Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (8): 55-59.doi: 10.19964/j.issn.1006-4990.2020-0562
• Research & Development • Previous Articles Next Articles
Study on thermodynamic model of vapor-liquid equilibrium of electrolyte system
Liu Zhiguo1( ),Zhang Na1,Chen Chuan1,Xu Xianzhen2
),Zhang Na1,Chen Chuan1,Xu Xianzhen2
- 1. Navel Aeronautical University,Qingdao Branch,Qingdao 266000,China
2. College of Chemistry and Chemical Engineering,Qingdao University
-
Received:2020-10-19Online:2021-08-10Published:2021-08-11
CLC Number:
Cite this article
Liu Zhiguo,Zhang Na,Chen Chuan,Xu Xianzhen. Study on thermodynamic model of vapor-liquid equilibrium of electrolyte system[J]. Inorganic Chemicals Industry, 2021, 53(8): 55-59.
share this article
Table 1
Model parameters for binary electrolyte solution"
| 体系 | h | τi,w(0) | τw,i(0) | τi,w(1) | τw,i(1) |
|---|---|---|---|---|---|
| BaBr2+H2O | 1.50 | -4.42 | 3.52 | -228.19 | 321.73 |
| CaBr2+H2O | 2.55 | -0.40 | -33.92 | -1 649.09 | 12 785.70 |
| CaCl2+H2O | 1.10 | 781.44 | -3 771.77 | -98.47 | -6 010.44 |
