Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (1): 100-105.doi: 10.19964/j.issn.1006-4990.2022-0211
• Research & Development • Previous Articles Next Articles
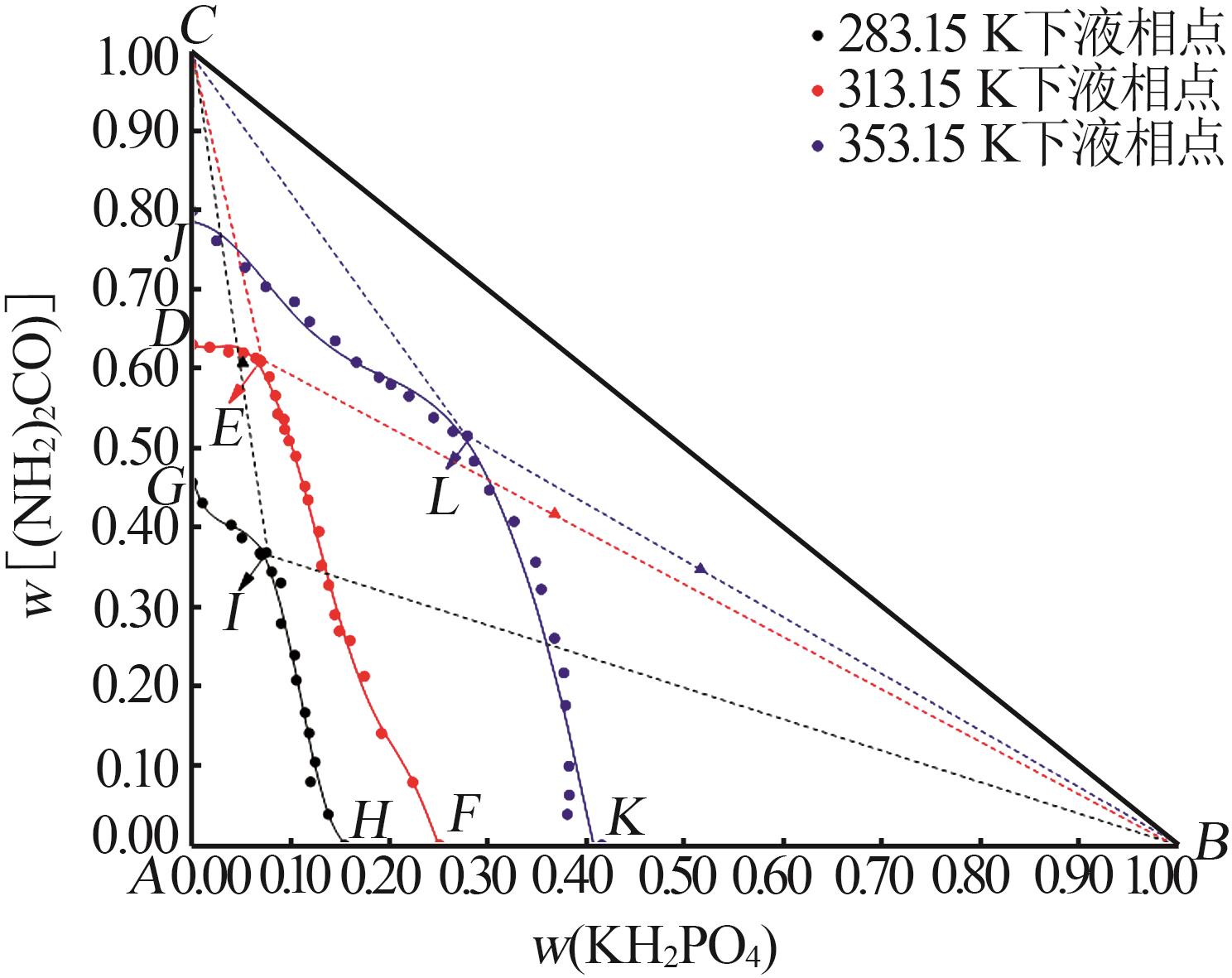
Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K
DENG Wenqing( ),ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang(
),ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang( )
)
- School of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
-
Received:2022-04-18Online:2023-01-10Published:2023-01-17 -
Contact:LI Tianxiang E-mail:1358413340@qq.com;txli@gzu.edu.cn
CLC Number:
Cite this article
DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K[J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105.
