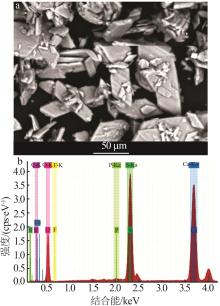
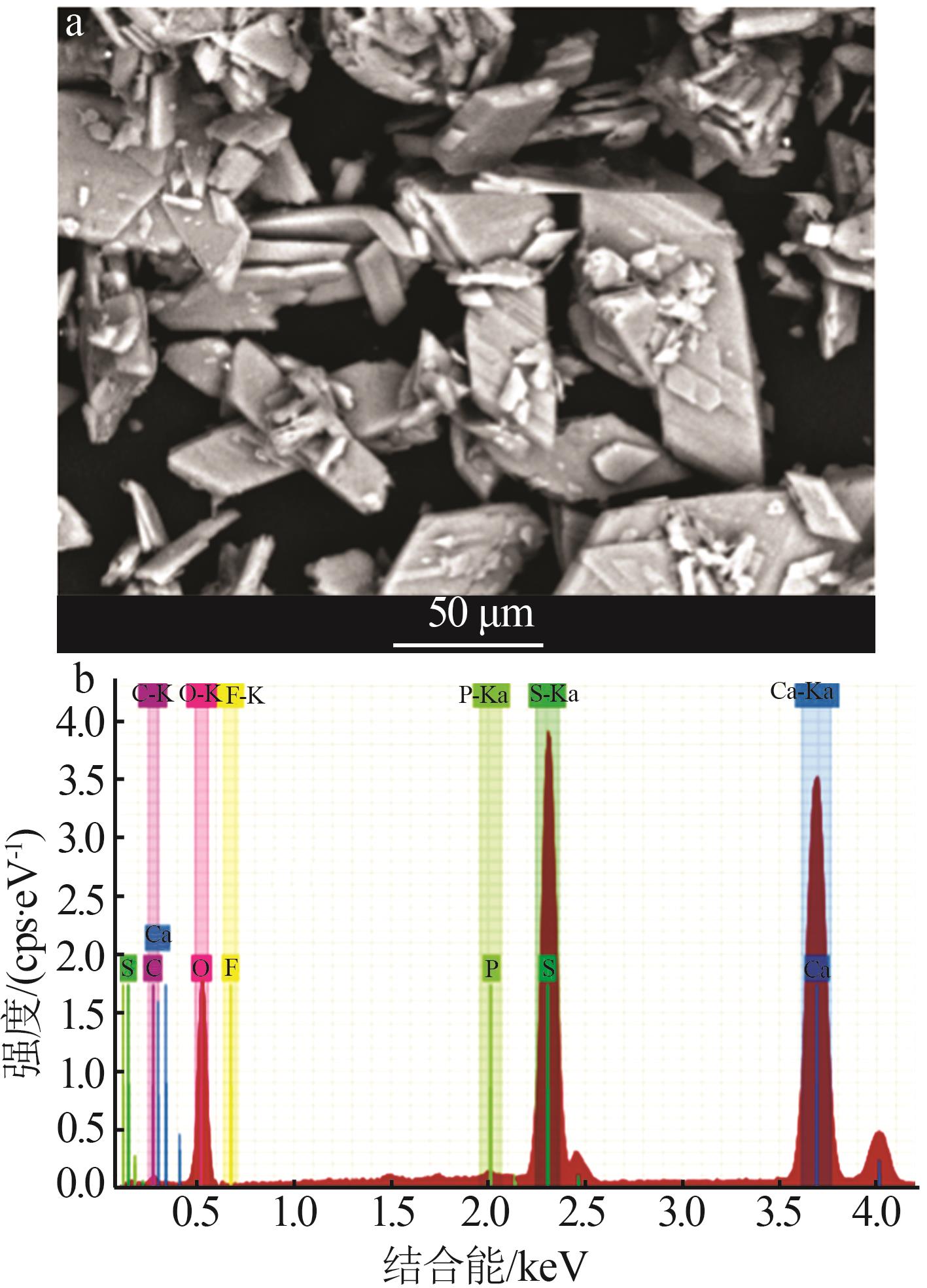