| CsBr+H2O | 0.50 | -4.34 | 4.12 | 423.85 | -480.68 |
| CsCl+H2O | 0.30 | -3.38 | 2.83 | -59.70 | -20.03 |
| CsI+H2O | 0.10 | -3.78 | -1.40 | 9.67 | 642.89 |
| K2SO4+H2O | 0.60 | -38.84 | 70.38 | 12 703.70 | -17 691.81 |
| KBr+H2O | 2.10 | 1.20 | -50.38 | -1 340.62 | 15 952.42 |
| KCl+H2O | 2.00 | -2.92 | 2.38 | -81.81 | 126.71 |
| KI+H2O | 3.00 | -3.63 | 3.64 | 199.06 | -326.81 |
| LiCl+H2O | 2.15 | -4.99 | 13.17 | -4.29 | 16.17 |
| LiBr+H2O | 0.80 | -5.47 | 56.87 | 510.23 | -23 153.41 |
| MgCl2+H2O | 3.47 | -6.26 | -58.68 | 453.19 | 19 585.20 |
| MgSO4+H2O | 0.41 | -2.63 | 3.29 | -696.95 | 2 367.36 |
| Na2SO4+H2O | 0.10 | 1.06 | -1.45 | -1 441.50 | 1 425.60 |
| NaBr+H2O | 4.00 | -1.50 | -0.69 | -456.26 | 567.10 |
| NaCl+H2O | 3.10 | -2.54 | 2.06 | 184.51 | -203.49 |
| NaI+H2O | 1.90 | -3.50 | 2.74 | -75.40 | -137.38 |
| RbCl+H2O | 2.70 | -0.94 | 0.66 | -607.71 | 993.37 |
| SrCl2+H2O | 2.00 | -6.85 | 30.00 | 539.38 | -8 296.66 |
| ZnCl2+H2O | 3.00 | -3.77 | 3.26 | -1.23 | 2.83 |
Table 2
Correlation of VLE data for binary electrolyte solution"
