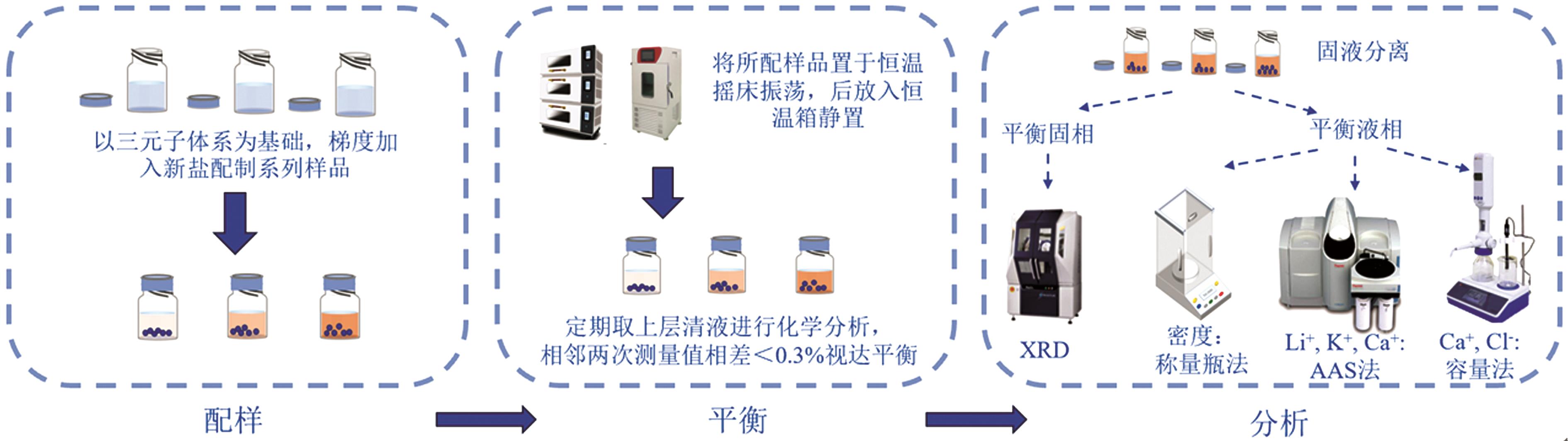
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (3): 30-35.doi: 10.19964/j.issn.1006-4990.2024-0304
• Research & Development • Previous Articles Next Articles
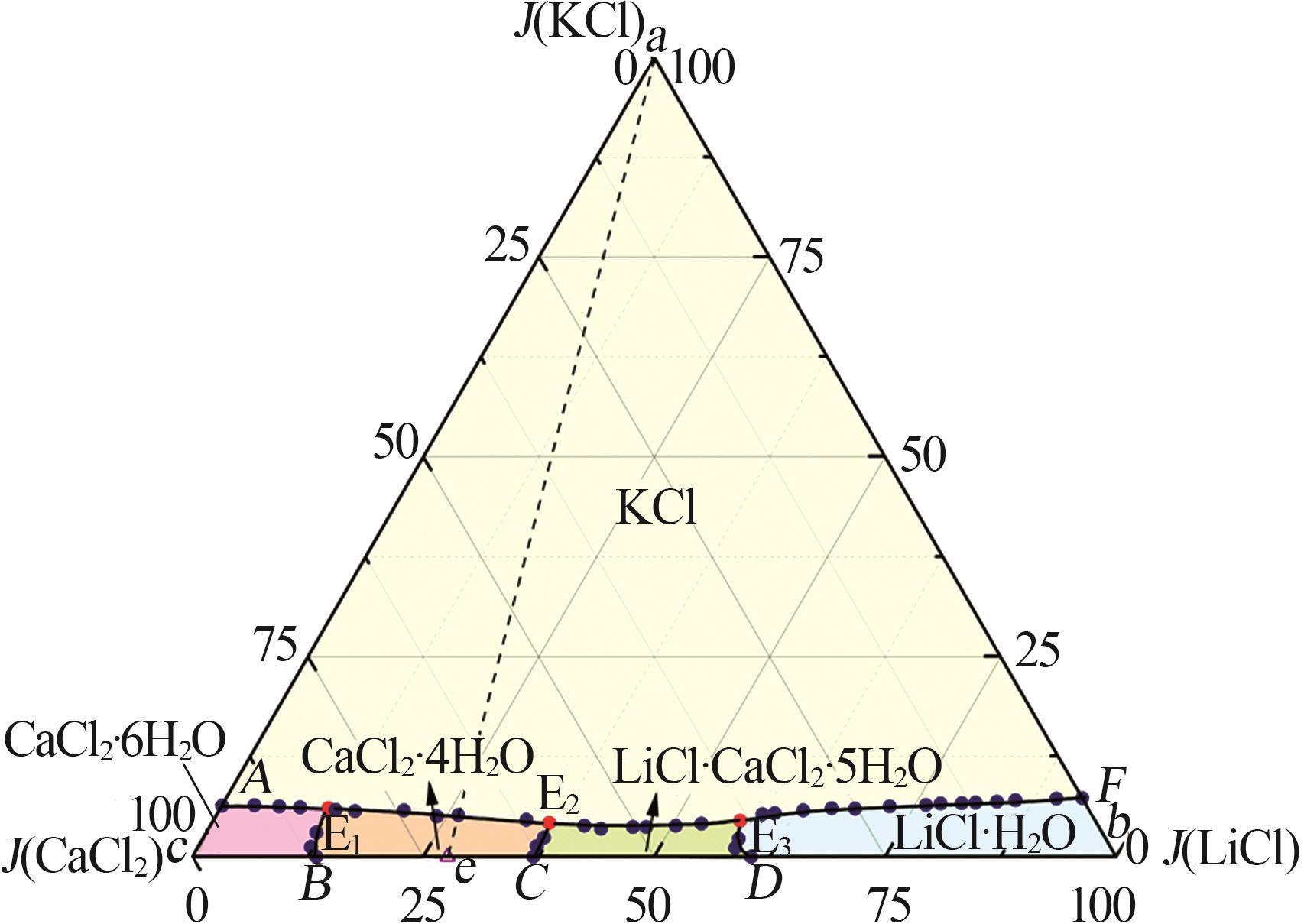
Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K
YU Xudong1,3( ), LI Jing1, REN Siying1,2, LUO Jun1,2, ZENG Ying1,3
), LI Jing1, REN Siying1,2, LUO Jun1,2, ZENG Ying1,3
- 1. College of Materials and Chemistry & Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
2. Qinghai Salt Lake Industrial Co. ,Ltd. ,Golmud 816000,China
3. Tianfu Yongxing Laboratory,Chengdu 610213,China
-
Received:2024-05-29Online:2025-03-10Published:2024-07-11
CLC Number:
Cite this article
YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K[J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35.