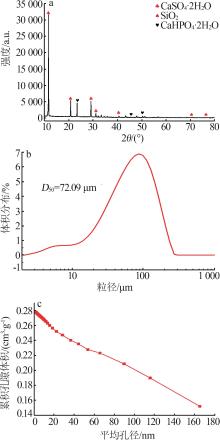
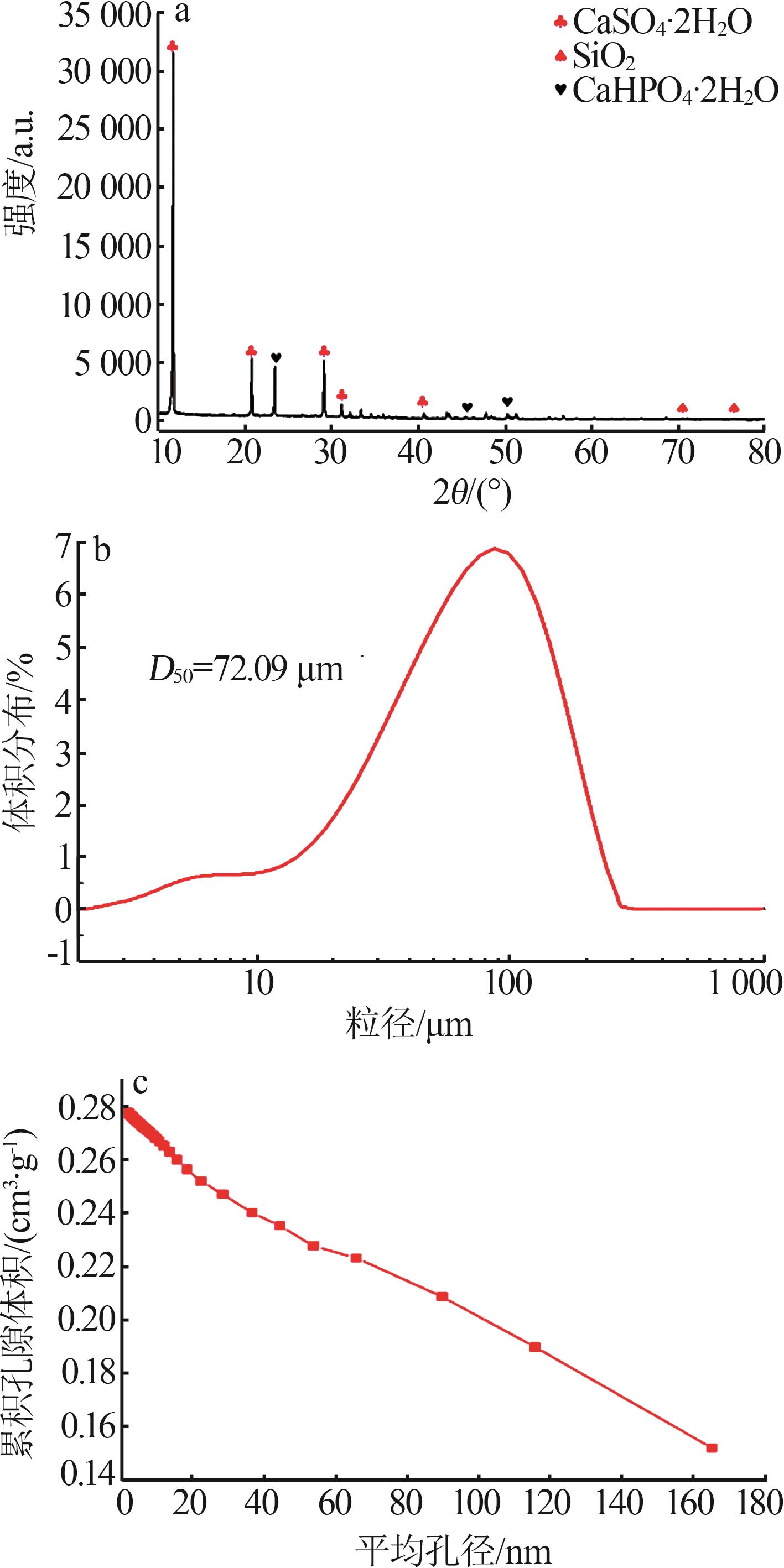