| 体系 | T/K | 数据 点 | 本实验 | |
|---|---|---|---|---|
| dY/kPa | dP/% | |||
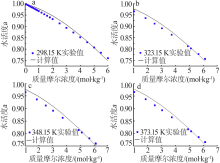
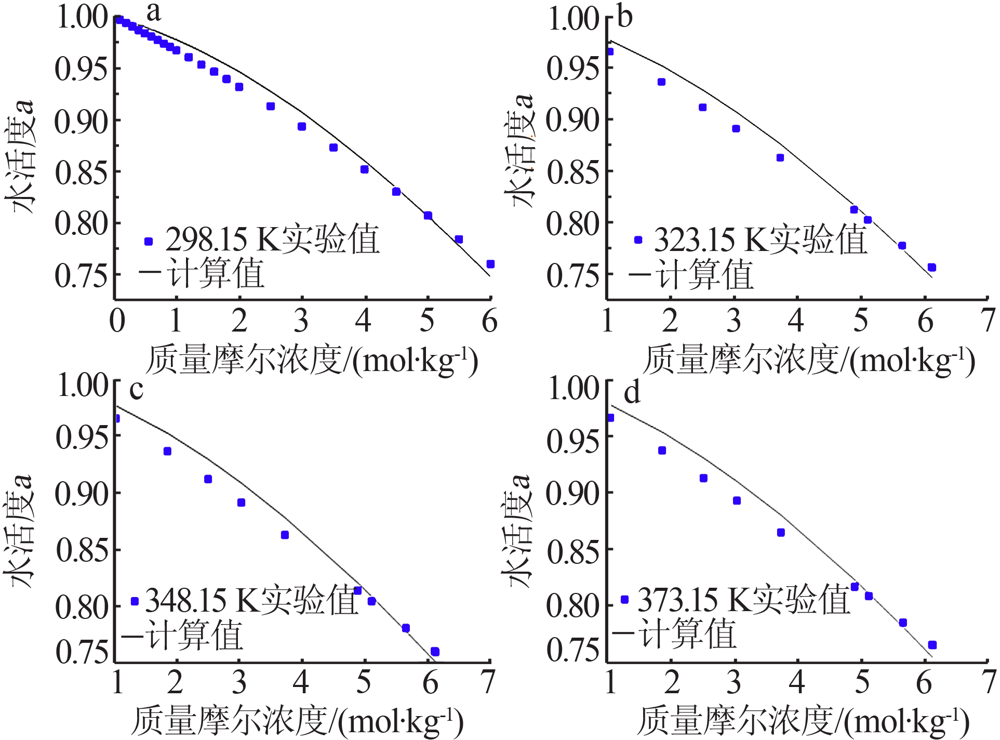
| LiCl+H2O[ | 298.15~394.40 | 47 | 0.018 | 1.250 |
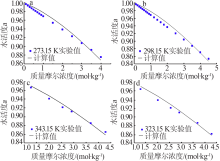
| LiBr+H2O[ | 298.15~377.85 | 182 | 0.070 | 0.860 |
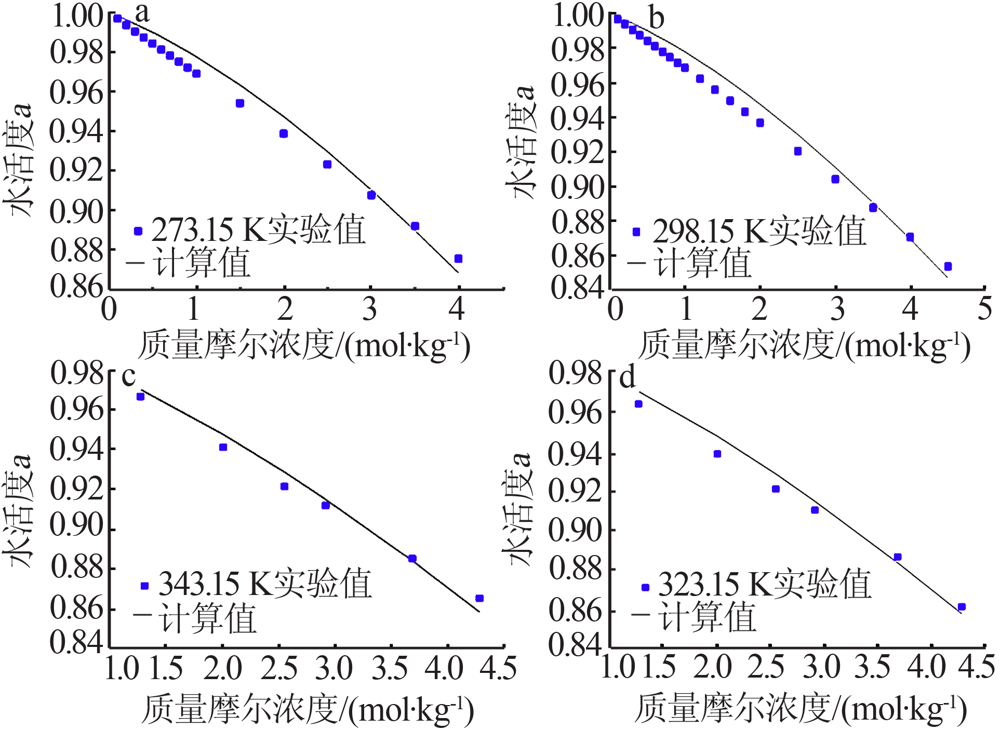
| MgCl2+H2O[ | 273.15~376.45 | 151 | 0.076 | 0.850 |
| MgSO4+H2O[ | 273.15~298.15 | 30 | 0.012 | 0.530 |
| Na2SO4+H2O[ | 298.15~343.15 | 49 | 0.048 | 0.750 |
| NaBr+H2O[ | 298.15~373.15 | 69 | 0.040 | 0.650 |
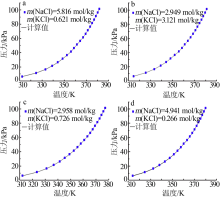
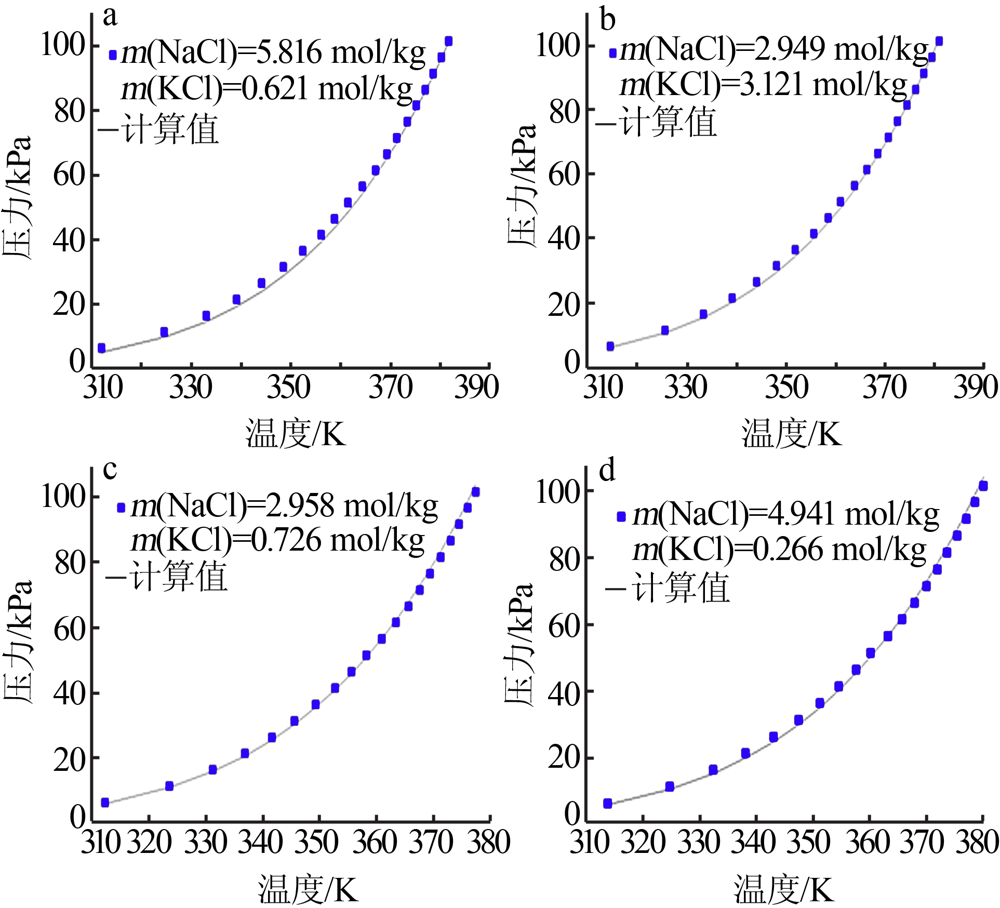
| NaCl+H2O[ | 298.15~343.15 | 58 | 0.090 | 0.929 |
| NaI+H2O[ | 298.15~343.15 | 51 | 0.061 | 0.690 |
| RbCl+H2O[ | 298.15~343.15 | 59 | 0.068 | 0.710 |
| SrCl2+H2O[ | 298.15~379.80 | 30 | 0.030 | 0.750 |
| ZnCl2+H2O[ | 298.15~379.80 | 30 | 0.030 | 0.910 |
| BaBr2+H2O[ | 298.15~353.15 | 55 | 0.079 | 0.780 |
| CaBr2+H2O[ | 298.15~353.15 | 63 | 0.049 | 1.100 |
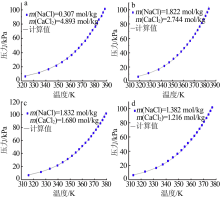
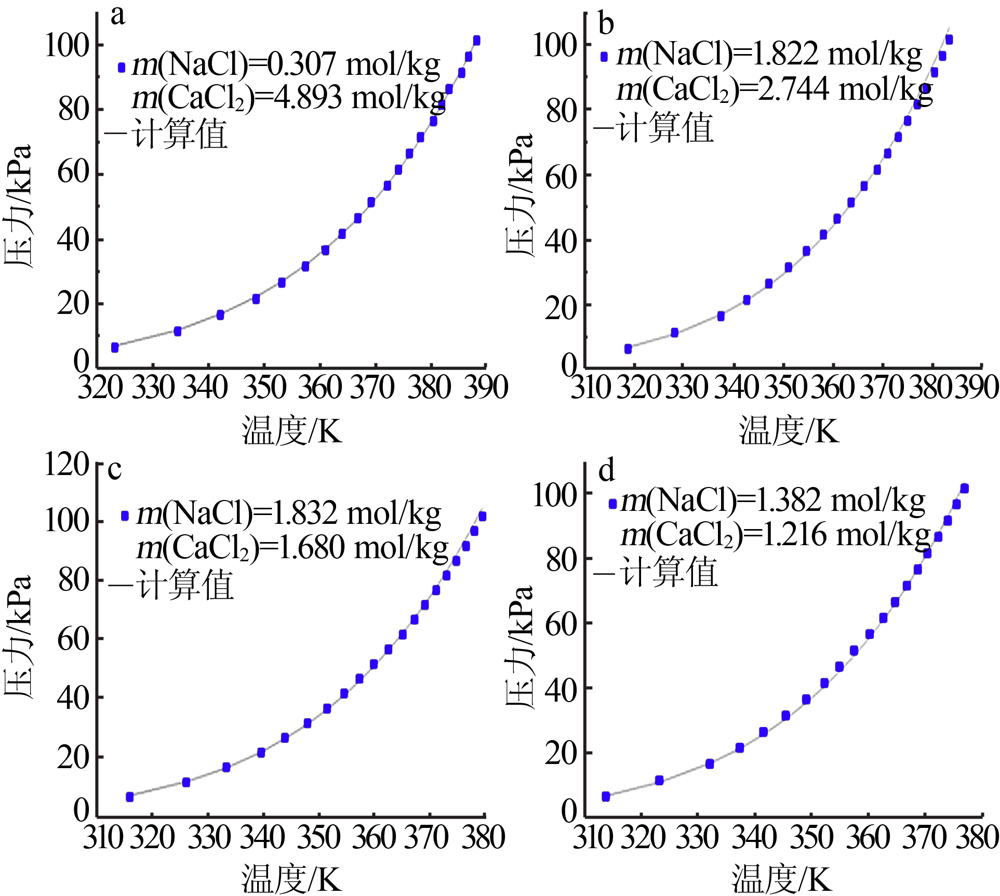