share this article
Table 1
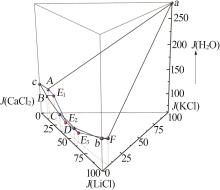
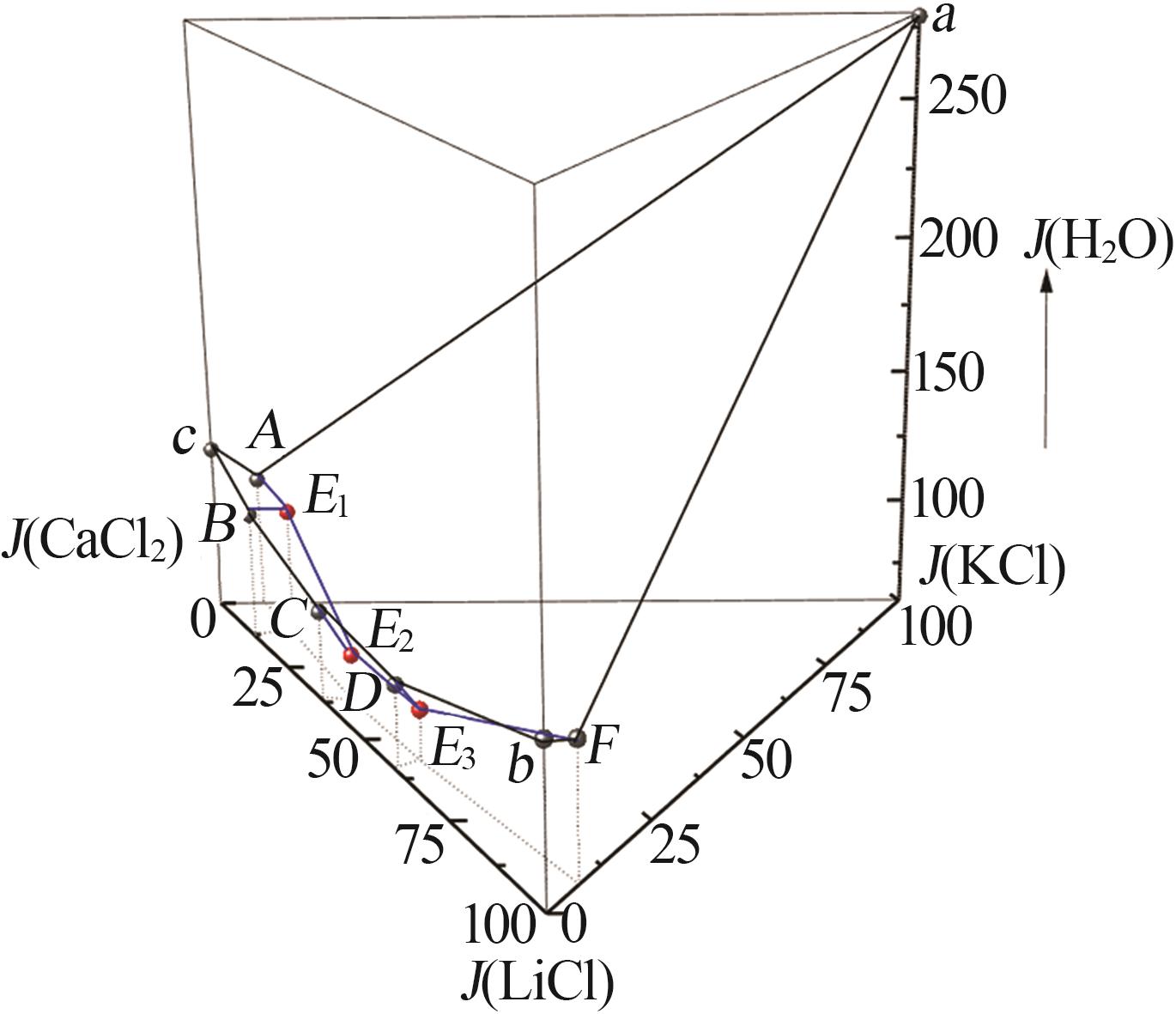
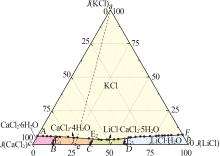
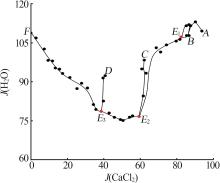
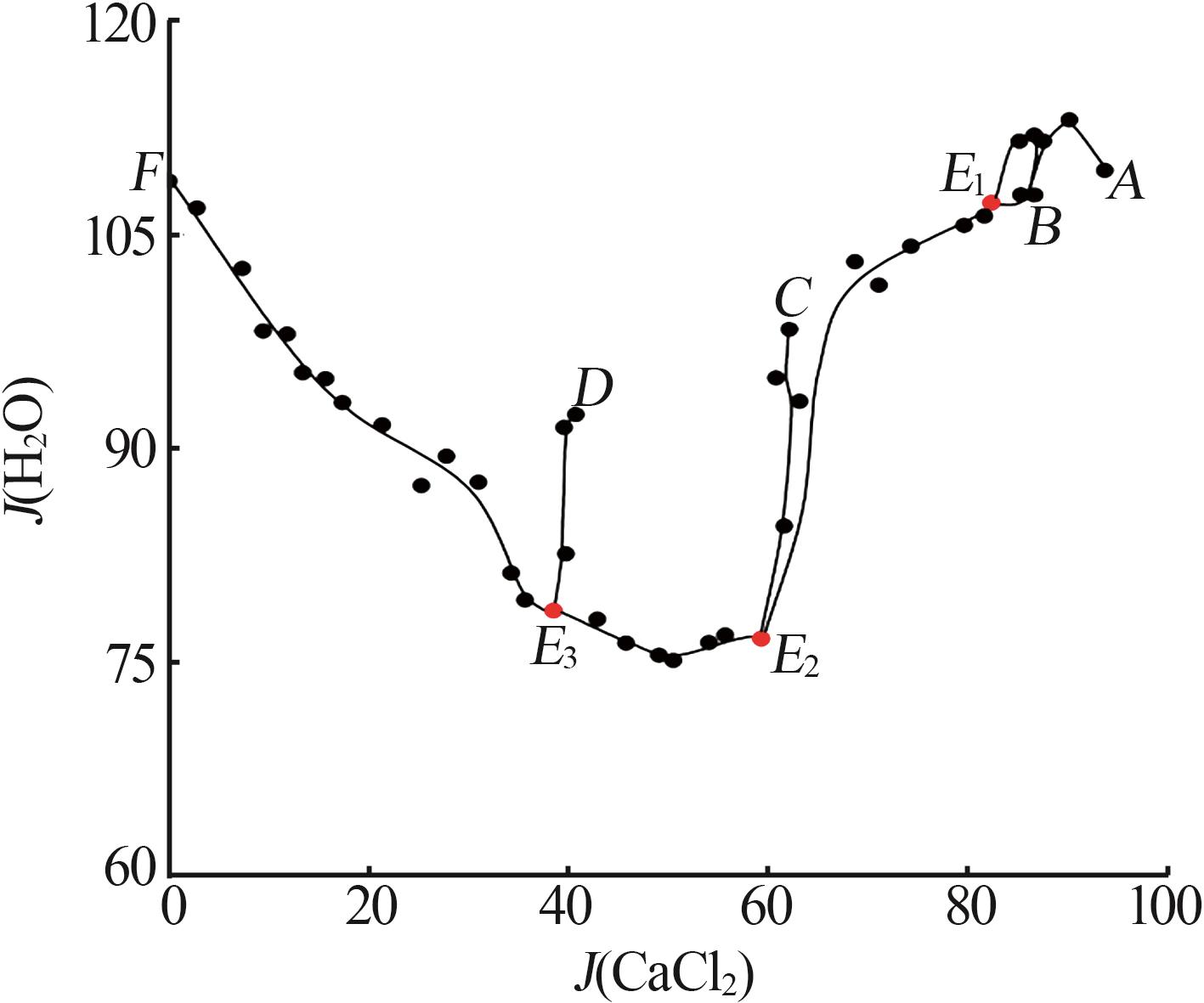
Solid-liquid phase equilibria data of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K a"
| 编号 | 密度/ (g·cm-3) | 液相组成 | 耶涅克指数 | 平衡固相 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| J(LiCl)+J(KCl)+J(CaCl2)=100 | |||||||||||
w(LiCl)× 102 | w(KCl) × 102 | w(CaCl2)× 102 | w(H2O)× 102 | J(LiCl) | J(KCl) | J(CaCl2) | J(H2O) | ||||
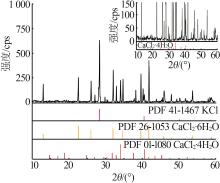
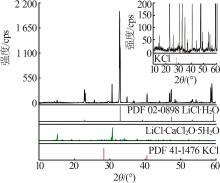
| a | — | 0.00 | 26.40 | 0.00 | 73.60 | 0.00 | 100.00 | 0.00 | 278.79 | KCl[ | |
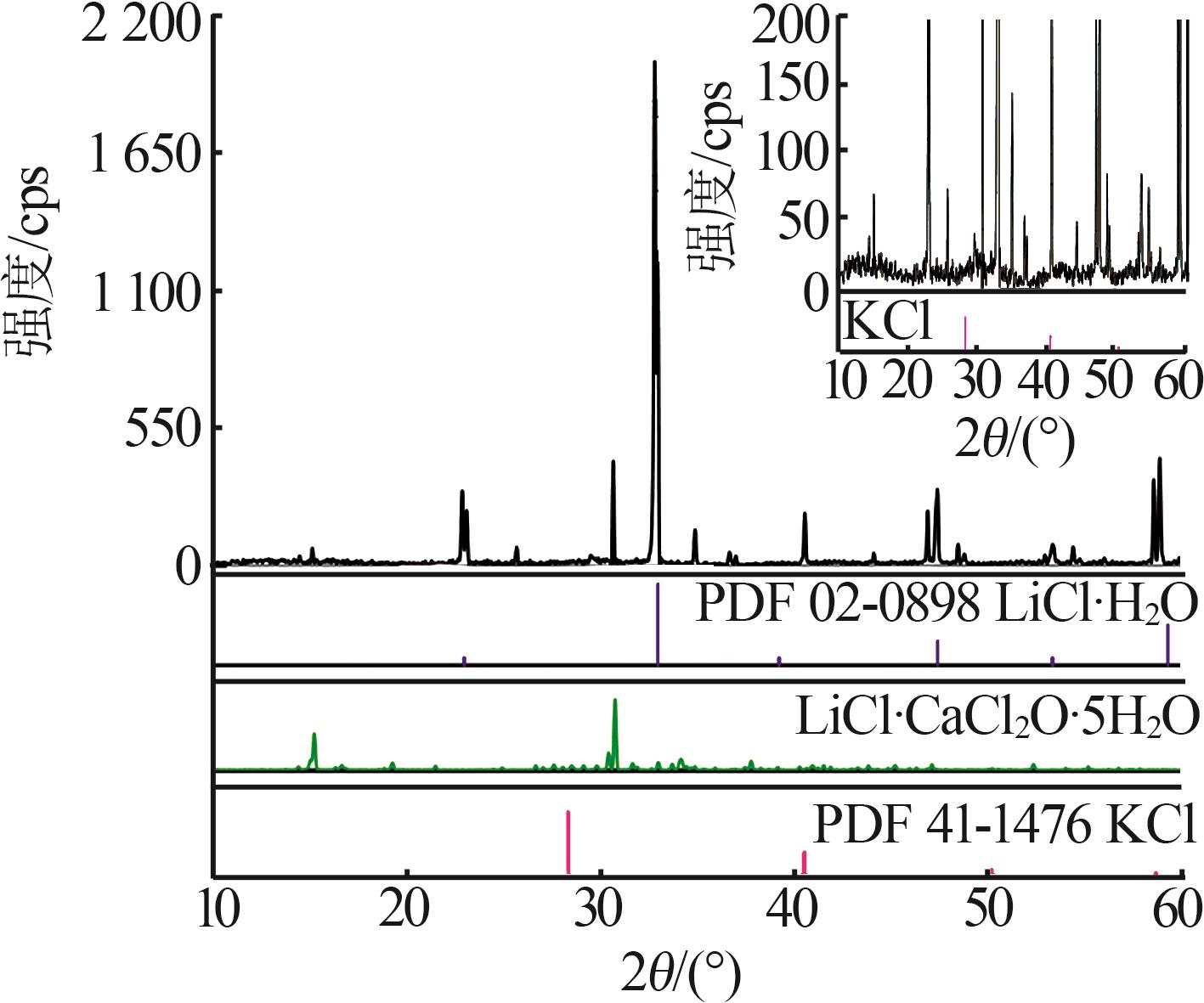
| b | — | 45.86 | 0.00 | 0.00 | 54.14 | 100.00 | 0.00 | 0.00 | 118.05 | LiCl·H2O[ | |
| c | — | 0.00 | 0.00 | 44.83 | 55.17 | 0.00 | 0.00 | 100.00 | 123.06 | CaCl2·6H2O[ | |
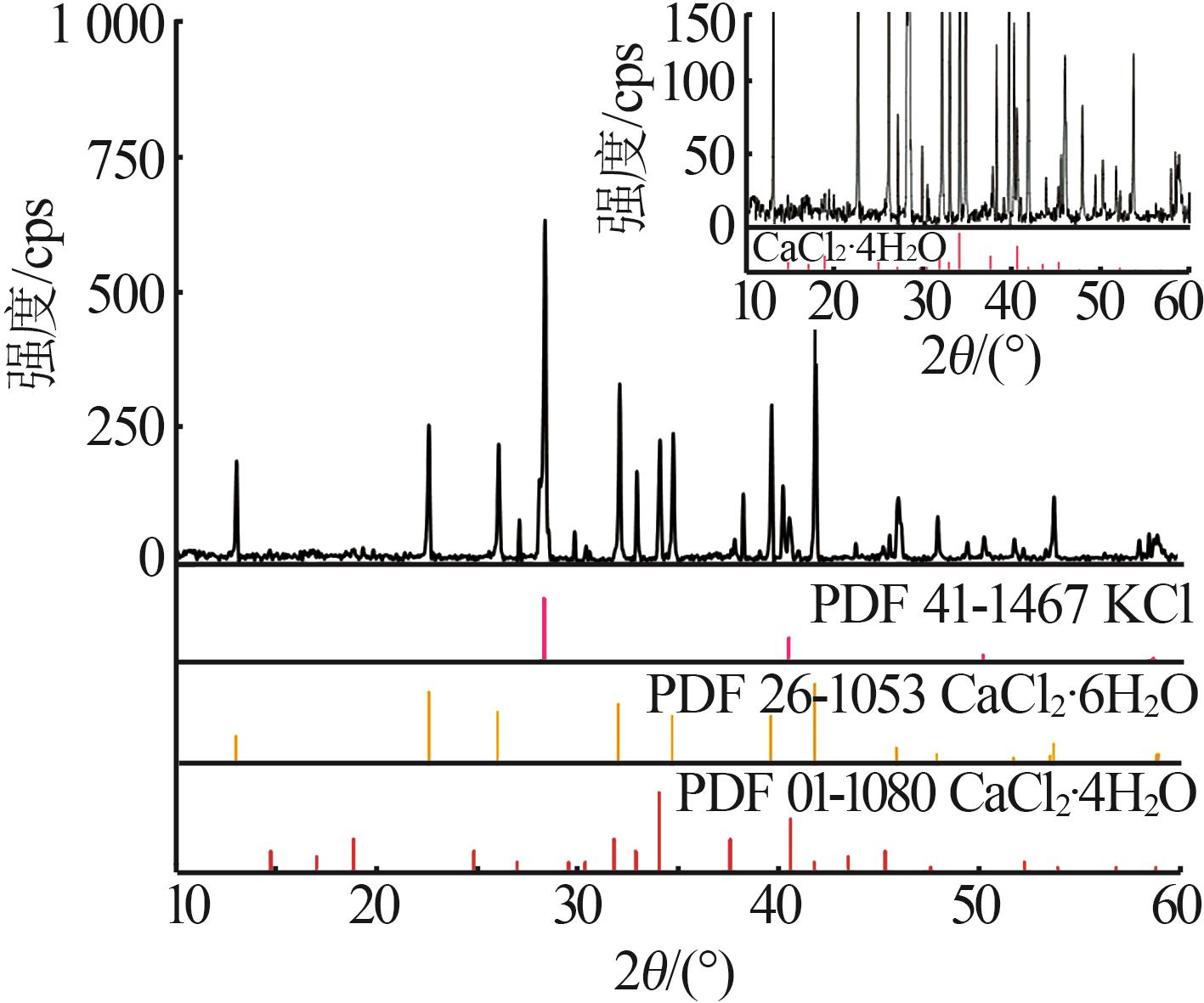
| 1,A | 1.466 4 | 0.00 | 3.06 | 44.68 | 52.26 | 0.00 | 6.41 | 93.59 | 109.47 | KCl +CaCl2·6H2O[ | |
| 2 | 1.436 2 | 1.64 | 3.03 | 42.28 | 53.05 | 3.49 | 6.45 | 90.06 | 112.99 | KCl +CaCl2·6H2O | |
| 3 | 1.435 0 | 2.95 | 2.98 | 41.35 | 52.72 | 6.24 | 6.30 | 87.46 | 111.51 | KCl +CaCl2·6H2O | |
| 4 | 1.441 9 | 4.13 | 2.98 | 41.02 | 51.87 | 8.58 | 6.19 | 85.23 | 107.77 | KCl +CaCl2·6H2O | |
| 5,E1 | 1.450 0 | 5.64 | 2.93 | 39.69 | 51.74 | 11.69 | 6.07 | 82.24 | 107.21 | KCl+CaCl2·6H2O+CaCl2·4H2O | |
| 6 | 1.435 0 | 6.10 | 2.84 | 39.54 | 51.52 | 12.58 | 5.86 | 81.56 | 106.27 | KCl+CaCl2·4H2O | |
| 7 | 1.431 2 | 7.18 | 2.78 | 38.67 | 51.37 | 14.76 | 5.72 | 79.52 | 105.63 | KCl+CaCl2·4H2O | |
| 8 | 1.425 3 | 9.80 | 2.84 | 36.34 | 51.02 | 20.01 | 5.80 | 74.19 | 104.16 | KCl+CaCl2·4H2O | |
