| 5 | 1.788 | 3.005 | 18.74 | 2.484 | 1.731 | 46.29 | 9.292 | 13.53 | 1 441 | Hy+S10+Ls | |
| 6 | 2.058 | 2.582 | 18.79 | 2.117 | 1.499 | 53.62 | 7.968 | 11.79 | 1 464 | Hy+S10+Ls | |
| 7 | 2.167 | 2.399 | 19.01 | 2.086 | 1.613 | 56.46 | 7.854 | 12.69 | 1 460 | Hy+S10+Ls | |
| 8 | 2.389 | 2.307 | 20.37 | 2.048 | 1.914 | 59.69 | 7.395 | 14.43 | 1 366 | Hy+S10+Ls | |
| 9 | 2.842 | 1.641 | 21.31 | 0.652 | 1.614 | 73.37 | 2.431 | 12.58 | 1 431 | Hy+S10+Ls | |
| 10 | 3.063 | 1.183 | 20.68 | 0.652 | 1.332 | 79.93 | 2.440 | 10.50 | 1 470 | Hy+S10+Ls | |
| 11 | 3.090 | 1.070 | 21.34 | 0.670 | 1.070 | 81.36 | 2.549 | 15.08 | 1 459 | Hy+S10+Ls | |
| 12 | 2.961 | 0.811 | 19.89 | 0.653 | 1.865 | 84.15 | 2.681 | 16.00 | 1 616 | Hy+S10+Ls | |
| 13,C1 | 3.052 | 0.000 | 17.59 | 2.336 | 2.416 | 90.04 | 9.956 | 21.52 | 1 696 | Hy+S10+Ls | |
| 14,C4[ | 0.000 | 7.944 | 23.12 | 0.477 | 0.192 | 0.00 | 1.497 | 1.261 | 1 275 | Hy+Eps+M8 | |
| 15 | 0.407 | 8.011 | 25.07 | 0.589 | 0.034 | 8.034 | 1.681 | 0.203 | 1 002 | Hy+Eps+M8 | |
| 16 | 0.797 | 7.760 | 26.51 | 0.522 | 0.119 | 15.03 | 1.421 | 0.680 | 934.0 | Hy+Eps+M8 | |
| 17 | 0.960 | 7.498 | 26.27 | 1.114 | 0.203 | 17.77 | 2.979 | 1.133 | 912.0 | Hy+Eps+M8 | |
| 18 | 1.205 | 7.038 | 26.51 | 1.219 | 0.470 | 22.31 | 3.262 | 2.638 | 906.9 | Hy+Eps+M8 | |
| 19 | 1.256 | 6.314 | 25.11 | 0.604 | 0.471 | 25.38 | 1.764 | 2.873 | 1 031 | Hy+Eps+M8 | |
| 20 | 1.380 | 6.505 | 26.70 | 0.743 | 0.794 | 26.53 | 2.064 | 4.606 | 946.1 | Hy+Eps+M8 | |
| 21,A2 | 1.482 | 6.439 | 27.00 | 0.389 | 0.606 | 28.41 | 1.079 | 3.505 | 946.8 | Hy+Eps+M8+Ls | |
| 22 | 2.360 | 5.200 | 27.90 | 0.280 | 0.573 | 43.95 | 0.753 | 3.218 | 913.8 | Hy+M8+Ls | |
| 23 | 3.150 | 4.314 | 29.19 | 0.063 | 0.365 | 56.02 | 0.162 | 1.957 | 862.1 | Hy+M8+Ls | |
| 24 | 3.360 | 3.960 | 29.43 | 0.038 | 0.482 | 59.71 | 0.097 | 2.586 | 858.9 | Hy+M8+Ls | |
| 25 | 3.420 | 3.900 | 29.70 | 0.028 | 0.567 | 60.52 | 0.073 | 3.030 | 850.5 | Hy+M8+Ls | |
| 26 | 3.907 | 3.198 | 30.37 | 0.025 | 0.714 | 68.10 | 0.063 | 3.760 | 829.8 | Hy+M8+Ls | |
| 27 | 3.964 | 3.213 | 30.55 | 0.020 | 0.611 | 68.32 | 0.049 | 3.180 | 818.5 | Hy+M8+Ls | |
| 28 | 4.680 | 2.508 | 31.66 | 0.028 | 0.300 | 76.52 | 0.065 | 1.480 | 766.3 | Hy+M8+Ls | |
| 29,A3 | 4.790 | 2.425 | 32.36 | 0.026 | 0.547 | 77.53 | 0.060 | 2.674 | 746.4 | Hy+M8+Ls+Lic | |
