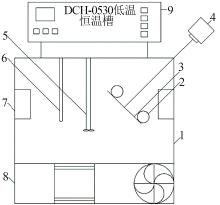
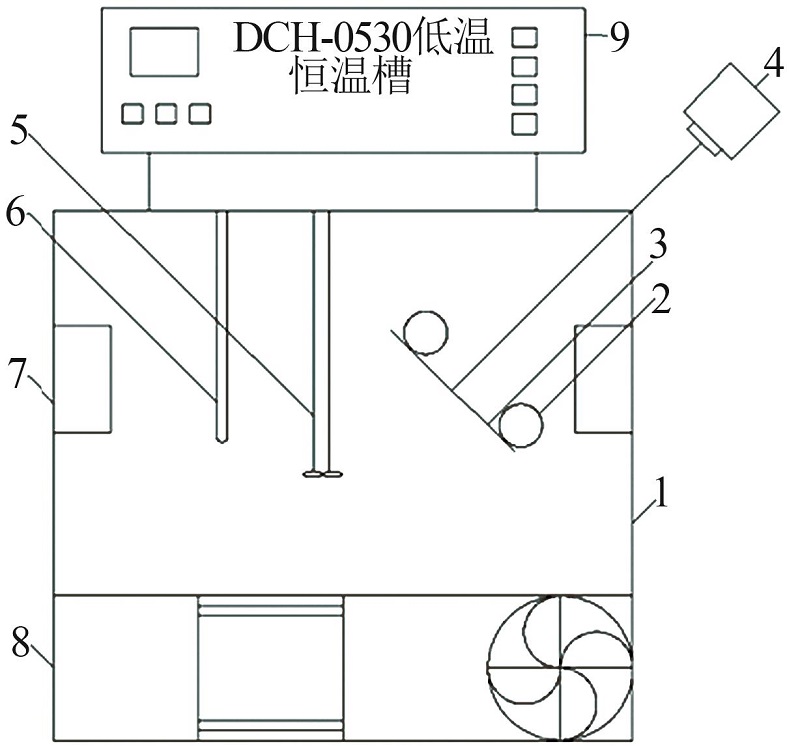
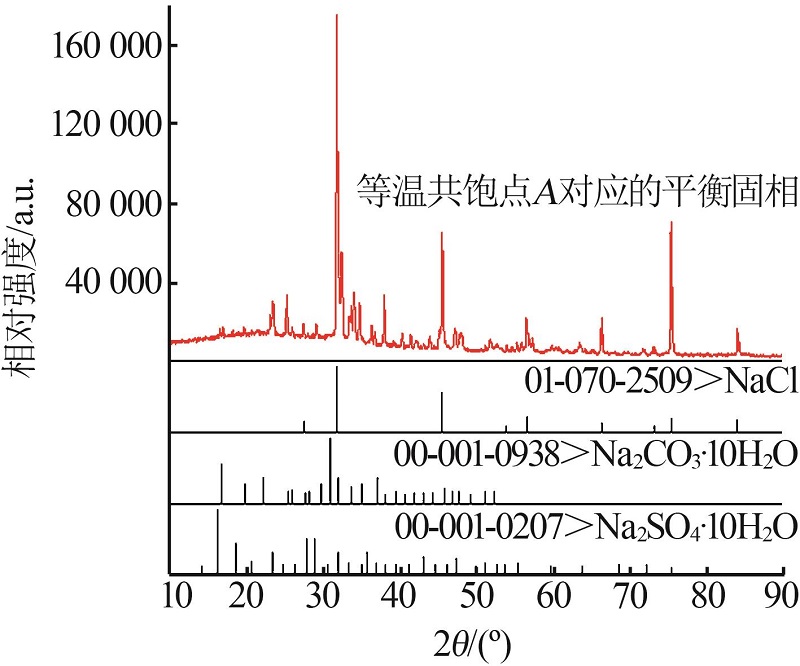
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (3): 55-59.doi: 10.19964/j.issn.1006-4990.2022-0289
• Research & Development • Previous Articles Next Articles
Study on crystallization process of Na2CO3 hydrate in seawater system and its application
GONG Xuemin( ), ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian
), ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian
- College of Chemical Engineering,North China University of Science and Technology,Tangshan 063210,China
-
Received:2022-05-12Online:2023-03-10Published:2023-03-17
CLC Number:
Cite this article
GONG Xuemin, ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian. Study on crystallization process of Na2CO3 hydrate in seawater system and its application[J]. Inorganic Chemicals Industry, 2023, 55(3): 55-59.