| 9 | 1.423 1 | 11.88 | 2.51 | 35.25 | 50.36 | 23.93 | 5.06 | 71.01 | 101.45 | KCl+CaCl2·4H2O | |
| 10 | 1.436 0 | 12.89 | 2.57 | 33.78 | 50.76 | 26.18 | 5.22 | 68.60 | 103.09 | KCl+CaCl2·4H2O | |
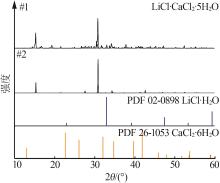
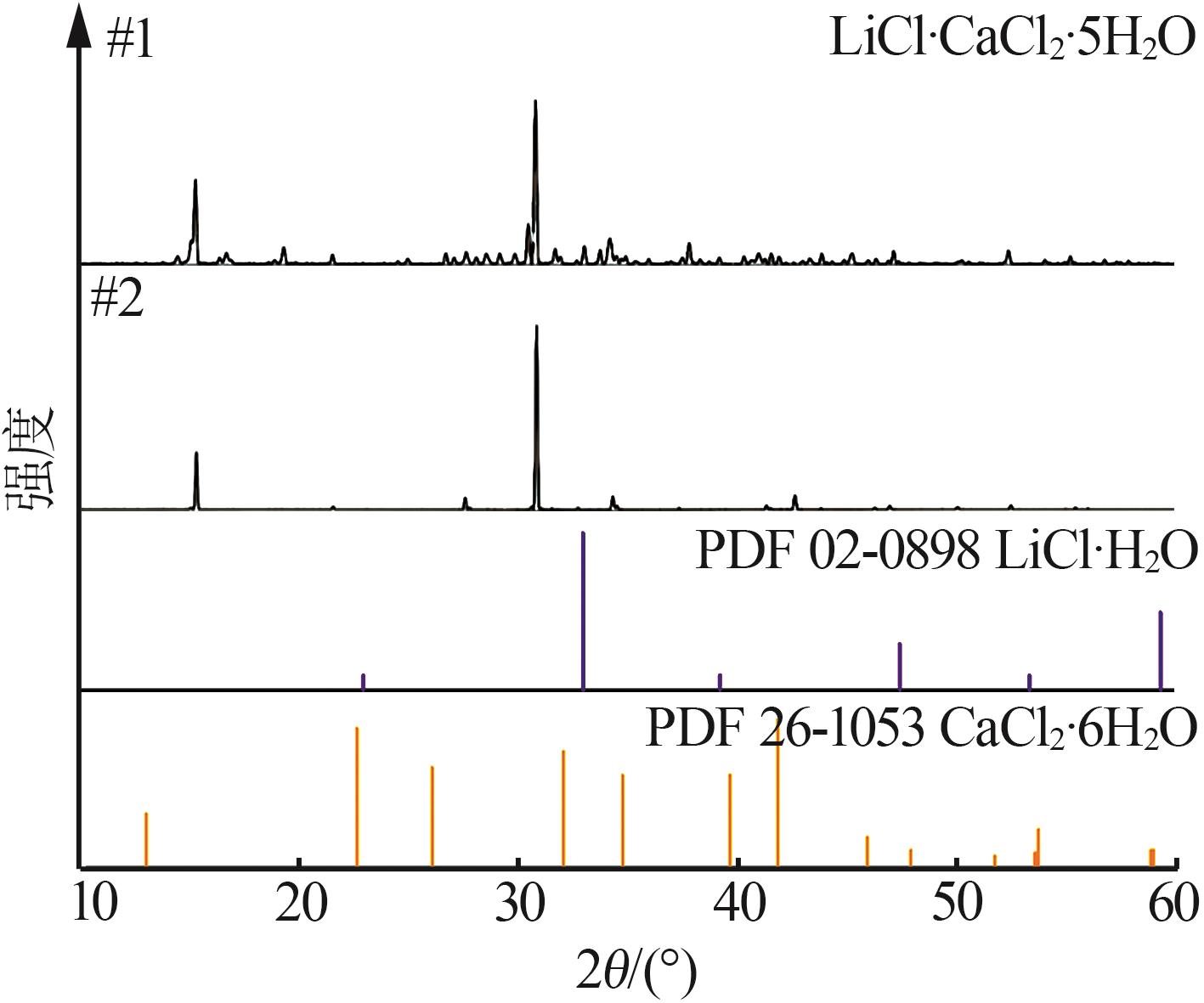
| 11,E2 | 1.469 6 | 20.66 | 2.40 | 33.53 | 43.41 | 36.51 | 4.24 | 59.25 | 76.71 | KCl+LCC+CaCl2·4H2O | |
| 12 | 1.469 1 | 22.88 | 2.18 | 31.44 | 43.50 | 40.50 | 3.86 | 55.64 | 76.99 | KCl+LCCb | |
| 13 | 1.468 6 | 24.08 | 1.98 | 30.61 | 43.33 | 42.49 | 3.49 | 54.02 | 76.46 | KCl+LCC | |
| 14 | 1.456 1 | 26.16 | 2.13 | 28.79 | 42.92 | 45.83 | 3.73 | 50.44 | 75.19 | KCl+LCC | |
| 15 | 1.449 1 | 26.92 | 2.13 | 27.91 | 43.04 | 47.26 | 3.74 | 49.00 | 75.56 | KCl+LCC | |
| 16 | 1.440 1 | 28.56 | 2.20 | 25.92 | 43.32 | 50.39 | 3.88 | 45.73 | 76.43 | KCl+LCC | |
| 17 | 1.435 5 | 29.77 | 2.33 | 24.05 | 43.85 | 53.02 | 4.15 | 42.83 | 78.09 | KCl+LCC | |
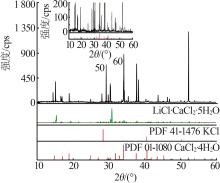
| 18,E3 | 1.426 0 | 31.93 | 2.51 | 21.52 | 44.04 | 57.05 | 4.49 | 38.46 | 78.70 | KCl+LCC+LiCl·H2O | |
| 19 | 1.305 9 | 43.47 | 3.51 | 1.36 | 51.66 | 89.93 | 7.26 | 2.81 | 106.87 | KCl+LiCl·H2O | |
| 20 | 1.319 1 | 42.23 | 3.50 | 3.62 | 50.65 | 85.57 | 7.09 | 7.34 | 102.63 | KCl+LiCl·H2O | |
| 21 | 1.323 2 | 42.20 | 3.49 | 4.75 | 49.56 | 83.66 | 6.92 | 9.42 | 98.26 | KCl+LiCl·H2O | |
| 22 | 1.338 0 | 41.11 | 3.43 | 5.96 | 49.50 | 81.41 | 6.79 | 11.80 | 98.02 | KCl+LiCl·H2O | |
| 23 | 1.342 1 | 40.93 | 3.42 | 6.85 | 48.80 | 79.94 | 6.68 | 13.38 | 95.31 | KCl+LiCl·H2O | |
| 24 | 1.305 9 | 43.47 | 3.51 | 1.36 | 51.66 | 89.93 | 7.26 | 2.81 | 106.87 | KCl+LiCl·H2O | |
| 25 | 1.351 3 | 39.86 | 3.41 | 8.04 | 48.69 | 77.68 | 6.65 | 15.67 | 94.89 | KCl+LiCl·H2O | |
| 26 | 1.356 1 | 39.42 | 3.35 | 8.98 | 48.25 | 76.17 | 6.47 | 17.36 | 93.24 | KCl+LiCl·H2O | |
