| [1] |
LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan.
Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash
[J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104.
|
| [2] |
ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong.
Study on preparation of low ash activated carbon by phosphoric acid method
[J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103.
|
| [3] |
HUANG Zhaojie, ZHAO Xiaoxu, WANG Haitao, CHANG Na, ZHANG Guoxin, XIE Yonglei, YIN Yanmei, LU Na, WANG Wei.
Study on ultrafiltration+nanofiltration double membrane treatment of iron process iron phosphate production wastewater
[J]. Inorganic Chemicals Industry, 2024, 56(11): 145-150.
|
| [4] |
JIN Shengshi, LIU Kaijie, LIU Qiuwen, ZHANG Yibo, YANG Xiangguang.
Study on catalytic performance of phosphoric acid modified CeO2 nanorod supported Pt catalyst for propane combustion
[J]. Inorganic Chemicals Industry, 2024, 56(1): 141-148.
|
| [5] |
ZHANG Ying, LI Jun, JIN Yang, HUANG Meiying.
Study on pyrolysis regeneration of activated carbon for Tributyl phosphate decolorization
[J]. Inorganic Chemicals Industry, 2024, 56(1): 59-66.
|
| [6] |
LI Jiaqiang, LI Jun, JIN Yang, CHEN Ming.
A gas chromatographic analysis method for acetic acid content in phosphoric acid and acetic acid mixed system
[J]. Inorganic Chemicals Industry, 2023, 55(5): 115-120.
|
| [7] |
TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin.
Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite
[J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65.
|
| [8] |
ZHOU Qinglie, WANG Baoqi, ZHANG Zhiye, ZHANG Yinghu, WANG Jian, YANG Lin.
Development of new process of removing metal cations from wet-process phosphoric acid by extraction
[J]. Inorganic Chemicals Industry, 2023, 55(3): 84-91.
|
| [9] |
TIAN Zhuangzhuang, CHEN Jianjun, JIN Yang, CHEN Ming, LI Jun, LIU Daijun.
Experimental study on removing metal ions from phosphoric acid by nanofiltration membrane
[J]. Inorganic Chemicals Industry, 2023, 55(12): 133-139.
|
| [10] |
HE Fuduo, YUAN Yifan, ZHOU Xiaohou, XU Lu, SUN Chongqing, WANG Xinlong, XU Dehua.
Preparation of monoammonium phosphate containing amino acid from waste feathers by in situ acid hydrolysis of wet process of phosphoric acid
[J]. Inorganic Chemicals Industry, 2023, 55(10): 100-105.
|
| [11] |
HE Lei,ZHU Ganyu,ZHENG Guangming,WU Wenfen,ZhANG Jianbo,LI Fang,LI Huiquan,CHEN Wen.
Study on crystallization process and mechanism of phosphogypsum in wet process phosphoric acid system
[J]. Inorganic Chemicals Industry, 2022, 54(7): 110-116.
|
| [12] |
FU Ziqi, ZHANG Cheng, SHENG Yong, JI Lijun.
Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents
[J]. Inorganic Chemicals Industry, 2022, 54(7): 129-134.
|
| [13] |
HE Ting,KONG Jiao,CUI Jingzhi,CHEN Zhihao,FU Tongtong,GUO Zirui,GU Shuai,YU Jianguo.
Study on leaching and thermodynamic of spent lithium-ion batteries with electrochemical reduction
[J]. Inorganic Chemicals Industry, 2022, 54(12): 34-43.
|
| [14] |
ZHENG Hanxiao,LÜ Li,TANG Shengwei,HE Yanjun,ZHANG Tao.