share this article
Table 1
Solubility data of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K"
| 序号 | 液相质量分数/% | 湿渣质量分数/% | 平衡 固相 | |||||
|---|---|---|---|---|---|---|---|---|
| KH2PO4 | (NH2)2CO | H2O | KH2PO4 | (NH2)2CO | H2O | |||
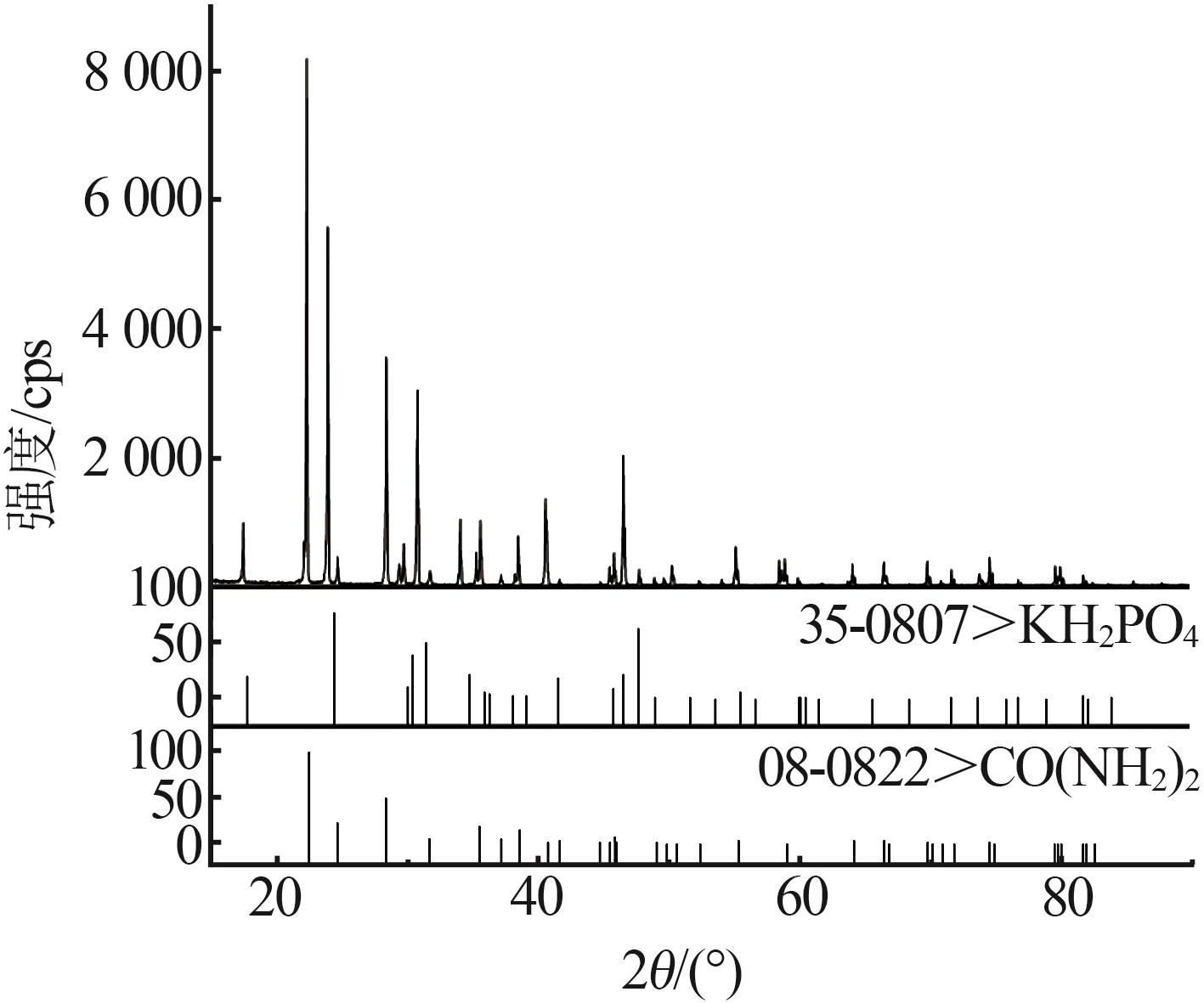
| 1,F | 25.09 | 0.00 | 74.91 | — | — | — | KH2PO4 | |
| 2 | 22.36 | 7.91 | 69.73 | 63.90 | 4.20 | 31.90 | KH2PO4 | |
| 3 | 19.21 | 14.06 | 66.73 | 62.98 | 7.18 | 29.84 | KH2PO4 | |
| 4 | 17.45 | 21.24 | 61.31 | 60.72 | 10.76 | 28.52 | KH2PO4 | |
| 5 | 16.00 | 25.75 | 58.24 | 60.98 | 11.26 | 27.76 | KH2PO4 | |
| 6 | 14.91 | 26.93 | 58.17 | 78.21 | 7.86 | 13.93 | KH2PO4 | |
| 7 | 14.45 | 29.00 | 56.55 | 69.91 | 10.21 | 19.88 | KH2PO4 | |
| 8 | 13.83 | 32.71 | 53.46 | 73.46 | 11.31 | 15.23 | KH2PO4 | |
| 9 | 13.12 | 35.20 | 51.69 | 55.51 | 19.12 | 25.37 | KH2PO4 | |
| 10 | 12.86 | 39.46 | 47.67 | 59.80 | 18.24 | 21.96 | KH2PO4 | |
| 11 | 11.75 | 43.45 | 44.80 | 78.31 | 11.23 | 10.46 | KH2PO4 | |
| 12 | 11.43 | 45.19 | 43.39 | 62.03 | 20.18 | 17.79 | KH2PO4 | |
| 13 | 10.48 | 48.98 | 40.54 | 66.21 | 18.21 | 15.58 | KH2PO4 | |
| 14 | 9.79 | 50.92 | 39.29 | 61.27 | 22.12 | 16.61 | KH2PO4 | |
| 15 | 9.38 | 52.34 | 38.28 | 75.30 | 15.42 | 9.28 | KH2PO4 | |
| 16 | 9.30 | 53.63 | 37.07 | 38.71 | 36.72 | 24.57 | KH2PO4 | |
| 17 | 8.67 | 54.27 | 37.06 | 78.51 | 14.82 | 6.67 | KH2PO4 | |