| 27 | 1.363 0 | 37.73 | 3.30 | 11.14 | 47.83 | 72.32 | 6.33 | 21.35 | 91.68 | KCl+LiCl·H2O | |
| 28 | 1.373 3 | 36.66 | 3.21 | 13.48 | 46.65 | 68.71 | 6.02 | 25.27 | 87.44 | KCl+LiCl·H2O | |
| 29 | 1.388 5 | 34.93 | 3.20 | 14.64 | 47.23 | 66.19 | 6.06 | 27.75 | 89.50 | KCl+LiCl·H2O | |
| 30 | 1.407 8 | 33.70 | 3.09 | 16.50 | 46.71 | 63.24 | 5.80 | 30.96 | 87.65 | KCl+LiCl·H2O | |
| 31 | 1.409 3 | 33.27 | 3.00 | 18.88 | 44.85 | 60.33 | 5.44 | 34.23 | 81.32 | KCl+LiCl·H2O | |
| 32,F | 1.273 4 | 44.39 | 3.52 | 0.00 | 52.09 | 92.65 | 7.35 | 0.00 | 108.72 | KCl+LiCl·H2O[ | |
| 33,B | 1.439 3 | 6.46 | 0.00 | 41.68 | 51.86 | 13.42 | 0.00 | 86.58 | 107.73 | CaCl2·6H2O+CaCl2·4H2O[ | |
| 34 | 1.435 4 | 5.78 | 0.57 | 40.83 | 52.82 | 12.25 | 1.21 | 86.54 | 111.95 | CaCl2·6H2O+CaCl2·4H2O | |
| 35 | 1.449 8 | 5.64 | 1.42 | 40.22 | 52.72 | 11.93 | 3.00 | 85.07 | 111.51 | CaCl2·6H2O+CaCl2·4H2O | |
| 36,C | 1.404 3 | 19.09 | 0.00 | 32.63 | 48.28 | 36.91 | 0.00 | 63.09 | 93.35 | CaCl2·4H2O+LCC[ | |
| 37 | 1.440 8 | 18.94 | 1.23 | 31.11 | 48.72 | 36.93 | 2.40 | 60.67 | 95.01 | CaCl2·4H2O+LCC | |
| 38 | 1.456 2 | 18.46 | 0.67 | 31.28 | 49.59 | 36.62 | 1.33 | 62.05 | 98.37 | CaCl2·4H2O+LCC | |
| 39 | 1.459 5 | 18.34 | 2.50 | 33.33 | 45.83 | 33.86 | 4.62 | 61.52 | 84.60 | CaCl2·4H2O+LCC | |
| 40,D | 1.373 5 | 31.59 | 0.00 | 20.64 | 47.77 | 60.48 | 0.00 | 39.52 | 91.46 | LiCl·H2O+LCC[ | |
| 41 | 1.395 5 | 30.25 | 0.57 | 21.15 | 48.03 | 58.20 | 1.10 | 40.70 | 92.42 | LiCl·H2O+LCC | |
| 42 | 1.410 5 | 31.77 | 1.23 | 21.75 | 45.25 | 58.02 | 2.25 | 39.73 | 82.65 | LiCl·H2O+LCC | |
| 1 | 郑明贵,于明,范秋蓉,等.中国2025—2035年碳酸锂需求预测:基于灰色关联分析和ARIMA-GM-BP神经网络的组合模型[J].地球科学进展,2023,38(4):377-387. |
| ZHENG Minggui, YU Ming, FAN Qiurong,et al.China′s lithium carbonate demand forecast 2025-2035:A combined model based on grey correlation analysis and the ARIMA-GM-BP neural network[J].Advances in Earth Science,2023,38(4):377-387. | |
