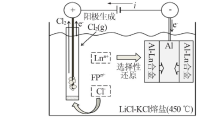
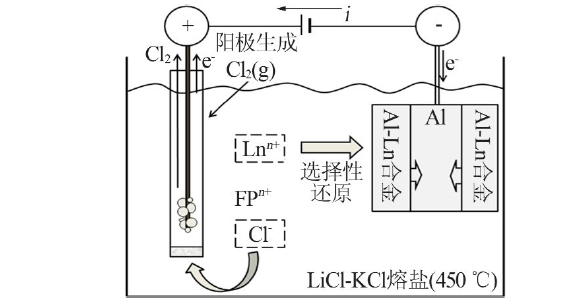
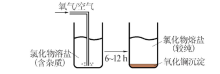
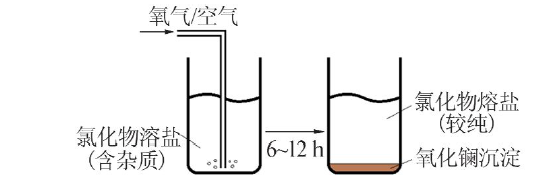
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (4): 81-87.doi: 10.19964/j.issn.1006-4990.2021-0577
• Reviews and Special Topics • Previous Articles Next Articles
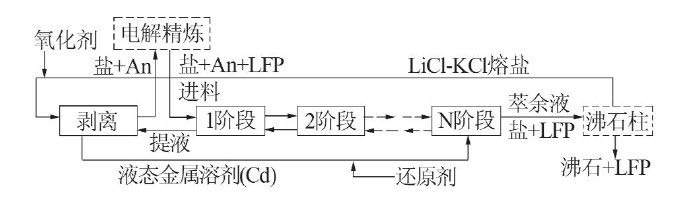
Research progress on purification process for active crack elements in waste salt by dry post-treatment
WU Sida( ),LIN Rushan(
),LIN Rushan( ),ZHANG Lei,JIA Yanhong
),ZHANG Lei,JIA Yanhong
- China Institute of Atomic Energy,Institute of Radiochemistry,Beijing 102413,China
-
Received:2021-09-23Online:2022-04-10Published:2022-04-18 -
Contact:LIN Rushan E-mail:wusida921@qq.com;lrsh3@163.com
CLC Number:
Cite this article
WU Sida,LIN Rushan,ZHANG Lei,JIA Yanhong. Research progress on purification process for active crack elements in waste salt by dry post-treatment[J]. Inorganic Chemicals Industry, 2022, 54(4): 81-87.
share this article
Table 1
Purification methods for different elements and their advantages and disadvantages"
| 净化方法 | 净化对象 | 优点 | 缺点 | |
|---|---|---|---|---|
| 熔盐萃取法 | 镧系元素 | 回收率高、净化效果好 | 需要严格控制Li用量 | |
| 熔盐电解法 | 镧系元素 | 操作简单、效率高 | 电解产生大量氯气,腐蚀设备,处理困难,有污染的风险 | |
| 沉 淀 法 | 磷酸盐沉淀法 | 镧系元素 | 反应高效、快捷 | 引入新杂质,分离Cs、Sr的效率低 |
| 碳酸盐沉淀法 | 镧系元素、碱土金属 | 不引入新杂质,可以沉淀碱土金属 | 除去碱土需要控制用量,且产生CO2废气 | |
| 氧化物沉淀法 | 镧系元素 | 操作简单、不引入新杂质、净化效率高 | 反应时间长、操作复杂、温度高;无法分离Cs、Sr | |
| 离子交换法 | 镧系元素、Cs、Sr | 低温熔盐离子交换技术成熟 | 分离产生大量废物,且难以适应高温熔盐 | |
| 结晶法 | 冷指分离法 | Cs、Sr | 不引入新的化学组成 | 操作效率低,分离的量有限,难以实现工程应用 |
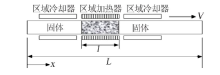
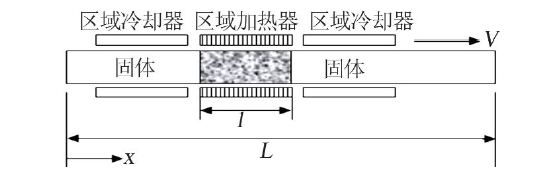
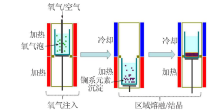
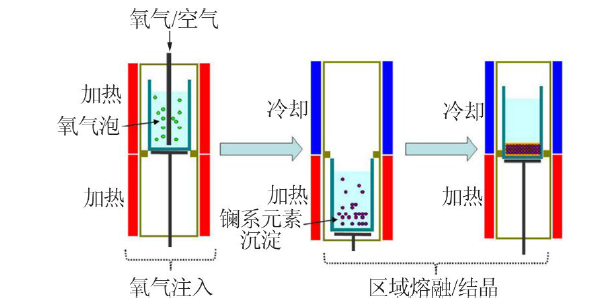
| 区域熔融/结晶法 | Cs、Sr | 不引入新的化学组成,且分离效果好,效率高 | 需要长时间的区域熔融/结晶过程才能得到较纯的分 离盐 | |
| [1] | 林如山, 何辉, 唐洪彬, 等. 我国乏燃料干法后处理技术研究现状与发展[J]. 原子能科学技术, 2020(S1):115-125. |
| [2] | 王有群, 何辉, 林如山, 等. 无机氯化物熔盐在乏燃料干法后处理中的应用进展[J]. 无机盐工业, 2016, 48(8):1-5. |
| [3] | 叶国安, 郑卫芳, 何辉, 等. 我国核燃料后处理技术现状和发展[J]. 原子能科学技术, 2020, 54(z1):75-83. |