| 18 | 8.45 | 56.60 | 34.95 | 37.26 | 38.97 | 23.77 | KH2PO4 | |
| 19 | 7.81 | 58.96 | 33.23 | 39.87 | 38.90 | 21.23 | KH2PO4 | |
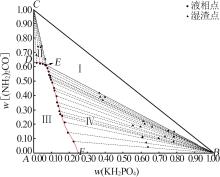
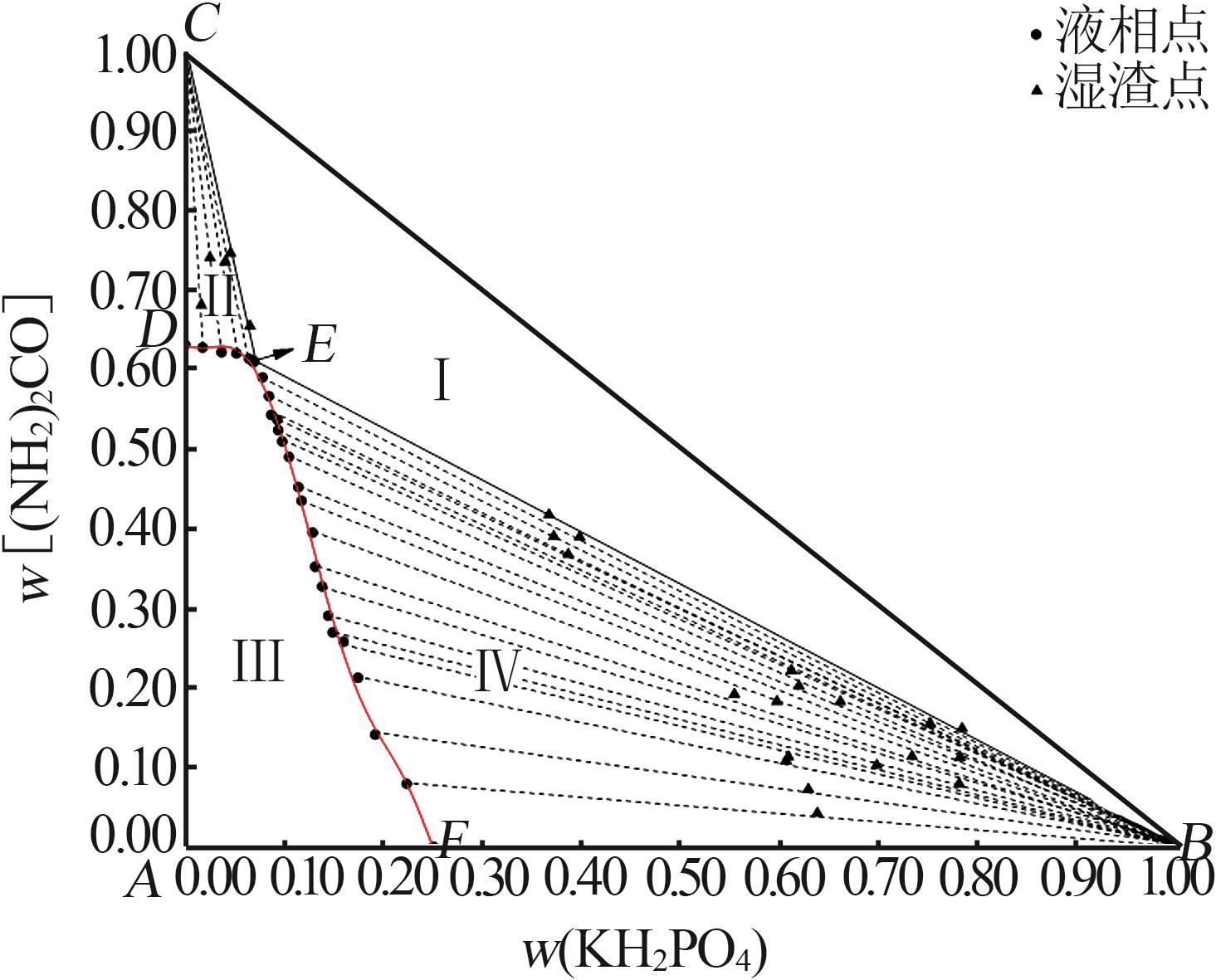
| 20,E | 6.96 | 60.99 | 32.05 | 36.78 | 41.69 | 21.53 | KH2PO4+(NH2)2CO | |
| 21 | 6.90 | 60.88 | 32.22 | 6.53 | 65.40 | 28.07 | (NH2)2CO | |
| 22 | 6.44 | 61.32 | 32.24 | 4.58 | 74.55 | 20.87 | (NH2)2CO | |
| 23 | 5.17 | 62.00 | 32.83 | 4.03 | 73.44 | 22.53 | (NH2)2CO | |
| 24 | 3.67 | 62.12 | 34.21 | 2.50 | 73.99 | 23.51 | (NH2)2CO | |
| 25 | 1.76 | 62.71 | 35.53 | 1.63 | 68.08 | 30.29 | (NH2)2CO | |
| 26,D | 0.00 | 63.05 | 36.95 | — | — | — | (NH2)2CO | |
Table 3
Wilson and NRTL binary interaction parameters of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K"
| i-j | Wilson | NRTL | |||||
|---|---|---|---|---|---|---|---|
| a | b | Λ | a | b | τ | ||
| KH2PO4-H2O | 5.87 | -3 860.72 | 197.52 | -1.47 | -491.56 | -3.04 | |
| H2O-KH2PO4 | 6.18 | -1 262.15 | 0.38 | -15.86 | 8 867.52 | 12.46 | |
| (NH2)2CO-H2O | -1.54 | -169.38 | 3.21 | 4.13 | -1 569.63 | -0.88 | |
| H2O-(NH2)2CO | 3.33 | -123.86 | 0.13 | 15.01 | -2 569.84 | 6.80 | |
KH2PO4- (NH2)2CO | -37.07 | 0.51 | 9.70×1015 | -19.87 | -3 310.29 | -30.44 | |
(NH2)2CO- KH2PO4 | -2.82 | 1.97 | 21.56 | -0.59 | -0.56 | -0.59 | |
Table 4
Wilson and NRTL model correlated calculation values of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K"