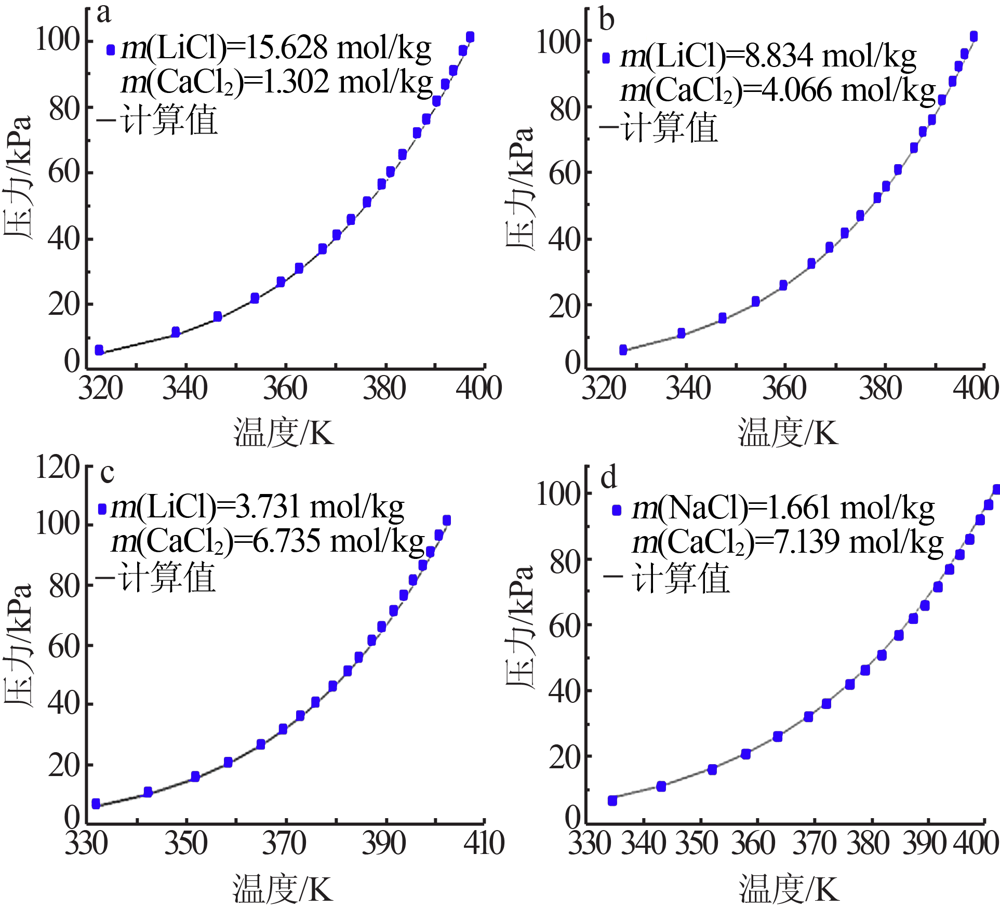
| CaCl2+H2O[ | 273.15~423.15 | 322 | 0.081 | 1.820 |
| CsBr+H2O[ | 298.15~353.15 | 51 | 0.097 | 1.010 |
| CsCl+H2O[ | 298.15~353.15 | 63 | 0.091 | 0.970 |
| CsI+H2O[ | 298.15~353.15 | 42 | 0.039 | 0.420 |
| K2SO4+H2O[ | 298.15~383.65 | 104 | 0.051 | 1.150 |
| KBr+H2O[ | 298.15~343.15 | 51 | 0.077 | 0.870 |
| KCl+H2O[ | 273.15~343.15 | 66 | 0.044 | 0.670 |
| KI+H2O[ | 298.15~343.15 | 60 | 0.097 | 0.950 |
| 平均值 | 0.059 | 0.880 | ||
Table 3
Correlation of VLE for mixed electrolyte solution system"
| 体系 | T/K | 数据点 | 本实验 | |
|---|---|---|---|---|
| dY/kPa | dP/% | |||
| NaCl-Na2SO4-H2O[ | 298.15 | 25 | 0.036 | 1.58 |
| MgSO4-Na2SO4-H2O[ | 298.15 | 30 | 0.024 | 0.82 |
| MgCl2-Na2SO4-H2O[ | 298.15 | 22 | 0.030 | 1.60 |
| NaCl-MgSO4-H2O[ | 298.15 | 30 | 0.044 | 1.55 |
| KCl-K2SO4-H2O[ | 280.15~323.15 | 50 | 0.021 | 0.85 |
| KCl-KBr-H2O[ | 279.55~323.35 | 48 | 0.075 | 0.95 |
| KCl-KNO3-H2O[ | 279.05~324.75 | 46 | 0.086 | 1.17 |
| K2SO4-MgSO4-H2O[ | 310~374.35 | 360 | 0.080 | 1.10 |