| [4] | 刘海军, 陈晓丽. 国内外乏燃料后处理技术研究现状[J]. 节能技术, 2021, 39(4):358-362. |
| [5] |
CHOI J H, LEE K R, KANG H W, et al. Reactive-crystallization method for purification of LiCl salt waste[J]. Journal of Radioanalytical and Nuclear Chemistry, 2020, 325(2):485-492.
doi: 10.1007/s10967-020-07235-0 |
| [6] |
INOUE T, KOCH L. Development of pyroprocessing and its future direction[J]. Nuclear Engineering and Technology, 2008, 40(3):183-190.
doi: 10.5516/NET.2008.40.3.183 |
| [7] | 梁红彦. 乏燃料电冶金废熔盐中放射性核素的脱除与固化[D]. 南宁:广西大学, 2015. |
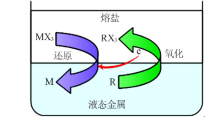
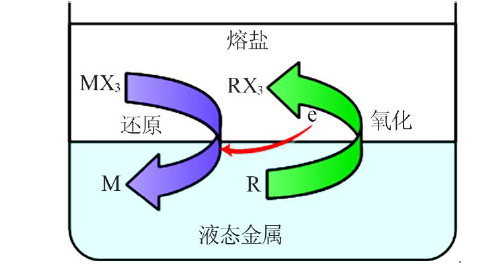
| [8] | 孙鹏院. 熔盐/液态金属(Bi-Li)还原萃取稀土Ce和Sm[D]. 哈尔滨:哈尔滨工程大学, 2015. |
| [9] | EUN H C, CHOI J H, CHO I H, et al. Purification of LiCl-KCl eutectic waste salt containing rare earth chlorides delivered from the pyrochemical process of used nuclear fuel using a reactive distillation process[J]. Journal of Radioanalytical and Nuclear Chemistry, 2016, 30:1419-1425. |
| [10] | 贾艳虹, 何辉, 林如山, 等. 用于熔盐体系的氮化硼隔膜Ag/AgCl参比电极性能[J]. 无机盐工业, 2015, 47(5):67-71. |
| [11] | MURAKAMI T, SAKAMURA Y, UOZUMI K, et al. Rare earth silicide formation on Si electrode in LiCl-KCl melt to establish a novel used salt treatment process[J]. ECS Transactions, 2020, 98(10):33-46. |
| [12] | 张凯, 肖益群, 林如山, 等. 俄罗斯氧化物乏燃料电沉积流程研究进展[J]. 核化学与放射化学, 2019, 41(3):233-241. |
| [13] |
SOUČEK P, MALMBECK R, MENDES E, et al. Exhaustive electrolysis for recovery of actinides from molten LiCl-KCl using solid aluminium cathodes[J]. Journal of Radioanalytical and Nuclear Chemistry, 2010, 286:823-828.
doi: 10.1007/s10967-010-0739-6 |
| [14] |
SONG K C, LEE H, HUR J M, et al. Status of pyroprocessing technology development in Korea[J]. Nuclear Engineering and Technology, 2010, 42(2):131-144.
doi: 10.5516/NET.2010.42.2.131 |
| [15] | 聂春晨. 稀土金属提纯现状及发展趋势[J]. 化工设计通讯, 2019, 45(5):67-68. |
| [16] |
IVANOV A B, BYZOVA E D, VOLKOVICH V A, et al. Application of phosphate precipitation for removing strontium and barium from alkali chloride based melts[J]. ECS Transactions, 2020, 98(10):283-294.