| 序号 | 液相物质的量 分数,m | Wilson计算值,m | NRTL计算值,m | |||||
|---|---|---|---|---|---|---|---|---|
| KH2PO4 | (NH2)2CO | KH2PO4 | (NH2)2CO | KH2PO4 | (NH2)2CO | |||
| 1,F | 0.042 5 | 0.000 0 | 0.039 2 | — | 0.040 3 | — | ||
| 2 | 0.039 4 | 0.031 6 | 0.037 1 | — | 0.037 7 | — | ||
| 3 | 0.034 6 | 0.057 4 | 0.035 6 | — | 0.039 7 | — | ||
| 4 | 0.033 0 | 0.091 0 | 0.033 4 | — | 0.035 0 | — | ||
| 5 | 0.031 1 | 0.113 5 | 0.032 0 | — | 0.033 0 | — | ||
| 6 | 0.028 9 | 0.118 4 | 0.031 8 | — | 0.035 3 | — | ||
| 7 | 0.028 5 | 0.129 5 | 0.031 1 | — | 0.033 3 | — | ||
| 8 | 0.028 1 | 0.150 7 | 0.029 8 | — | 0.029 3 | — | ||
| 9 | 0.027 1 | 0.165 0 | 0.028 9 | — | 0.027 8 | — | ||
| 10 | 0.027 8 | 0.193 4 | 0.027 1 | — | 0.022 0 | — | ||
| 11 | 0.026 2 | 0.219 5 | 0.025 7 | — | 0.020 1 | — | ||
| 12 | 0.025 9 | 0.231 9 | 0.024 9 | — | 0.018 9 | — | ||
| 13 | 0.024 5 | 0.259 5 | 0.023 4 | — | 0.017 5 | — | ||
| 14 | 0.023 2 | 0.273 4 | 0.022 7 | — | 0.017 9 | — | ||
| 15 | 0.022 5 | 0.284 3 | 0.022 1 | — | 0.018 0 | — | ||
| 16 | 0.022 6 | 0.295 8 | 0.021 4 | — | 0.016 9 | — | ||
| 17 | 0.021 1 | 0.298 8 | 0.021 4 | — | 0.019 5 | — | ||
| 18 | 0.021 1 | 0.320 1 | 0.020 2 | — | 0.018 0 | — | ||
| 19 | 0.019 9 | 0.340 4 | 0.019 2 | — | 0.019 9 | — | ||
| 20,E | 0.018 0 | 0.356 9 | — | 0.358 1 | — | 0.356 5 | ||