share this article
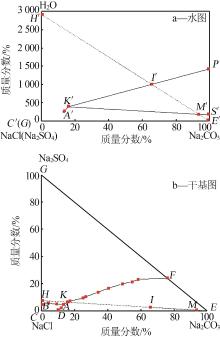
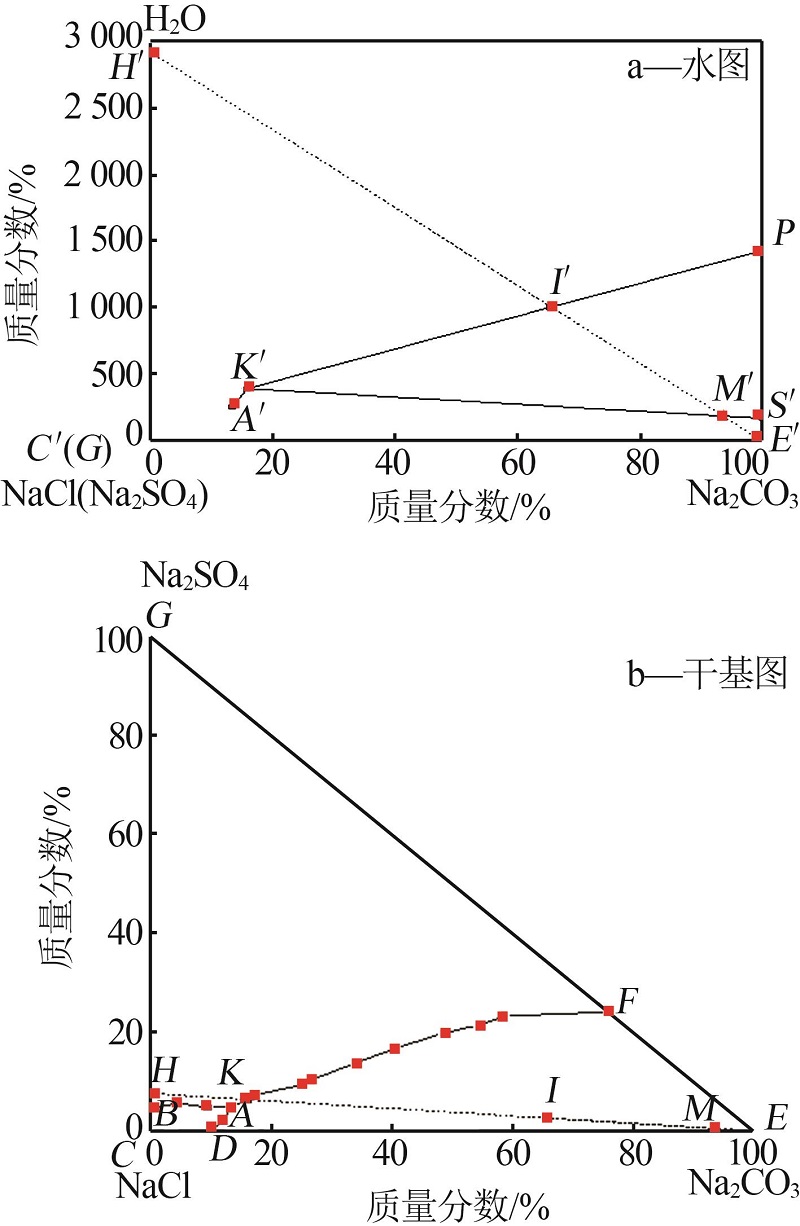
Table 1
Phase equilibrium solubility data of NaCl- Na2SO4-Na2CO3-H2O system at 0 ℃"
| 相图点 | 液相组成质量分数/% | 液相干盐组成/% | 平衡 固相 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NaCl | Na2CO3 | Na2SO4 | H2O | NaCl | Na2CO3 | Na2SO4 | |||
| B | 25.65 | 0.00 | 1.20 | 73.15 | 95.53 | 0.00 | 4.47 | Nl+Ns | |
| 24.50 | 1.20 | 1.53 | 72.77 | 89.97 | 4.41 | 5.62 | Nl+Ns | ||
| 23.04 | 2.54 | 1.28 | 73.14 | 85.78 | 9.46 | 4.77 | Nl+Ns | ||
| A | 23.03 | 3.79 | 1.38 | 71.80 | 81.67 | 13.44 | 4.89 | Nc+Ns+Nl | |
| 13.32 | 3.05 | 1.27 | 82.36 | 75.51 | 17.29 | 7.20 | Nc+Ns | ||
| 20.00 | 7.78 | 2.89 | 69.33 | 65.21 | 25.37 | 9.42 | Nc+Ns | ||
| 14.13 | 5.99 | 2.32 | 77.57 | 62.98 | 26.69 | 10.33 | Nc+Ns | ||
| 10.88 | 7.09 | 2.87 | 79.16 | 52.21 | 34.02 | 13.77 | Nc+Ns | ||
| 4.47 | 4.27 | 1.76 | 89.50 | 42.57 | 40.67 | 16.76 | Nc+Ns | ||
| 4.77 | 7.39 | 3.02 | 84.83 | 31.42 | 48.68 | 19.89 | Nc+Ns | ||
| 3.53 | 7.97 | 3.13 | 85.37 | 24.13 | 54.48 | 21.39 | Nc+Ns | ||
| 2.68 | 8.61 | 3.41 | 85.30 | 18.23 | 58.57 | 23.20 | Nc+Ns | ||
| F | 0.00 | 10.85 | 3.45 | 85.70 | 0.00 | 75.87 | 24.13 | Nc+Ns | |
| D | 24.24 | 2.74 | 0.00 | 73.02 | 89.84 | 10.16 | 0.00 | Nc+Nl | |
| 23.73 | 3.31 | 0.63 | 72.33 | 85.76 | 11.96 | 2.28 | Nc+Nl | ||
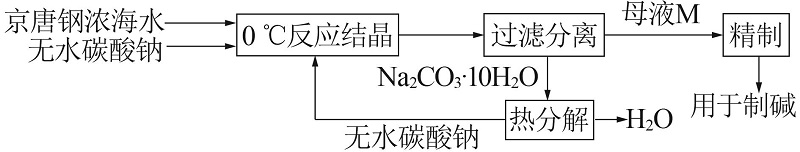
Table 3
Calculation of seawater concentration by sodium carbonate hydrate method at 0 ℃"
| 工艺过程 | 体系 | 各组成质量分数/% | 质量/ kg | |||
|---|---|---|---|---|---|---|
| Na2CO3 | Na2SO4 | NaCl | H2O | |||
| 增浓过程 | 京唐钢浓海水 | 0 | 0.25 | 3.06 | 96.69 | 1 000.00 |
| 加入Na2CO3 | 100 | 0 | 0 | 0 | 483.95 | |
| 混合体系 | 32.61 | 0.17 | 2.06 | 65.16 | 1 483.95 | |
| 母液 | 3.23 | 1.30 | 15.74 | 79.73 | 194.41 | |
| 生成Na2CO3·10H2O | 37.04 | 0 | 0 | 62.96 | 1 289.54 | |
Na2CO3·10H2O 热分解过程 | 无水碳酸钠 | 100 | 0 | 0 | 0 | 477.65 |
| H2O | 0 | 0 | 0 | 100 | 811.89 | |
| 1 | 李卜义,王建友.浓海水处理及综合利用技术的新进展[J].化工进展,2014,33(11):3067-3074. |
| LI Boyi, WANG Jianyou.Progress on treatment and comprehensive utilization of concentrated seawater[J].Chemical Industry and Engineering Progress,2014,33(11):3067-3074. | |