| 2 | 郑明贵,刘丽珍,陶思敏,等.中国碳酸锂经济安全预警研究[J].盐湖研究,2024,32(2):117-126. |
| ZHENG Minggui, LIU Lizhen, TAO Simin,et al.Economic security warning of lithium carbonate in China[J].Journal of Salt Lake Research,2024,32(2):117-126. | |
| 3 | 杨游胜,姚智豪,赵志星,等.富锂硫酸盐型盐湖卤水蒸发实验研究进展[J].无机盐工业,2024,56(4):1-7. |
| YANG Yousheng, YAO Zhihao, ZHAO Zhixing,et al.Research progress of lithium-rich sulfate type salt lake brine evaporation experiment[J].Inorganic Chemicals Industry,2024,56(4):1-7. | |
| 4 | 韩佳欢,郑绵平,乜贞,等.我国深层地下卤水钾、锂资源及其开发前景[J].盐湖研究,2024,32(2):90-100. |
| HAN Jiahuan, ZHENG Mianping, NIE Zhen,et al.Lithium and potassium resources of oilfield brine and development prospects in China[J].Journal of Salt Lake Research,2024,32(2):90-100. | |
| 5 | 成怀刚,程芳琴.水盐体系相分离[M].北京:冶金工业出版社,2022. |
| 6 | 杨森楠,张子义,李栋婵.三元体系LiCl-KCl-H2O和LiCl-SrCl2-H2O在288.15 K时稳定相平衡研究[J].化学工程,2019,47(12):54-58. |
| YANG Sennan, ZHANG Ziyi, LI Dongchan.Phase equilibria in ternary systems LiCl-KCl-H2O and LiCl-SrCl2-H2O at 288.15 K[J].Chemical Engineering (China),2019,47(12):54-58. | |
| 7 | FARELO F, FERNANDES C, AVELINO A.Solubilities for six ternary systems:NaCl+NH4Cl+H2O,KCl+NH4Cl+H2O,NaCl+LiCl+H2O,KCl+LiCl+H2O,NaCl+AlCl3+H2O,and KCl+AlCl3+H2O at T=(298 to 333) K[J].Journal of Chemical & Engineering Data,2005,50(4):1470-1477. |
| 8 | WANG Xia, ZHAO Kaiyu, GUO Yafei,et al.Experimental determination and thermodynamic model of solid-liquid equilibria in the ternary system(LiCl+SrCl2+H2O) at 273.15 K and its application in industry[J].Journal of Solution Chemistry,2019,48(4):528-545. |
| 9 | LI Long, YUAN Fei, GUO Yafei,et al.Experimental and predictive equilibrium thermodynamics of the aqueous ternary system(LiCl+CaCl2+H2O) at T=288.15 K[J].Journal of Chemical & Engineering Data,2020,65(9):4369-4377. |
