| [1] |
郑绵平, 侯献华.青海盐湖资源综合利用与可持续发展战略[J].科技导报, 2017, 35(12): 11-13.
|
| [2] |
赵露, 王晓, 马玉军, 等. 西台吉乃尔湖尾闾盐沼区水资源中有价元素分布特征研究[J].青海大学学报, 2020, 38(3): 101-106.
|
|
ZHAO Lu, WANG Xiao, MA Yujun, et al.Distribution characteristics of valuable elements in water resources from the downstream salt marsh of West Taijinar Lake[J].Journal of Qinghai University, 2020, 38(3): 101-106.
|
| [3] |
张福祥, 赵莎, 刘卓, 等. 全球硼矿资源现状与利用趋势[J].矿产保护与利用, 2019, 39(6): 142-151.
|
|
ZHANG Fuxiang, ZHAO Sha, LIU Zhuo, et al.Current situation and utilization trend of the global boron resources[J].Conservation and Utilization of Mineral Resources, 2019, 39(6): 142-151.
|
| [4] |
焦森, 郑厚义, 屈云燕, 等. 全球硼矿资源供需形势分析[J].国土资源情报, 2020(10): 85-89.
|
|
JIAO Sen, ZHENG Houyi, QU Yunyan, et al.Supply and demand situation of global boron resources[J].Land and Resources Information, 2020(10): 85-89.
|
| [5] |
YUAN Fei, LI Hua, LI Long, et al.Solid-liquid phase equilibria of the ternary system(KBO2+K2SO4+H2O) at 288.15,308.15 K,and 0.1 MPa[J].Journal of Chemical & Engineering Data, 2021, 66(4): 1703-1708.
|
| [6] |
WANG Shiqiang, SHI Chuncheng, YANG Juan, et al.Solid-liquid phase equilibria in the ternary systems(LiBO2+NaBO2+H2O) and (LiBO2+KBO2+H2O) at 288.15 K and 0.1 MPa[J].Journal of Solution Chemistry, 2020, 49(3): 353-364.
|
| [7] |
LI Jun, GAO Shiyang, XIA Shuping, et al.Thermochemistry of hydrated magnesium borates[J].The Journal of Chemical Thermodynamics, 1997, 29(4): 491-497.
|
| [8] |
CHEN Shangqing, WANG Mengxue, HU Jiayin, et al.Phase equilibria in the aqueous ternary systems(NaCl+NaBO2+H2O) and (Na2SO4+NaBO2+H2O) at 298.15 K and 0.1 MPa[J].Journal of Chemical and Engineering Data(the ACS Journal for Data), 2018, 63(12): 4662-4668.
|
| [9] |
CUI Wanjing, HOU Hongfang, HU Jiayin, et al.Phase equilibria and phase diagrams for the aqueous ternary system containing sodium,chloride,and metaborate ions at 288.15 and 308.15 K and 0.1 MPa[J].Journal of Chemistry, 2019.Doi:10.1155/2019/ 1983051 .
doi: 10.1155/2019/ 1983051
|
| [10] |
YANG Lan, LI Dan, ZHANG Tao, et al.Thermodynamic phase equilibria in the aqueous ternary system NaCl-NaBO2-H2O:Experimental data and solubility calculation using the Pitzer mo-del[J].The Journal of Chemical Thermodynamics, 2020,142.Doi:10.1016/j.jct.2019.106021 .
doi: 10.1016/j.jct.2019.106021
|
| [11] |
BU Baihui, LI Long, ZHANG Nan, et al.Solid-liquid metastable phase equilibria for the ternary system(Li2SO4+K2SO4+H2O) at 288.15 and 323.15 K,p=0.1 MPa[J].Fluid Phase Equilibria, 2015, 402: 78-82.
|
| [12] |
WANG Shiqiang, YANG Juan, SHI Chuncheng, et al.Solubilities,densities,and refractive indices in the ternary systems(LiBO2+NaBO2+H2O) and(LiBO2+KBO2+H2O) at 298.15 K and 0.1 MPa[J].Journal of Chemical & Engineering Data, 2019, 64(7): 3122-3127.
|
| [13] |
中国科学院青海盐湖研究所分析室.卤水和盐的分析方法[M].2版.北京:科学出版社, 1988.