| 2 | 刘元元,范天博,张亚南,等.FTC海水淡化浓盐水蒸发结晶析盐规律研究[J].无机盐工业,2016,48(6):16-19. |
| LIU Yuanyuan, FAN Tianbo, ZHANG Ya′nan,et al.Study on salt precipitation law in evaporation crystallization process of concentrated brine from FTC seawater desalination[J].Inorganic Chemicals Industry,2016,48(6):16-19. | |
| 3 | 郑智颖,李凤臣,李倩,等.海水淡化技术应用研究及发展现状[J].科学通报,2016,61(21):2344-2370. |
| ZHENG Zhiying, LI Fengchen, LI Qian,et al.State-of-the-art of R & D on seawater desalination technology[J].Chinese Science Bulletin,2016,61(21):2344-2370. | |
| 4 | 陈嘉琳,张鑫,龚久炎,等.海水淡化浓盐水制备复合阻燃材料研究[J].无机盐工业,2017,49(3):47-51. |
| CHEN Jialin, ZHANG Xin, GONG Jiuyan,et al.Study on preparation of composite flame retardant materials with concentrated seawater after desalination[J].Inorganic Chemicals Industry,2017,49(3):47-51. | |
| 5 | 王建行,赵颖颖,李佳慧,等.二氧化碳的捕集、固定与利用的研究进展[J].无机盐工业,2020,52(4):12-17. |
| WANG Jianhang, ZHAO Yingying, LI Jiahui,et al.Research progress of carbon dioxide capture,fixation and utilization[J].Inorganic Chemicals Industry,2020,52(4):12-17. | |
| 6 | 李佳乐,王新宇,陈天艺,等.连续鼓泡法海水碳化制备碳酸钙文石的研究[J].无机盐工业,2021,53(2):42-46. |
| LI Jiale, WANG Xinyu, CHEN Tianyi,et al.Preparation of calcium carbonate aragonite by continuous bubbling carbonization in seawater[J].Inorganic Chemicals Industry,2021,53(2):42-46. | |
| 7 | 云玉娥,刘艳娟,王秉钧.浓海水化盐精制工艺实验研究[J].山东化工,2016,45(16):39-41. |
| YUN Yue, LIU Yanjuan, WANG Bingjun.Study on the refining process of desalination seawater[J].Shandong Chemical Industry,2016,45(16):39-41. | |
| 8 | 黄鹏飞,王小军,张寅,等.我国海水淡化现状与开发方案研究[J].盐科学与化工,2020,49(8):6-10. |
| HUANG Pengfei, WANG Xiaojun, ZHANG Yin,et al.The state of the art of desalination development and research on exploration scheme in China[J].Journal of Salt Science and Chemical Industry,2020,49(8):6-10. | |
| 9 | 王亚敏,刘杰,袁俊生.海水淡化副产浓海水资源化利用制备NaCl[J].水处理技术,2020,46(5):60-64. |
| WANG Yamin, LIU Jie, YUAN Junsheng.Preparation of NaCl from concentrated seawater of seawater desalination by-products[J].Technology of Water Treatment,2020,46(5):60-64. | |
| 10 | 徐政涛,谢应明,孙嘉颖,等.水合物法海水淡化技术研究进展及展望[J].热能动力工程,2020,35(7):1-11. |
| XU Zhengtao, XIE Yingming, SUN Jiaying,et al.Research progress and prospect of hydrate based desalination technology[J].Journal of Engineering for Thermal Energy and Power,2020,35(7):1-11. | |