| NaCl-KCl-H2O[ | 298.15~382.25 | 180 | 0.091 | 1.80 |
| NaCl-CaCl2-H2O[ | 313.25~388.25 | 240 | 0.091 | 1.10 |
| NaCl-KCl-MgCl2-H2O[ | 298.15 | 136 | 0.026 | 0.80 |
| MgCl2-MgSO4-H2O[ | 298.15 | 20 | 0.100 | 1.70 |
| NaCl-MgCl2-H2O[ | 298.15 | 20 | 0.080 | 1.70 |
| NaCl-KCl-MgCl2-CaCl2- MgSO4[ | 306.75~373.25 | 60 | 0.070 | 1.07 |
| 平均值 | 0.061 | 1.27 | ||
Table 4
Prediction of VLE for mixed electrolyte solution systems"
| 体系 | T/K | 数据 点 | 本实验 | |
|---|---|---|---|---|
| dY/kPa | dP/% | |||
| NaCl-Na2SO4-H2O[ | 298.15 | 25 | 0.072 | 2.58 |
| MgSO4-Na2SO4-H2O[ | 298.15 | 30 | 0.037 | 1.22 |
| MgCl2-Na2SO4-H2O[ | 298.15 | 22 | 0.080 | 2.60 |
| NaCl-MgSO4-H2O[ | 298.15 | 30 | 0.074 | 2.55 |
| KCl-K2SO4-H2O[ | 280.15~323.15 | 50 | 0.031 | 1.50 |
| KCl-KBr-H2O[ | 279.55~323.35 | 48 | 0.150 | 2.50 |
| KCl-KNO3-H2O[ | 279.05~324.75 | 46 | 0.160 | 2.71 |
| K2SO4-MgSO4-H2O[ | 310~374.35 | 360 | 0.180 | 3.10 |
| NaCl-KCl-H2O[ | 298.15~382.25 | 180 | 0.170 | 2.80 |
| NaCl-CaCl2-H2O[ | 313.25~388.25 | 240 | 0.140 | 1.90 |
| NaCl-KCl-MgCl2-H2O[ | 298.15 | 136 | 0.046 | 1.50 |