doi: 10.1149/09810.0283ecst |
| [17] |
CHO Y Z, PARK G H, YANG H C, et al. Minimization of eutectic salt waste from pyroprocessing by oxidative precipitation of lanthanides[J]. Journal of Nuclear Science and Technology, 2009, 46(10):1004-1011.
doi: 10.1080/18811248.2009.9711610 |
| [18] |
LEE T K, CHO Y Z, EUN H C, et al. Study on the phosphate reaction characteristics of lanthanide chlorides in molten salt with operating conditions[J]. Journal of Nuclear Science and Technology, 2013, 50(7):742-750.
doi: 10.1080/00223131.2013.799396 |
| [19] |
CHO Y Z, LEE T K, EUN H C, et al. Purification of used eutectic (LiCl-KCl) salt electrolyte from pyroprocessing[J]. Journal of Nuclear Materials, 2013, 437(1/2/3):47-54.
doi: 10.1016/j.jnucmat.2013.01.344 |
| [20] |
RILEY B J. Electrochemical salt wasteform development:A review of salt treatment and immobilization options[J]. Industrial and Engineering Chemistry Research, 2020, 59(21):9760-9774.
doi: 10.1021/acs.iecr.0c01357 |
| [21] |
CHOI J H, EUN H C, LEE K R, et al. Fabrication of rare earth calcium phosphate glass waste forms for the immobilization of rare earth phosphates generated from pyrochemical process[J]. Journal of Non-Crystalline Solids, 2016, 434:79-84.
doi: 10.1016/j.jnoncrysol.2015.12.017 |
| [22] |
KIM E H, PARK G I, CHO Y Z, et al. A new approach to minimize pyroprocessing waste salts through a series of fission product removal process[J]. Nuclear Technology, 2008, 162(2):208-218.
doi: 10.13182/NT08-A3949 |
| [23] |
LAN Y P, SOHN H Y, MURALI A, et al. The formation and growth of CeOCl crystals in a molten KCl-LiCl flux[J]. Applied Physics A, 2018, 124(10).Doi: 10.1007/s00339-018-2122-3.
doi: 10.1007/s00339-018-2122-3 |
| [24] |
LIU Y, LIU K, LUO L, et al. Direct separation of uranium from lanthanides (La,Nd,Ce,Sm) in oxide mixture in LiCl-KCl eutectic melt[J]. Electrochimica Acta, 2018, 275:100-109.
doi: 10.1016/j.electacta.2018.04.140 |
| [25] |
LEE H S, GYU-HWAN O H, LEE Y S, et al. Concentrations of CsCl and SrCl2 from a simulated LiCl salt waste generated by pyroprocessing by using Czochralski method[J]. Journal of Nuclear Science and Technology, 2009, 46(4):392-397.
doi: 10.1080/18811248.2007.9711545 |
| [26] |
KIM I S, CHUNG D Y, PARK M S, et al. Evaporation of CsCl,BaCl2, and SrCl2 from the LiCl-Li2O molten salt of the electrolytic reduction process[J]. Journal of Radioanalytical and Nuclear Chemistry, 2015, 303(1):223-227.
doi: 10.1007/s10967-014-3330-8 |
| [27] | YOO T S, FRANK S M, SIMPSON M F, et al. Salt-zeolite ion-exchange equilibrium studies for a complete set of fission products in molten LiCl-KCl[J]. Nuclear Technology:A journal of the American Nuclear Society, 2010, 171(3):306-315. |
| [28] |
SACHDEV P, SIMPSON M F, FRANK S M, et al. Selective separation of Cs and Sr from LiCl-based salt for electrochemical processing of oxide spent nuclear fuel[J]. Separation Science and Technology, 2008, 43(9/10):2709-2721.
doi: 10.1080/01496390802122212 |
| [29] |
LEXA D, JOHNSON I. Occlusion and ion exchange in the molten (lithium chloride-potassium chloride-alkali metal chloride) salt+zeolite 4A system with alkali metal chlorides of sodium,rubidium,and cesium[J]. Metallurgical and Materials Transactions B, 2001, 32(3):429-435.
doi: 10.1007/s11663-001-0028-4 |
| [30] |
LEXA D. Occlusion and ion exchange in the molten(lithium chloride+potassium chloride+alkaline-earth chloride) salt+zeolite 4A system with alkaline-earth chlorides of calcium and strontium and in the molten(lithium chloride+potassium chloride+actinide chloride) salt+zeolite 4A system with the actinide chloride of uranium[J]. Metallurgical and Materials Transactions B, 2003, 34(2):201-208.