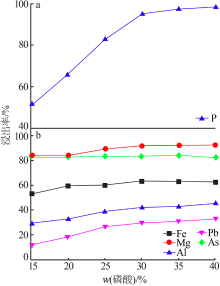
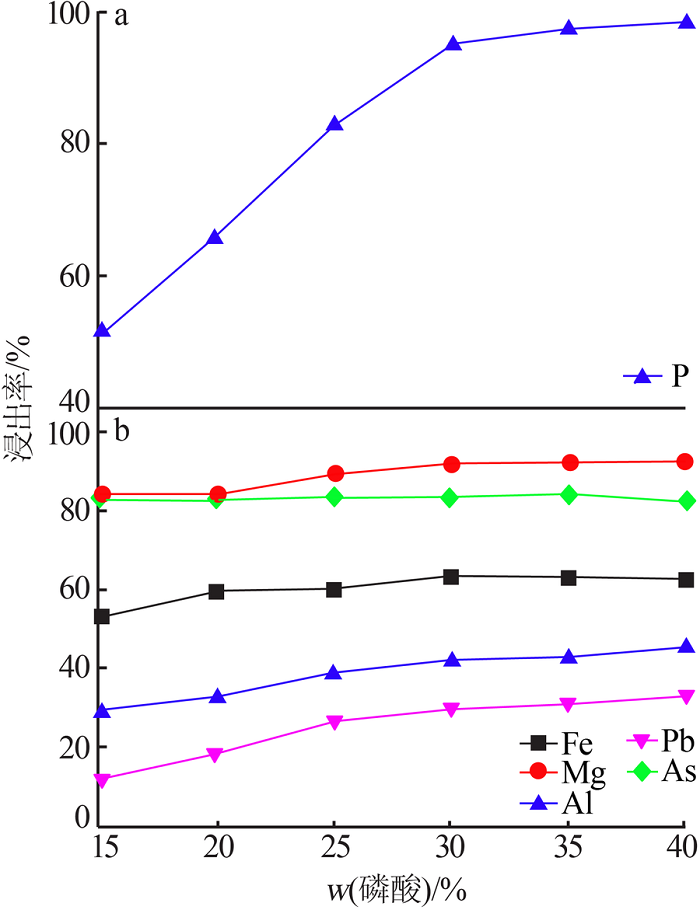
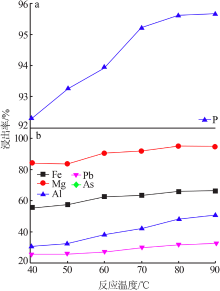
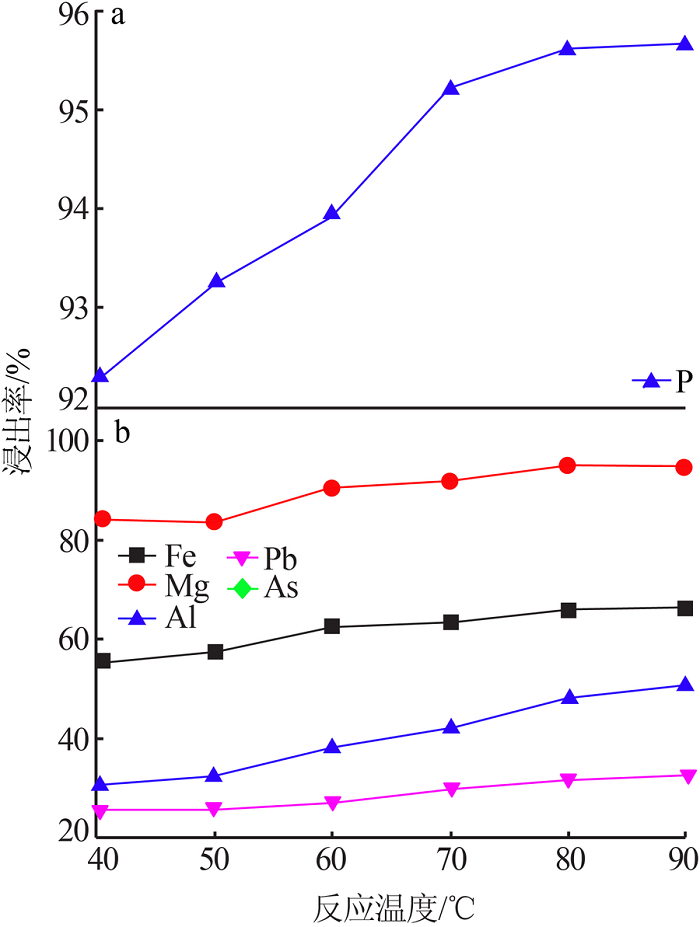
Leaching behavior of phosphate ore in phosphoric acid
[J]. Inorganic Chemicals Industry, 2022, 54(10): 96-101.
|
| [15] |
XIAO Yong,YANG Xiushan,XU Dehua,WANG Xinlong,ZHANG Zhiye.
Study on treatment of medium and low grade high magnesium collophanite by nitric acid method
[J]. Inorganic Chemicals Industry, 2022, 54(1): 71-76.
|
 ),XIE Tian2,4(
),XIE Tian2,4( ),YANG Lin1,HE Bingbing3,4
),YANG Lin1,HE Bingbing3,4