| MgCl2-MgSO4-H2O[ | 298.15 | 20 | 0.160 | 2.70 |
| NaCl-MgCl2-H2O[ | 298.15 | 20 | 0.180 | 2.75 |
| NaCl-KCl-MgCl2-CaCl2- MgSO4[ | 306.75~373.25 | 60 | 0.070 | 1.07 |
| 平均值 | 0.117 | 2.36 | ||
| [1] | Robinson R A, Stokes R H. Electrolyte Solutions[M].2nd ed. London: Courier Dover Publications, 2002. |
| [2] |
Patil K R, Tripathi A D, Pathak G, Katti S S. Thermodynamic proper-ties of aqueous electrolyte solutions,vapor pressure of aqueous so-lutions of sodium bromide,sodium iodide,potassium chloride,pota-ssium bromide,potassium iodide,rubidium chloride,cesium chlo-ride,cesium bromide,cesium iodide,magnesium chloride,calcium chloride,calcium bromide,calcium iodide,strontium chloride,st-rontium bromide,strontium iodide,barium chloride,and barium bromide[J]. Journal of Chemical and Engineering Data, 1991, 36:225-230.
doi: 10.1021/je00002a021 |
| [3] |
Dinane A, Guendouzi M E, Mounir A. Hygrometric determination of water activities,osmotic and activity coefficients of (NaCl+KCl)(aq) at T=298.15 K[J]. Journal of Chemical Thermodynamics, 2002, 34:423-441.