| 21 | 0.017 8 | 0.355 3 | — | 0.358 0 | — | 0.356 5 | ||
| 22 | 0.016 6 | 0.357 3 | — | 0.356 6 | — | 0.356 7 | ||
| 23 | 0.013 1 | 0.356 9 | — | 0.352 8 | — | 0.355 1 | ||
| 24 | 0.009 1 | 0.3494 | — | 0.349 2 | — | 0.351 4 | ||
| 25 | 0.004 3 | 0.344 7 | — | 0.344 3 | — | 0.344 9 | ||
| 26,D | 0.000 0 | 0.338 6 | — | 0.340 1 | — | 0.338 0 | ||
| RAD/% | 3.34 0.17 | 10.53 0.38 | ||||||
| RMSD/% | ||||||||
| 1 | 张婉玉, 魏君英. 我国农药化肥减量效应与推进对策[J].北方园艺, 2021(18):167-172. |
| ZHANG Wanyu, WEI Junying. Research on the reducing effect of pesticides and fertilizers in my country and the promoting countermeasures[J].Northern Horticulture, 2021(18):167-172. | |
| 2 | KHAN H I. Appraisal of biofertilizers in rice:To supplement inorganic chemical fertilizer[J].Rice Science, 2018, 25(6):357-362. |
| 3 | XIE Xiaofei. Study on the effect of chemical fertilizer reduction on new agricultural operation subject[C]//Proceedings of 2nd International Conference on the Frontiers of Innovative Economics and Management(FIEM 2021.Jiangsu,China,2021. |
| 4 | GUO Yanle, ZHANG Min, LIU Zhiguang, et al. Applying and optimizing water-soluble,slow-release nitrogen fertilizers for water-saving agriculture[J].ACS Omega, 2020, 5(20):11342-11351. |
| 5 | SAVCI S. Investigation of effect of chemical fertilizers on environment[J].APCBEE Procedia, 2012, 1:287-292. |
| 6 | 梁嘉敏, 杨虎晨, 张立丹, 等. 我国水溶性肥料及水肥一体化的研究进展[J].广东农业科学, 2021, 48(5):64-75. |