| 30,A4 | 4.985 | 2.452 | 32.62 | 0.027 | 0.017 | 78.02 | 0.061 | 0.078 | 722.3 | Hy+Ls+Lic+Lc | |
| 31 | 5.000 | 2.188 | 32.30 | 0.025 | 0.255 | 79.96 | 0.057 | 1.233 | 742.1 | Hy+Ls+Lc | |
| 32 | 5.201 | 1.080 | 32.56 | 0.013 | 1.850 | 89.37 | 0.032 | 9.599 | 785.1 | Hy+Ls+Lc | |
| 33 | 5.352 | 0.518 | 32.11 | 0.012 | 2.120 | 94.74 | 0.030 | 11.33 | 816.7 | Hy+Ls+Lc | |
| 34,C2 | 6.343 | 0.000 | 32.42 | 0.086 | 0.054 | 99.81 | 0.194 | 0.258 | 740.6 | Hy+Ls+Lc | |
| 1 | 杨卉芃,柳林,丁国峰.全球锂矿资源现状及发展趋势[J].矿产保护与利用,2019,39(5):26-40. |
| YANG Huipeng, LIU Lin, DING Guofeng.Present situation and development trend of lithium resources in the world[J].Conservation and Utilization of Mineral Resources,2019,39(5):26-40. | |
| 2 | THOMPSON C A.Mineral commodity summaries 2021[R].Virginia:United States Geological Survey,2021. |
| 3 | 崔瑞芝,李武,董亚萍,等.298 K四元体系LiCl-MgCl2-CaCl2-H2O相平衡实验及溶解度计算[J].化工学报,2018,69(10):4148-4155. |
| CUI Ruizhi, LI Wu, DONG Yaping,et al.Measurements and calculations of solid-liquid equilibria in quaternary system LiCl-MgCl2-CaCl2-H2O at 298 K[J].CIESC Journal,2018,69(10):4148-4155. | |
| 4 |
CUI Ruizhi, ZHANG Yongming, DONG Yaping,et al.Solid-Liquid equilibria of two quaternary systems LiBr-NaBr-Li2SO4-Na2SO4-H2O and LiBr-KBr-Li2SO4-K2SO4-H2O at 298.15 K[J].The Journal of Chemical Thermodynamics,2022,165.Doi:10.1016/j.jct.2021.106665 .
doi: 10.1016/j.jct.2021.106665 |
| 5 | ZHUGE Fuyu, YU Xuefeng, ZENG Ying,et al.Phase equilibria for the reciprocal aqueous quaternary system Li+,Rb+//Cl-,borate-H2O at 323.2 K[J].Journal of Solution Chemistry,2020,49(11):1349-1359. |
| 6 |
YUAN Fei, JIANG Jie, WANG Shiqiang,et al.Phase equilibria and thermodynamic model of the quinary system(Li+,Na+,Mg2+//Cl-,SO4 2--H2O) at 273.15 K and 0.1 MPa[J].Journal of Molecular Liquids,2021,337.Doi:10.1016/j.molliq.2021.116334 .
doi: 10.1016/j.molliq.2021.116334 |
| 7 | ZENG Ying, LIN Xiaofeng, YU Xudong.Study on the solubility of the aqueous quaternary system Li2SO4+Na2SO4+K2SO4+H2O at 273.15 K[J].Journal of Chemical & Engineering Data,2012,57(12):3672-3676. |
| 8 |
QI Xiaoyun, HE Chunxia, SANG Shihua,et al.Solid-liquid equilibria in the quaternary system LiBr-NaBr-KBr-H2O and its two ternary subsystems at 288.15 K[J].Asia-Pacific Journal of Chemical Engineering,2021,16(2).Doi:10.1002/apj.2595 .