| 11 | 薛倩,王晓霖,李遵照,等.水合物利用技术应用进展[J].化工进展,2021,40(2):722-735. |
| XUE Qian, WANG Xiaolin, LI Zunzhao,et al.Research progresses in hydrate based technologies and processes[J].Chemical Industry and Engineering Progress,2021,40(2):722-735. | |
| 12 | FAKHARIAN H, GANJI H, NADERIFAR A.Saline produced water treatment using gas hydrates[J].Journal of Environmental Chemical Engineering,2017,5(5):4269-4273. |
| 13 | 喻志广,祁影霞,姬利明,等.CO2水合物法淡化海水影响因素的实验研究[J].低温与特气,2013,31(1):21-25. |
| YU Zhiguang, QI Yingxia, JI Liming,et al.Experimental study of effects on hydrate seawater desalination by CO2 [J].Low Temperature and Specialty Gases,2013,31(1):21-25. | |
| 14 | 李栋梁,龙臻,梁德青.水合冷冻法海水淡化研究[J].水处理技术,2010,36(6):65-68. |
| LI Dongliang, LONG Zhen, LIANG Deqing.Preliminary study of seawater desalination using gas hydrate technology[J].Technology of Water Treatment,2010,36(6):65-68. | |
| 15 | 任宏波,相凤奎,张磊,等.水合物法海水淡化技术应用进展[J].海洋地质前沿,2011,27(6):74-78. |
| REN Hongbo, XIANG Fengkui, ZHANG Lei,et al.Application of hydrate to seawater desalination[J].Marine Geology Frontiers,2011,27(6):74-78. | |
| 16 | 刘骆峰,张雨山,黄西平,等.淡化后浓海水化学资源综合利用技术研究进展[J].化工进展,2013,32(2):446-452. |
| LIU Luofeng, ZHANG Yushan, HUANG Xiping,et al.Research progress of multipurpose utilization technologies of brine from seawater desalination plant[J].Chemical Industry and Engineering Progress,2013,32(2):446-452. | |
| 17 | 巩学敏,王岭,王秉钧,等.硫酸钠水合物法增浓精制海水技术研究[J].化学工程,2021,49(5):6-10. |
| GONG Xuemin, WANG Ling, WANG Bingjun,et al.Technological base of refined seawater enrichment by Na2SO4 hydrate method[J].Chemical Engineering(China),2021,49(5):6-10. | |
| 18 | 吕晓英,王秉钧,王海芳.硫酸钠溶液在盐水精制过程中的应用[J].纯碱工业,2015(5):21-22. |
| LV Xiaoying, WANG Bingjun, WANG Haifang.Application of sodium sulfate solution in brine refining process[J].Soda Industry,2015(5):21-22. | |
| 19 | 邓天龙,周桓,陈侠.水盐体系相图及应用[M].2版.北京:化学工业出版社,2020. |
| 20 | 四川大学化学工程学院,浙江大学化学系.分析化学实验[M].4版.北京:高等教育出版社,2015. |
| [1] | WANG You, LIAO Lianzhen, CHEN Zheng, GAO Youjun. Effect of surfactants on electrocrystallization of Ni(OH)2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 58-63. |