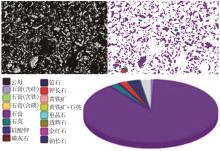
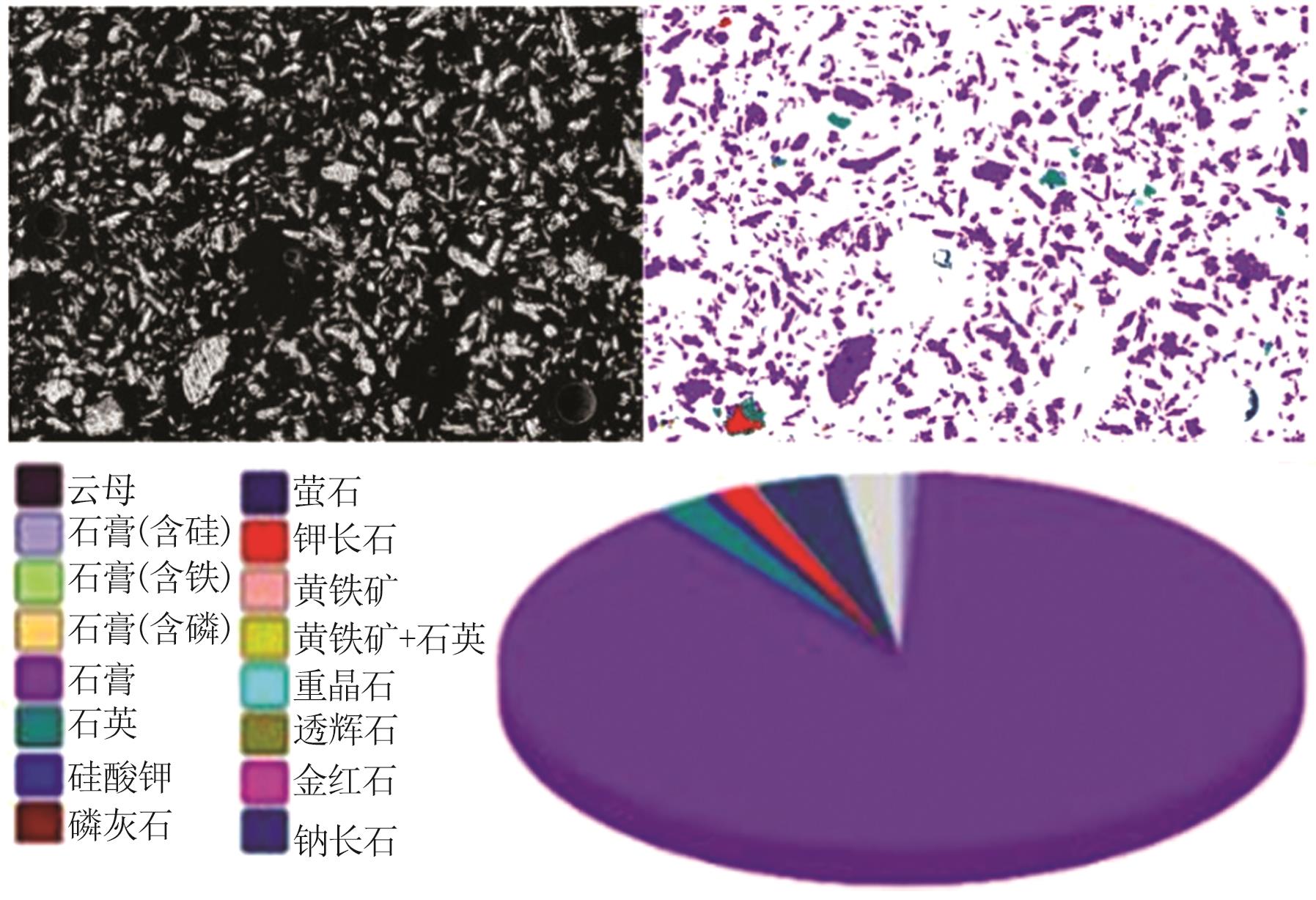
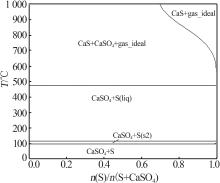