doi: 10.1002/apj.2595 |
| 9 | 王雪莹,黄雪莉,黄河,等.-15 ℃下Na+,K+,Mg2+//Cl-,NO3 -,SO4 2--H2O体系相平衡研究[J].化工学报,2020,71(11):5059-5066. |
| WANG Xueying, HUANG Xueli, HUANG He,et al.Study on phase equilibrium of system Na+,K+,Mg2+//Cl-,NO3 -,SO4 2--H2O at -15 ℃[J].CIESC Journal,2020,71(11):5059-5066. | |
| 10 | 朱巧丽,黄雪莉.-15 ℃下Na+,K+,Mg2+//Cl-,SO4 2--H2O体系相平衡[J].化工学报,2015,66(4):1252-1257. |
| ZHU Qiaoli, HUANG Xueli.Liquid-solid phase equilibrium of Na+,K+,Mg2+// Cl-,SO4 2--H2O system at -15 ℃[J].CIESC Journal,2015,66(4):1252-1257. | |
| 11 | 牛自得,程芳琴.水盐体系相图及其应用[M].天津:天津大学出版社,2002:172. |
| 12 | 中科院青海盐湖所.卤水和盐的分析方法[M].北京:科学与技术出版社,1984:61-295. |
| 13 | 任效京,黄雪莉.273.15 K下四元体系Li+,Mg2+//Cl-,SO4 2--H2O相平衡研究[J].无机盐工业,2016,48(7):13-15,28. |
| REN Xiaojing, HUANG Xueli.Phase equilibria in the quatemary system of Li+,Mg2+//Cl-,SO4 2--H2O at 273.15 K[J].Inorganic Chemicals Industry,2016,48(7):13-15,28. | |
| 14 | 廖玲,黄雪莉,宋欢.258.15 K下五元体系Na+,K+//Cl-,NO3 -,SO4 2--H2O相平衡研究[J].高校化学工程学报,2016,30(1):7-12. |
| LIAO Ling, HUANG Xueli, SONG Huan.Phase equilibria of quinary system Na+,K+//Cl-,NO3 -,SO4 2--H2O at 258.15 K[J].Journal of Chemical Engineering of Chinese Universities,2016,30(1):7-12. | |
| 15 | 郑绵平,刘喜方.青藏高原盐湖水化学及其矿物组合特征[J].地质学报,2010,84(11):1585-1600. |
| ZHENG Mianping, LIU Xifang.Hydrochemistry and minerals assemblages of salt lakes in the Qinghai-Tibet plateau,China[J].Acta Geologica Sinica,2010,84(11):1585-1600. |
| [1] | WANG Minrui, TIAN Guiying, ZHANG Ao, GE Junjie, ZHANG Lei, XIANG Jun, TANG Na. Study on granulation optimization for Al-based lithium adsorbent and its lithium recovery performance from brine [J]. Inorganic Chemicals Industry, 2025, 57(3): 36-42. |
| [2] | MA Jingyuan, LI Yan, ZHOU Hanjie, LI Jiangang. Research progress of PEO based organic/inorganic composite solid electrolyte [J]. Inorganic Chemicals Industry, 2025, 57(3): 1-8. |
| [3] | LI Chao, WANG Liping, GAO Guimei, ZHANG Yunfeng, HONG Yu, LIU Darui, XU Lijun, CUI Yongjie. Study on reaction mechanism of acid leaching lithium from circulating fluidized bed fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 101-107. |
| [4] | CHEN Xue, JIANG Guanghui, OUYANG Quansheng, SHAO Jiaojing. Recent research progress of lithium sulfur batteries under lean electrolyte based on sulfur electrode design [J]. Inorganic Chemicals Industry, 2025, 57(2): 1-13. |
| [5] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [6] | ZHANG Shanshan, ZENG Yule, ZHANG Ting, LIN Sen, LIU Chenglin. Research progress of cathode pre-lithiation technology for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2025, 57(1): 1-13. |
| [7] | KONG Lingjie, LI Guangbi, XIE Jiahao, YANG Xinhui, BAI Xiaoqin. Research progress on lithium extraction technology from salt lake brine [J]. Inorganic Chemicals Industry, 2025, 57(1): 14-26. |
| [8] | TIAN Peng, ZHANG Haoran, XU Jingang, MOU Chenxi, XU Qianjin, NING Guiling. Study on aluminum sol modified anode and cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2024, 56(9): 44-53. |
| [9] | ZHOU Wanji, LI Sixia. Study on extraction lithium from brine with high magnesium using ionic liquid/metal salt extraction system [J]. Inorganic Chemicals Industry, 2024, 56(9): 54-59. |
| [10] | MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru. Kinetic study of lithium extraction from solution with iron-based ionic liquid system [J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66. |
| [11] | CHEN Xue, OUYANG Quansheng, SHAO Jiaojing. Recent research progress of lithium-sulfur batteries based on solid-solid reaction mechanism [J]. Inorganic Chemicals Industry, 2024, 56(9): 12-23. |
| [12] | XU Jing, WANG Dahui, CHEN Huaijing, GUO Yongqi, ZHENG Yang. Research progress on regeneration utilization of LiFePO4 materials in retired power batteries of electric vehicles [J]. Inorganic Chemicals Industry, 2024, 56(8): 1-8. |
| [13] | XUE Shan, LIU Lu, DAI Jiansheng, LI Qing, FENG Ze, LI Yineng. Study on electrochemical properties of europium⁃doped LiFePO4 cathode material for lithium⁃ion battery [J]. Inorganic Chemicals Industry, 2024, 56(8): 67-73. |
| [14] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [15] | ZHU Zongjiang, WANG Gang, WEI Yuanfeng, TANG Yanhong, KAKUTA Cheng, LIU Chengbin. Research progress and prospect of resourceful recycling technology of electrolyte from decommissioned lithium⁃ion battery [J]. Inorganic Chemicals Industry, 2024, 56(7): 11-17. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||