|
| [14] |
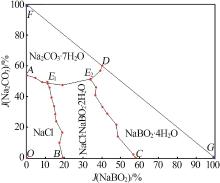
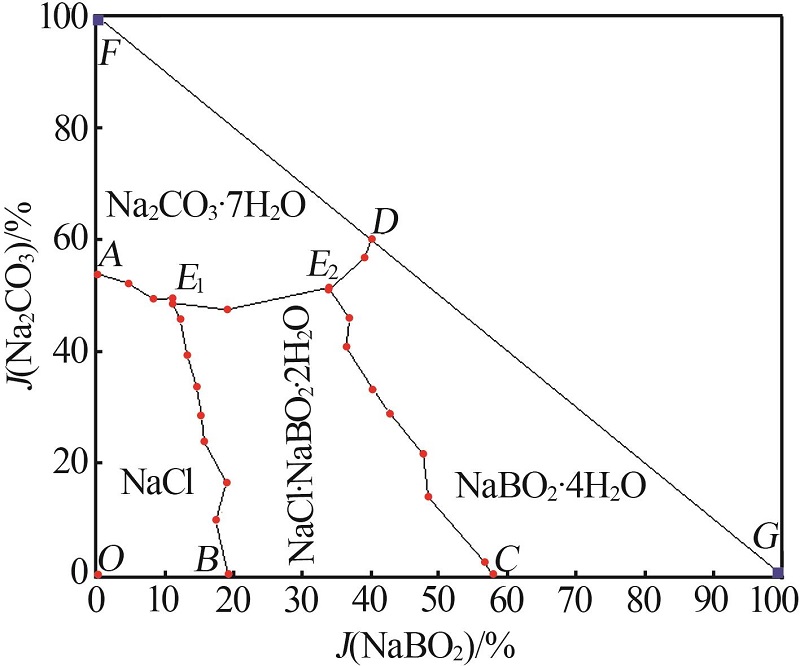
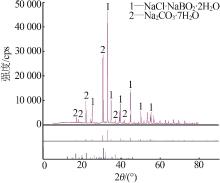
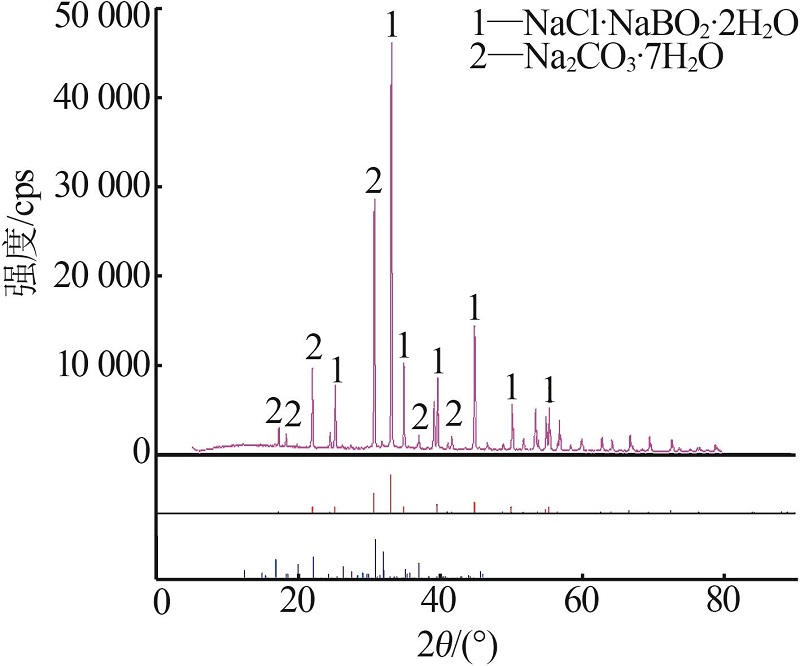
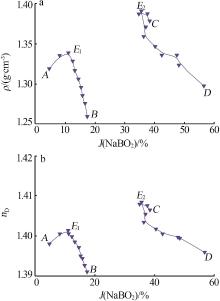
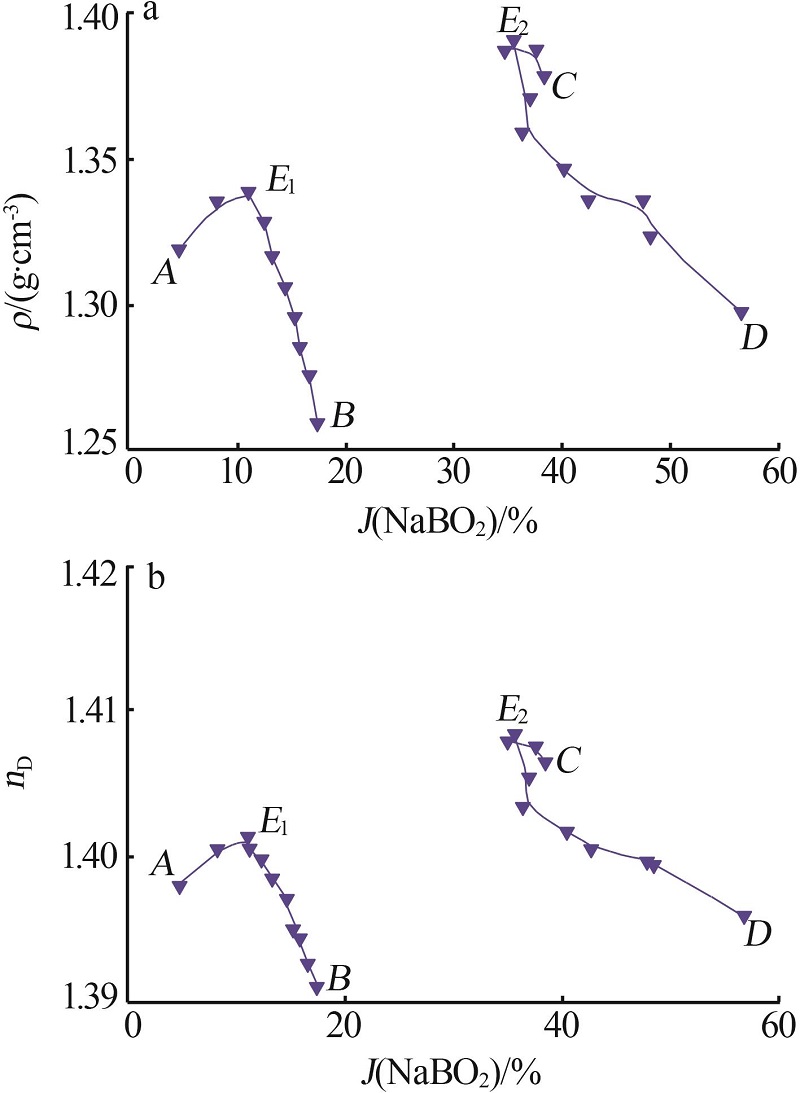
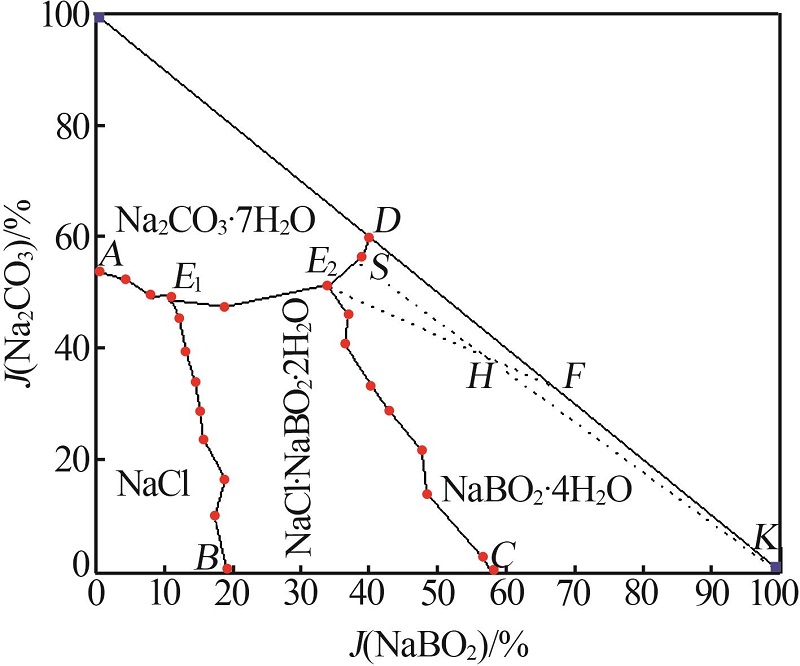
杨琴, 李君, 李恒欣, 等. 三元体系Na2B4O7-NaHCO3-H2O和Na2CO3-NaBO2-H2O等温溶解度研究[J].盐湖研究, 2001, 9(4): 24-29.
|
|
YANG Qin, LI Jun, LI Hengxin, et al.Study on the isothermal solubility for the ternary system Na2B4O7-NaHCO3-H2O and Na2CO3-NaBO2-H2O[J].Journal of Salt Lake Research, 2001, 9(4): 24-29.
|
 ),WANG Xiao(
),WANG Xiao( ),LI Shuya,ZHANG Fukang,LI Juan
),LI Shuya,ZHANG Fukang,LI Juan