| LIANG Jiamin, YANG Huchen, ZHANG Lidan, et al. Research progress of water-soluble fertilizer and fertigation in China[J].Guangdong Agricultural Sciences, 2021, 48(5):64-75. | |
| 7 |
SUN Guangzhao, HU Tiantian, LIU Xiaogang, et al. Optimizing irrigation and fertilization at various growth stages to improve mango yield,fruit quality and water-fertilizer use efficiency in xerothermic regions[J].Agricultural Water Management, 2022, 260.Doi:10.1016/j.agwat.2021.107296.
doi: 10.1016/j.agwat.2021.107296 |
| 8 | 付强强, 郑瑞永, 李万和, 等. 固体水溶性肥料生产工艺现状[J].磷肥与复肥, 2019, 34(5):20-22. |
| FU Qiangqiang, ZHENG Ruiyong, LI Wanhe, et al. Production process for solid water-soluble fertilizer[J].Phosphate & Compound Fertilizer, 2019, 34(5):20-22. | |
| 9 | PAWAR N, SAHA A, NANDAN N, et al. Solution cocrystallization:A scalable approach for cocrystal production[J].Crystals, 2021, 11(3):303-321. |
| 10 | 蒋成君, 程桂林. 共结晶分离技术研究进展[J].化工进展, 2020, 39(1):311-319. |
| JIANG Chengjun, CHENG Guilin. Progress in co-crystallization as a separation technology[J].Chemical Industry and Engineering Progress, 2020, 39(1):311-319. | |
| 11 |
GUI Lintao, YANG Haitao, HUANG Hui, et al. Liquid solid fluidized bed crystallization granulation technology:Development,applications,properties,and prospects[J].Journal of Water Process Engineering, 2022, 45.Doi:10.1016/j.jwpe.2021.102513.
