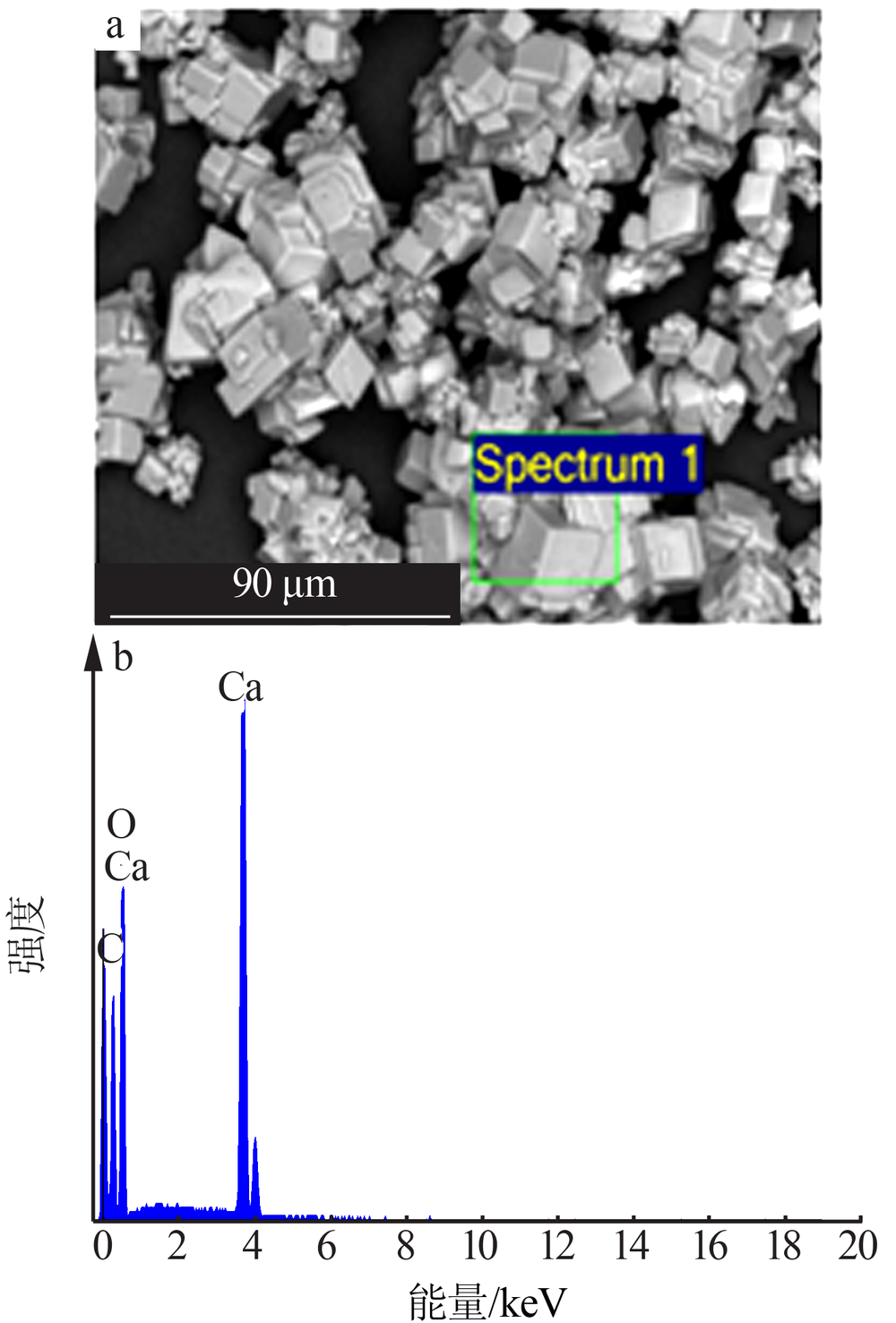
| [1] |
许博超, 于志刚, 刘东升 , 等. 莱州湾南岸地下卤水高浓度溶解铀及其成因研究[J]. 中国海洋大学学报:自然科学版, 2011,41(11):81-85.
|
| [2] |
韩立民, 李大海, 于会娟 . 加快推进山东半岛蓝色经济区建设的对策研究[J]. 山东经济, 2011,27(1):115-119.
|
| [3] |
Ji Y L, Qian W J, Yu Y W , et al. Recent developments in nanofiltration membranes based on nanomaterials[J]. Chinese Journal of Chemical Engineering, 2017,25(11):1639-1652.
|
| [4] |
高从堦, 陈益棠 . 纳滤膜及其应用[J]. 中国有色金属学报, 2004(S1):310-316.
|
| [5] |
周金盛, 陈观文 . 纳滤膜技术的研究进展[J]. 膜科学与技术, 1999(4):1-11.
|
| [6] |
刘建路, 迟庆峰, 张浩波 , 等. 一种利用提溴卤水生产精制盐水的方法和装置:中国, 104743582A[P]. 2015-07-01.
|
| [7] |
刘俊强, 路建新, 刘琪 , 等. 纳滤卤水精制技术在纯碱生产中的应用[J]. 纯碱工业, 2015(4):15-17.
|
| [8] |
李昆, 王健行, 魏源送 . 纳滤在水处理与回用中的应用现状与展望[J]. 环境科学学报, 2016,36(8):2714-2729.
|
| [9] |
刘昌胜, 邬行彦, 潘德维 , 等. 膜的污染及其清洗[J]. 膜科学与技术, 1996(2):26-31.
|
| [10] |
Ahmed Saleh Al-Amoudi . Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes:A review[J]. Desalination, 2010,259(1):1-10.
|
| [11] |
Her N, Amy G, Jarusutthirak C . Seasonal variations of nanofiltration (NF) foulants:Identification and control[J]. Desalination, 2000,132(1):143-160.
doi: 10.1016/S0011-9164(00)00143-0
|
| [12] |
王红庆, 包宗宏, 段东平 , 等. 硫酸钙在卤水输送管道中的结垢抑制[J]. 中国有色金属学报, 2008(z1):92-95.
|
| [13] |
颜亚盟, 张仂 . 硫酸钙在盐水中的溶解度及溶度积实验研究[J]. 盐业与化工, 2014,43(11):27-30.
|
| [14] |
姜欣欣 . 碳酸钙结晶动力学研究[J]. 西安石油大学学报:自然科学版, 2015,30(6):89-92,11.
|
| [15] |
邢晓凯, 马重芳, 陈永昌 . 溶液pH值对碳酸钙结垢的影响[J]. 石油化工设备, 2004(5):11-14.
|
| [16] |
于剑峰, 霍静, 唐仕明 , 等. 卤水管道中影响碳酸钙垢溶解度的因素及规律[J]. 盐业与化工, 2011,40(4):20-23.
|
| [17] |
牛俊坡 . 石膏型卤水加热管结垢的原因分析及预防[J]. 盐业与化工, 2014,43(6):53-54.
|