| [2] | GUO Yingjun, WU Songsong, DING Chunyan, ZHAO Shikai, SONG Tao, WEN Guangwu. Preparation of SSZ-13 zeolite membrane from glass-ceramics-strontium feldspar by crystal transformation method [J]. Inorganic Chemicals Industry, 2025, 57(2): 76-82. |
| [3] | SHEN Xiaoqian, ZHOU Fei, LIU Wanchen, XU Lu, WU Junshu. Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance [J]. Inorganic Chemicals Industry, 2025, 57(2): 57-67. |
| [4] | ZHU Jicheng, YANG Qixin, LIANG Haoquan, WANG Zengkun, OUYANG Fugui, DI Jing, GAI Xikun. Effect of confined catalyst Ni@S2 on performance of methane dry reforming reaction [J]. Inorganic Chemicals Industry, 2025, 57(2): 138-146. |
| [5] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [6] | ZHANG Jianle, CAO Yapeng, ZU Minghua, ZHANG Zhikun, LIU Yumin, HAN Jilong. Study on morphology of calcium carbonate controlled by sodium dodecyl sulfate and amino acid double template [J]. Inorganic Chemicals Industry, 2025, 57(1): 58-63. |
| [7] | ZOU Yang, LU Zhiyan, HU Zhilin, SUN Ze. Study on metastable zone width and primary nucleation kinetics for cooling crystallization of KNO3 [J]. Inorganic Chemicals Industry, 2024, 56(9): 67-74. |
| [8] | LI Shuai, LI Tianxiang, ZHU Jing, LIU Songlin. Study on purification process of sodium fluoride [J]. Inorganic Chemicals Industry, 2024, 56(9): 90-97. |
| [9] | WANG Jianjie, SHU Xiaolong, XIAO Xia, WANG Peng, FAN Xiaoqiang, KONG Lian, XIE Zean, ZHAO Zhen. Study on synthesis of hierarchical flower⁃like ZSM-5 zeolite and its catalytic performance for n-octane cracking [J]. Inorganic Chemicals Industry, 2024, 56(8): 139-146. |
| [10] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [11] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [12] | HU Cheng, LIU Meng, XIANG Weiheng, CHEN Ping, WANG Neng, LU Guanju, ZHOU Jinlan. Preparation of α-hemihydrous gypsum from CaCl2 and MgCl2 and their composite solution [J]. Inorganic Chemicals Industry, 2024, 56(7): 112-117. |
| [13] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [14] | LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing. Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72. |
| [15] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||