doi: 10.1016/j.jwpe.2021.102513 |
| 12 | 邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M].北京:化学工业出版社, 2013. |
| 13 | WALAS S M. Phase equilibria in chemical engineering[M].Boston:Butterworth, 1985. |
| 14 | 王肖丽, 朱静, 吴强, 等. NH4 +,K+//H2PO4 -,CO(NH2)2-H2O四元体系298.15 K相平衡研究[J].化学工程, 2021, 49(8):39-44. |
| WANG Xiaoli, ZHU Jing, WU Qiang, et al. Phase equilibrium of quaternary system NH4 +,K+//H2PO4 -,CO(NH2)2-H2O at 298.15 K[J].Chemical Engineering(China), 2021, 49(8):39-44. | |
| 15 | 黄林川, 李天祥, 杨家敏, 等. 三元体系KH2PO4-CO(NH2)2-H2O在283.15 K的固液相平衡测定与关联[J].化学工业与工程, 2021, 38(3):64-69. |
| HUANG Linchuan, LI Tianxiang, YANG Jiamin, et al. Determination and correlation of solid-liquid equilibrium of ternary system KH2PO4-CO(NH2)2-H2O at 283.15 K[J].Chemical Industry and Engineering, 2021, 38(3):64-69. | |
| 16 | 杨家敏, 朱静, 胡雪, 等. 283.15 K下三元KCl-NH4Cl-H2O和KH2PO4-NH4H2PO4-H2O体系固-液相平衡测定与关联[J].无机盐工业, 2021, 53(1):30-35. |
| YANG Jiamin, ZHU Jing, HU Xue, et al. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K[J].Inorganic Chemicals Industry, 2021, 53(1):30-35. | |
| 17 | 于梅. 钾-铵基固溶体水盐体系的相平衡、结构特性及其分离过程研究[D].银川:宁夏大学, 2019. |
| YU Mei. Research on phase equilibrium,structural properties and separation process of potassium-ammonium based solid solution in aqueous solid solution[D].Yinchuan:Ningxia University, 2019. | |
| 18 | EYSSELTOVÁ J, DIRKSE T P. IUPAC-NIST solubility data series 66.Ammonium phosphates[J].Journal of Physical and Che- mical Reference Data, 1998, 27(6):1289-1470. |
| 19 | 叶祥盛, 童军, 赵竹青. 流动注射分析法与钼锑抗比色法分析土壤有效磷含量的比较[J].河北农业科学, 2011, 15(1):160-164. |
| YE Xiangsheng, TONG Jun, ZHAO Zhuqing. Detection of soil available phosphorus content by flow injection analysis method and Mo-Sb antispetrophotography method[J].Journal of Hebei Agricultural Sciences, 2011, 15(1):160-164. | |
| 20 | 苗晓杰, 蒋恩臣, 王佳, 等. 对二甲氨基苯甲醛显色分光光度法检测水溶液中常微量尿素[J].东北农业大学学报, 2011, 42(8):87-92. |
| MIAO Xiaojie, JIANG Enchen, WANG Jia, et al. Using spectrophotometry with para-dimethyl-amino-benzaldehyde as chromogenic agent to determine macro and micro urea in aqueous solution[J].Journal of Northeast Agricultural University, 2011, 42(8):87-92. | |
| 21 | MEHTA A P, MAKOGON T Y, BURRUSS R C, et al. A composite phase diagram of structure H hydrates using Schreinemakers' geometric approach[J].Fluid Phase Equilibria, 1996, 121(1/2):141-165. |
| 22 | 王肖丽. K+//Cl-,H2PO4 -,(NH2)2CO-H2O和NH4 +,K+//Cl-,(NH2)2CO-H2O体系在 353.15 K下相平衡研究[D].贵阳:贵州大学, 2021. |
| 23 | WANG Jian, XU Renjie, XU Anli, et al. Thermodynamic modelling for solubility of 5-chloro-1-methyl-4-nitroimidazole in eleven organic solvents from T=(283.15 to 318.15) K[J].The Journal of Chemical Thermodynamics, 2017, 105:58-70. |
| 24 | WILSON G M. Vapor-liquid equilibrium.XI.A new expression for the excess free energy of mixing[J].Journal of the American Chemical Society, 1964, 86(2):127-130. |
| 25 | RENON H, PRAUSNITZ J. Estimation of parameters for the NRTL equation for excess Gibbs energies of strongly nonideal liquid mixtures[J].Industrial & Engineering Chemistry Process Design and Development, 1969, 8:413-419. |
| 26 |
HUANG Chunxiang, FANG Yun, WANG Jun, et al. Measurement and modelling of ternary solid-liquid phase equilibrium of (DL-malic acid+maleic acid+water) from 283.15 K to 333.15 K[J].The Journal of Chemical Thermodynamics, 2021, 159.Doi:10.1016/j.jct.2021.106470.