doi: 10.1006/jcht.2001.0845 |
| [4] | Kolár P, Nakata H, Tsuboi A, et al. Measurement and modeling of vapor-liquid equilibria at high salt concentrations[J]. Fluid Phase Equilibria, 2005, 228:493-497. |
| [5] |
Apelblat A, Korin E. Temperature dependence of vapor pressures over saturated aqueous solutions at invariant points of the NaCl+KCl+H2O,NaCl+NaNO3+H2O,KCl+KBr+H2O,KCl+KI+H2O,KCl+KNO3+H2O,and KCl+K2SO4+H2O systems[J]. Journal of Chemical and Engineering Data, 2009, 54:1619-1624.
doi: 10.1021/je800963g |
| [6] |
Chen C C, Britt H I, Boston J F. Extension and application of the pi-tzer equation for vapor-liquid equilibrium of aqueous electrolyte systems with molecular solutes[J]. AIChE Journal, 1979, 25(5):820-831.
doi: 10.1002/(ISSN)1547-5905 |
| [7] |
Pitzer K S. Thermodynamics of electrolytes,I.Theoretical basis and general equations[J]. Journal of Physical Chemistry, 1973, 77(2):268-277.
doi: 10.1021/j100621a026 |
| [8] |
Clegg S L, Pitzer K S. Thermodynamics of multicomponent,miscible,ionic solutions:Generalize equations for symmetrical electrolytes[J]. Journal of Physical Chemistry, 1992, 96(8):3513-3520.
doi: 10.1021/j100187a061 |
| [9] |
Chen C C, Britt H I, Boston J F, et al. Local composition model for excess Gibbs energy of electrolyte systems; part Ⅰ:Single solvent,single completely dissociated electrolyte systems[J]. AIChE Journal, 1982, 28(4):588-596.
doi: 10.1002/(ISSN)1547-5905 |
| [10] |
Chen C C, Evans L B. A local composition model for the excess gi-bbs energy of aqueous electrolyte systems[J]. AIChE Journal, 1986, 32(3):444-454.
doi: 10.1002/(ISSN)1547-5905 |
| [11] |
Lu X H, Maurer G. Model for describing activity coefficients in mi-xed electrolyte aqueous solutions[J]. AIChE Journal, 1993, 39(9):1527-1538.
doi: 10.1002/aic.v39:9 |
| [12] | Lu X, Zhang L, Wang Y, et al. Prediction of activity coefficients of electrolytes in aqueous solutions at high temperatures[J]. Indus-trial & Engineering Chemistry Research, 1996, 35(5):1777-1784. |
| [13] |
Wang P, Anderko A, Young R D. A speciation-based model for mi-xed-solvent electrolyte systems[J]. Fluid Phase Equilibria, 2002, 203(1/2):141-176.
doi: 10.1016/S0378-3812(02)00178-4 |
| [14] |
Thomsen K, Rasmussen P. Modeling of vaporliquid-solid equilibri-um in gas-aqueous electrolyte systems[J]. Chemical Engineering Science, 1999, 54(12):1787-1802.
doi: 10.1016/S0009-2509(99)00019-6 |
| [15] |
Renon H, Prausnitz J M. Local compositions in thermodynamic ex-cess functions for liquid mixtures[J]. AIChE Journal, 1968, 14(1),135-144.
doi: 10.1002/(ISSN)1547-5905 |
| [16] | Xu X, Wang Y, Sun X, et al. Vapor-liquid equilibria study of LiCl+CaCl2+H2O system[J]. ACS Omega, 2019(4):4390-4396. |
| [17] |
Platford R F. Osmotic coefficients of aqueous solutions of seven co-mpounds at 0 deg[J]. Journal of Chemical and Engineering Data, 1973, 18(2):215-217.
doi: 10.1021/je60057a017 |
| [18] |
Xu X, Hu Y, Wu L, et al. Experimental and modeling of vapor-liquid equilibria for electrolyte solution systems[J]. Journal of Chemical and Engineering Data, 2014, 59(11):3741-3748.
doi: 10.1021/je500623w |
| [19] |
Xu X, Hu Y, Wang X, et al. Experimental and modeling of vapor-liquid equilibria for mixed electrolyte solution systems[J]. Journal of Chemical and Engineering Data, 2016, 61(7):2311-2320.