| 10 | CHRISTOV C, VELIKOVA S, IVANOVA K.Study of (m1LiX+m2CaX2)(aq) where mi denotes molality and X denotes Cl,or Br at the temperature 298.15 K[J].The Journal of Chemical Thermodynamics,2000,32(11):1505-1512. |
| 11 | ZENG Dewen, XU Wenfang, VOIGT W,et al.Thermodynamic study of the system(LiCl+CaCl2+H2O)[J].The Journal of Chemical Thermodynamics,2008,40(7):1157-1165. |
| 12 | 孟浩,罗军,任思颖,等.三元体系LiCl+CaCl2+H2O 348.2 K相平衡研究[J].矿产保护与利用,2023,43(6):114-119. |
| MENG Hao, LUO Jun, REN Siying,et al.Phase equilibria of aqueous ternary system LiCl+CaCl2+H2O at 348.2 K[J].Conservation and Utilization of Mineral Resources,2023,43(6):114-119. | |
| 13 | 乌志明,邓小川.盐析作用及其在盐卤分离中应用探讨[J].海湖盐与化工,2000,29(5):11-14. |
| WU Zhiming, DENG Xiaochuan.Discussion on salting-out and its application in brine separation[J].Sea-Lake Salt and Chemical Industry,2000,29(5):11-14. | |
| 14 | LI Dongdong, ZENG Dewen, YIN Xia,et al.Phase diagrams and thermochemical modeling of salt lake brine systems.Ⅱ.NaCl+H2O,KCl+H2O,MgCl2+H2O and CaCl2+H2O systems[J].Calp-had,2016,53:78-89. |
| 15 | 于旭东,李琪,陈念粗,等.三元体系KCl+CaCl2+H2O 298.2、323.2及348.2 K相平衡研究及计算[J].化工学报,2023,74(8):3256-3265. |
| YU Xudong, LI Qi, CHEN Niancu,et al.Phase equilibria and calculation of aqueous ternary system KCl+CaCl2+H2O at 298.2,323.2,and 348.2 K[J].CIESC Journal,2023,74(8):3256-3265. | |
| 16 | 国家市场监督管理总局,国家标准化管理委员会.铝及铝合金化学分析方法 第9部分:锂含量的测定 火焰原子吸收光谱法:GB/T 20975.9—2020[S].北京:中国标准出版社,2020. |
| 17 | 国家市场监督管理总局,国家标准化管理委员会.铝及铝合金化学分析方法 第33部分:钾含量的测定 火焰原子吸收光谱法:GB/T 20975.33—2020[S].北京:中国标准出版社,2020. |
| 18 | 国家市场监督管理总局,国家标准化管理委员会.铝及铝合金化学分析方法 第21部分:钙含量的测定:GB/T 20975.21—2020[S].北京:中国标准出版社,2020. |
| 19 | 李红霞,姚燕,曾德文.水盐体系中有干扰Li+离子存在下测定Mg2+浓度的掩蔽法研究[J].盐湖研究,2012,20(2):24-30. |