doi: 10.1007/s11663-003-0007-z |
| [31] |
SHALTRY M, PHONGIKAROON S, SIMPSON M F. Ion exchange kinetics of fission products between molten salt and zeolite-A[J]. Microporous and Mesoporous Materials, 2012, 152:185-189.
doi: 10.1016/j.micromeso.2011.11.035 |
| [32] | CHO Y Z, LEE T K, CHOI J H, et al. Study on LiCl waste salt treatment process by layer melt crystallization[C]// Salt Lake City.International nuclear fuel cycle conference,GLOBAL 2013:Nuclear energy at a crossroads, 2013:297-299. |
| [33] | POGLYAD S S, ANKUDINOVA N S, NECHAEV P I, et al. The cesium precipitation from the spent electrolyte LiCl-KCl composition simulator[J]. Journal of Physics:Conference Series, 2018, 1133(1):12-22. |
| [34] |
CHO Y Z, AHN B G, EUN H C, et al. Melt crystallization process treatment of LiCl salt waste generated from electrolytic reduction process of spent oxide fuel[J]. Energy Procedia, 2011, 7(1):525-528.
doi: 10.1016/j.egypro.2011.06.072 |
| [35] |
VERSEY J R, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from LiCl-CsCl molten salt by cold finger melt crystallization[J]. Nuclear Engineering and Technology, 2014, 46(3):395-406.
doi: 10.5516/NET.06.2013.082 |
| [36] |
CHOI J H, CHO Y Z, LEE T K, et al. Inclusion behavior of Cs,Sr, and Ba impurities in LiCl crystal formed by layer-melt crystallization:Combined first-principles calculation and experimental study[J]. Journal of Crystal Growth, 2013, 371:84-89.
doi: 10.1016/j.jcrysgro.2013.02.013 |
| [37] |
CHOI J H, LEE T K, LEE K R, et al. Melt-crystallization monitoring system for the purification of 10 kg-scale LiCl salt waste[J]. Nuclear Engineering and Design, 2018, 326:1-6.
doi: 10.1016/j.nucengdes.2017.10.016 |
| [38] | 任永胜, 李军, 马睿, 等. 区域熔融法提纯工业黄磷的数学模型与实验研究[J]. 高校化学工程学报, 2009(6):933-938. |
| [39] |
GHOSH K, MANI V N, DHAR S. Numerical study and experimental investigation of zone refining in ultra-high purification of gallium and its use in the growth of GaAs epitaxial layers[J]. Journal of Crystal Growth, 2009, 311(6):1521-1528.
doi: 10.1016/j.jcrysgro.2009.01.102 |
| [40] |
CHO Y Z, LEE T K, CHOI J H, et al. Eutectic(LiCl-KCl) waste salt treatment by sequencial separation process[J]. Nuclear Engineering and Technology, 2013, 45(5):675-682.
doi: 10.5516/NET.06.2013.022 |
| [41] | WILLIAMS A N, PACK M, PHONGIKAROON S. Separation of SrCl2 and CsCl from ternary SrCl2-LiCl-KCl and quaternary SrCl2-CsCl-LiCl-KCl molten salts via melt crystallization[J]. Transactions of the American Nuclear Society, 2014, 111(1):431-433. |
| [42] | 周骏宏, 李军, 任永胜. 区域熔融法净化磷酸的初步研究[J]. 无机盐工业, 2010, 42(7):23-25. |
| [43] | 张先锋. 区域熔融温度场数值模拟与实验研究[D]. 沈阳:东北大学, 2006. |
| [44] |
CHO Y Z, LEE T K, EUN H C, et al. Purification of used eutectic (LiCl-KCl) salt electrolyte from pyroprocessing[J]. Journal of Nuclear Materials, 2013, 437(1/2/3):47-54.
doi: 10.1016/j.jnucmat.2013.01.344 |
| [45] |
SHIM M, CHOI H G, CHOI J H, et al. Separation of Cs and Sr from LiCl-KCl eutectic salt via a zone-refining process for pyroprocessing waste salt minimization[J]. Journal of Nuclear Materials, 2017, 491:9-17.
doi: 10.1016/j.jnucmat.2017.04.036 |
| [46] |
CHOI H G, SHIM M, LEE J H, et al. Numerical analysis of impurity separation from waste salt by investigating the change of concentration at the interface during zone refining process[J]. Journal of Crystal Growth, 2017, 474:69-75.
doi: 10.1016/j.jcrysgro.2016.12.083 |
| [47] |
SHIM M, KIM Y M, LEE H H, et al. Separation behavior of impurities and selenium reduction by the reactive zone refining process using high-frequency induction heating to purify Te[J]. Journal of Crystal Growth, 2016, 455:6-12.