doi: 10.1021/acs.jced.5b01028 |
| [20] | Wu Y C, Rush R M, Scatchard G. Osmotic and activity coefficients for binary mixtures of sodium chloride,sodium sulfate,magnesium sulfate,and magnesium chloride in water at 25 deg.I.Isopiestic mea-surements on the four systems with common ions[J]. Journal of Phy-sical Chemistry, 1968, 72:4048-4053. |
| [21] |
Wu Y C, Rush R M, Scatchard G. Osmotic and activity coefficients for binary mixtures of sodium chloride,sodium sulfate,magnesium sulfate,and magnesium chloride in water at 25 deg.Ⅱ.Isopiestic and electromotive force measurements on the two systems without common ions[J]. Journal of Physical Chemistry, 1969, 73:2047-2053.
doi: 10.1021/j100726a068 |
| [22] | Saad D, Padova J, Marcus Y. Thermodynamics of mixed electrolyte solutions.Ⅵ.An isopiestic study of a pseudo-ternary system:NaCl-KCl-MgCl2·H2O at 25 ℃[J]. Journal of Solution Chemistry, 1975(4):983-993. |
| [23] | Padova J, Saad D, Rosenzweig D. Thermodynamics of mixed elec-trolyte solutions.X.An isopiestic study of the uaternary system NaCl-KCl-MgCl2·H2O at 25 ℃[J]. Journal of Solution Chemistry, 1977(6):309-325. |
| [24] | Saad D, Padova J. Thermodynamics of mixed electrolyte solutions.Ⅸ.Calculation of the osmotic and activity coefficients in a pseudo-ternary system(NaCl-nKCl)-MgCl2-H2O at 298.15 K[J]. Journal of Solution Chemistry, 1977(6):191-201. |
| [1] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [2] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [3] | YANG Bo, MA Zhen, ZENG Ying, YAN Xiongzhong, LI Qi, HOU Yuansheng, YU Xudong. Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K [J]. Inorganic Chemicals Industry, 2024, 56(11): 116-122. |
| [4] | GONG Xuemin, ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian. Study on crystallization process of Na2CO3 hydrate in seawater system and its application [J]. Inorganic Chemicals Industry, 2023, 55(3): 55-59. |
| [5] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [6] | YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K [J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66. |
| [7] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [8] | LU Ziyu,LIU Zhaogang,WU Jinxiu,HU YanHong,LIU Xingyu. Study on phase equilibrium of ternary system of Na2CO3-NaF-H2O at 298.15 K and 318.15 K [J]. Inorganic Chemicals Industry, 2022, 54(3): 66-70. |
| [9] | HE Ting,KONG Jiao,CUI Jingzhi,CHEN Zhihao,FU Tongtong,GUO Zirui,GU Shuai,YU Jianguo. Study on leaching and thermodynamic of spent lithium-ion batteries with electrochemical reduction [J]. Inorganic Chemicals Industry, 2022, 54(12): 34-43. |
| [10] | FAN Xiaojuan,ZHU Jing,DENG Wenqing,CHEN Yan,LI Tianxiang. Study on phase equilibria of reciprocal quaternary system of K+,NH4+//Cl-,H2PO4--H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 102-108. |
| [11] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [12] | Liang Ning,Mo Fujin,Zhou Jierong,Wang Junzheng. Study on preparation of sludge biochar and its adsorption performance for phosphorus [J]. Inorganic Chemicals Industry, 2021, 53(6): 174-179. |
| [13] | Li Fei,Zhou Jianmin,Li Chengyu,Xu Wentao,Zhang Xinyue,Li Xinwei,Wang Jun,Ji Zhiyong,Zhao Yingying,Guo Xiaofu,Yuan Junsheng. Study on mechanism and conditions of the new process of ammonium chloride conversion [J]. Inorganic Chemicals Industry, 2021, 53(3): 48-53. |
| [14] | LIANG Chengbo,XIE Tian,YANG Lin,HE Bingbing. Study on leaching law of metal elements in process of decomposing phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2021, 53(11): 49-54. |
| [15] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||