| LI Hongxia, YAO Yan, ZENG Dewen.Measurement of concentrations of magnesium ion by masking method in presence of the interference lithium ion in salt water system[J].Journal of Salt Lake Research,2012,20(2):24-30. | |
| 20 | 中国科学院青海盐湖研究所分析室.卤水和盐的分析方法[M].2版.北京:科学出版社,1988. |
| 21 | 中国合格评定国家认可委员会.化学分析测量不确定度的评估指南[M].北京:中国计量出版社,2019. |
| 22 | ELLISON S L, WILLIAMS A.EURACHEM/CITAC Guide quantifying uncertainty in analytical measurement[M].3nd ed.London:A Focus for Analytical Chemistry in Europe,2011. |
| 23 | 任思颖,于旭东,罗军,等.298.2 K四元体系Li+,K+,NH4 +//Cl--H2O相平衡研究[J].化工学报,2022,73(10):4335-4344. |
| REN Siying, YU Xudong, LUO Jun,et al.Phase equilibria of aqueous quaternary system Li+,K+,NH4 +//Cl--H2O at 298.2 K[J].CIESC Journal,2022,73(10):4335-4344. | |
| 24 | 崔瑞芝,李武,董亚萍,等.298 K四元体系LiCl-MgCl2-CaCl2-H2O相平衡实验及溶解度计算[J].化工学报,2018,69(10):4148-4155. |
| CUI Ruizhi, LI Wu, DONG Yaping,et al.Measurements and calculations of solid-liquid equilibria in quaternary system LiCl-MgCl2-CaCl2-H2O at 298 K[J].CIESC Journal,2018,69(10):4148-4155. | |
| 25 | 阳海棠,刘鹏程,曾德文,等.锂光卤石X-ray衍射标准图谱[J].盐湖研究,2016,24(4):32-36. |
| YANG Haitang, LIU Pengcheng, ZENG Dewen,et al.The identification map of lithium carnallite by X-ray diffraction[J].Journal of Salt Lake Research,2016,24(4):32-36. | |
| 26 | LUO Jun, REN Siying, ZHENG Qiufeng,et al.Solid-liquid phase equilibria determination of quaternary system NH4 +,Mg2+,Ca2+//Cl–-H2O at T=298.2 and 323.2 K and P=94.77 kPa[J].Journal of Chemical & Engineering Data,2023,68(3):769-779. |
| [1] | SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system [J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46. |
| [2] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [3] | YANG Yousheng, YAO Zhihao, ZHAO Zhixing, FENG Xia, ZENG Ying, YU Xudong. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment [J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. |