doi: 10.1016/j.jcrysgro.2016.09.032 |
| [48] |
CHO Y Z, PARK G H, LEE H S, et al. Concentration of cesium and strontium elements involved in a LiCl waste salt by a melt crystallization process[J]. Nuclear Technology, 2010, 171(3):325-334.
doi: 10.13182/NT09-7 |
| [49] |
WILLIAMS A N, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from a ternary CsCl-LiCl-KCl salt via a melt crystallization technique for pyroprocessing waste minimization[J]. Chemical Engineering Science, 2013, 89:258-263.
doi: 10.1016/j.ces.2012.12.012 |
| [50] | 付海英, 耿俊霞, 杨洋, 等. 乏燃料干法后处理中的熔盐减压蒸馏技术[J]. 核技术, 2018(4):5-12. |
| [1] | LI Shuai, LI Tianxiang, ZHU Jing, LIU Songlin. Study on purification process of sodium fluoride [J]. Inorganic Chemicals Industry, 2024, 56(9): 90-97. |
| [2] | YANG Li, LUO Xiaoxiong, HUANG Junli, LI Jing, LUO Minzhu. Study on purification process of sulfur by solvent method [J]. Inorganic Chemicals Industry, 2024, 56(7): 69-73. |
| [3] | LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing. Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72. |
| [4] | DENG Fuli, XIA Zhixiang, LONG Bingwen, ZHANG Yi, DAI Yafen, WANG Bin, DING Yigang. Study on purification process of phosphogypsum by reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(5): 115-120. |
| [5] | BAI Chaopeng, PENG Hui, CHEN Yuting, LUO Jiang, ZHANG Shengming. Research progress of recovery,purification and recycling of ruthenium from scrap ruthenium materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 24-33. |
| [6] | TAO Longhai, WANG Yongjie, DUAN Jiatang, SHU Yizhou, WAN Banglong, HUANG Chengdong, ZHANG Yueqiang, LU Zhenya. Research progress and prospect of sludge acid and raffinate acid by-product of wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 12-18. |
| [7] | SU Xin, LAI Dongmei, ZHOU Yuan, ZHU Xiaping. Study on preparation process of strontium rich functional salt from Zigong underground brine [J]. Inorganic Chemicals Industry, 2024, 56(3): 45-50. |
| [8] | LÜ Lingshuang, CAO Wenhao, YU Ruixian, WANG Shouzhi, WANG Guodong, ZHU Yajun, DU Jiachen, XU Xiangang, ZHANG Lei. Research on purification process of AlN source material by two-step sintering method [J]. Inorganic Chemicals Industry, 2024, 56(12): 51-55. |
| [9] | CHEN Xiaohong, YU Yi, ZHU Miao, YE Hengpeng, CHEN Shaohua, LI Yubiao. Study on co⁃reverse flotation process of phosphogypsum for impurity removal and whitening [J]. Inorganic Chemicals Industry, 2024, 56(10): 86-94. |
| [10] | WANG Zihan, LI Jun, CHEN Ming, ZHOU Qingyu. Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid [J]. Inorganic Chemicals Industry, 2023, 55(7): 51-57. |
| [11] | CHEN Xinyi, ZHANG Hua, FANG Wei, XU Xiaofeng. Application of metal-organic frameworks in fields of natural gas purification and storage [J]. Inorganic Chemicals Industry, 2023, 55(4): 13-19. |
| [12] | LAN Yinghua, CHEN Yanmei, MA Ruixiao, ZHANG Yanhui. Preparation and photocatalytic performance of Ce-Ti oxide-attapulgite composites [J]. Inorganic Chemicals Industry, 2023, 55(4): 133-140. |
| [13] | WU Hao,LI Xi,ZHANG Jun,DUAN Siyu. Study on impurity removal process of prereduction-oxalic acid precipitation in stainless steel coloring ageing liquid [J]. Inorganic Chemicals Industry, 2023, 55(2): 119-125. |
| [14] | HU Guangshou, LI Huping, ZHOU Wanchun, LI Xiangdong, MA Xuhua. Study on origin of tetravalent cerium in extraction and separation of rare earth [J]. Inorganic Chemicals Industry, 2023, 55(11): 64-69. |
| [15] | HE Zhengyan,LI Changwei,TIAN Peng,BI Shengnan,ZHU Peihan,LI Xinzhu,YE Junwei,NING Guiling. Investigation on holding mechanism and removal method of trace calcium in high purity boric acid prepared by esterification method [J]. Inorganic Chemicals Industry, 2022, 54(7): 49-54. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||