doi: 10.1016/j.jct.2021.106470 |
| 27 |
LI Rongrong, JIN Yanxian, YU Binbin, et al. Solubility determination and thermodynamic properties calculation of macitentan in mixtures of ethyl acetate and alcohols[J].The Journal of Chemical Thermodynamics, 2021, 156.Doi:10.1016/j.jct.2020.106344.
doi: 10.1016/j.jct.2020.106344 |
| 28 | 刘光启, 马连湘, 刘杰. 化学化工物性数据手册-无机卷[M].北京:化学工业出版社, 2002. |
| 29 | 中国寰球化学工程公司. 氮肥工艺设计手册[M].北京:化学工业出版社, 1988. |
| [1] | YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K [J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35. |
| [2] | LUO Chengling, FAN Xiaofan. Research progress of microstructure-regulated catalysts for urea oxidation reactions [J]. Inorganic Chemicals Industry, 2025, 57(2): 26-35. |
| [3] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [4] | WANG Ping, XU Rongsheng, SUN Dong, SHI Xiaohong, XU Wei, LI Mei. Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(9): 117-127. |
| [5] | PANG Hongchang, LIU Shuai, TIAN Peng, NING Guiling. Study on application of modified halloysite nanotubes in polyurea⁃based fireproof coatings [J]. Inorganic Chemicals Industry, 2024, 56(8): 27-32. |
| [6] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [7] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [8] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [9] | YANG Bo, MA Zhen, ZENG Ying, YAN Xiongzhong, LI Qi, HOU Yuansheng, YU Xudong. Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K [J]. Inorganic Chemicals Industry, 2024, 56(11): 116-122. |
| [10] | FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K [J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. |
| [11] | GONG Xuemin, ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian. Study on crystallization process of Na2CO3 hydrate in seawater system and its application [J]. Inorganic Chemicals Industry, 2023, 55(3): 55-59. |
| [12] | YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K [J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66. |
| [13] | LI Yan,WANG Yansong,CHENG Huaigang,KANG Jin,LI Enze,LÜ Hongzhou,LIU Qian. Rapid quality inspection of high-purity lithium carbonate based on determination of magnesium by water-soluble probe method [J]. Inorganic Chemicals Industry, 2023, 55(1): 87-92. |
| [14] | SONG Zhi, LIU Boxia, CHEN Yaoyao. Study on synthesis of FeWO4/WO3 complex by sol-gel and degradation of textile dye wastewater [J]. Inorganic Chemicals Industry, 2022, 54(5): 131-137. |
| [15] | CHEN Shuai,YANG Bo,CHEN Niancu,LUO Jun,REN Siying,ZENG Ying,YU Xudong. Study on phase equilibria of ternary system NH4Cl+MgCl2+H2O at 323.2 K [J]. Inorganic Chemicals Industry, 2022, 54(4): 100-103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||