| [4] | FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K [J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. |
| [5] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [6] | LI Yan,WANG Yansong,CHENG Huaigang,KANG Jin,LI Enze,LÜ Hongzhou,LIU Qian. Rapid quality inspection of high-purity lithium carbonate based on determination of magnesium by water-soluble probe method [J]. Inorganic Chemicals Industry, 2023, 55(1): 87-92. |
| [7] | CHEN Shuai,YANG Bo,CHEN Niancu,LUO Jun,REN Siying,ZENG Ying,YU Xudong. Study on phase equilibria of ternary system NH4Cl+MgCl2+H2O at 323.2 K [J]. Inorganic Chemicals Industry, 2022, 54(4): 100-103. |
| [8] | WU Liping,YUAN Hongzhan,JIN Fang. Study on natural evaporation experimental of deep brine in Yahu structure [J]. Inorganic Chemicals Industry, 2022, 54(11): 71-78. |
| [9] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [10] | ZHANG Huanhuan,CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen. Determination and correlation of solubility of potassium dihydrogen phosphate in acetonitrile-water solvent [J]. Inorganic Chemicals Industry, 2022, 54(1): 83-85. |
| [11] | Wang Bin,Deng Xiaochuan,Shi Yifei,Dong Chaochao,Fan Faying,Zhu Chaoliang,Fan Jie. Online determination of the solubility of lithium carbonate in water and NaCl-KCl solution system [J]. Inorganic Chemicals Industry, 2021, 53(7): 73-79. |
| [12] | CHEN Yunfei,MAO Lili,LIU Dan,WANG Haizeng. Measurement and correlation of solubility of magnesium chloride hexahydrate in six polyols [J]. Inorganic Chemicals Industry, 2021, 53(12): 85-90. |
| [13] | JIN Fang,LI Hongpu,CHANG Donghai. Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin [J]. Inorganic Chemicals Industry, 2021, 53(11): 86-90. |
| [14] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [15] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||