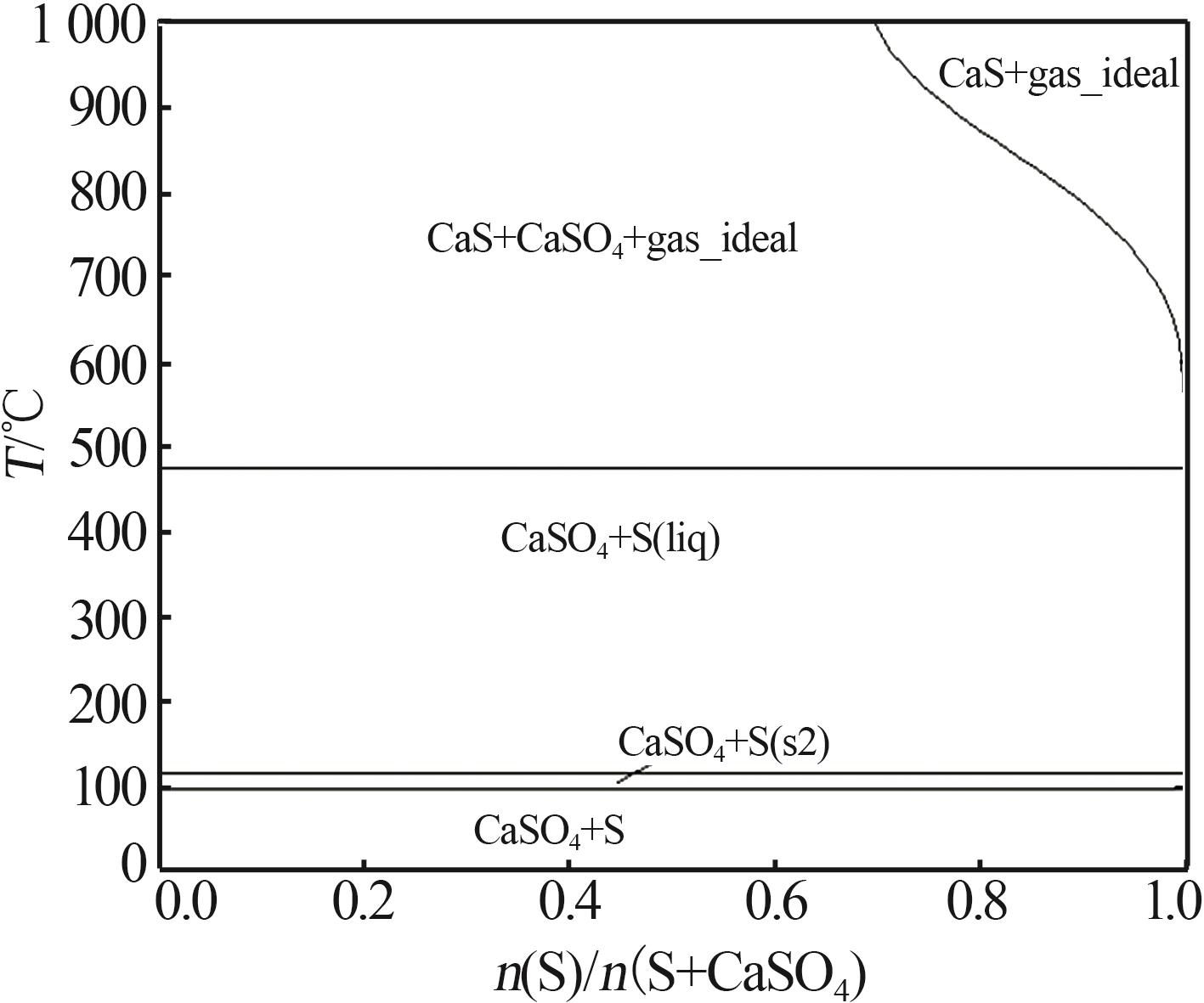
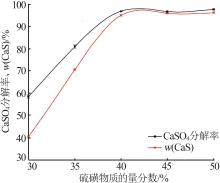
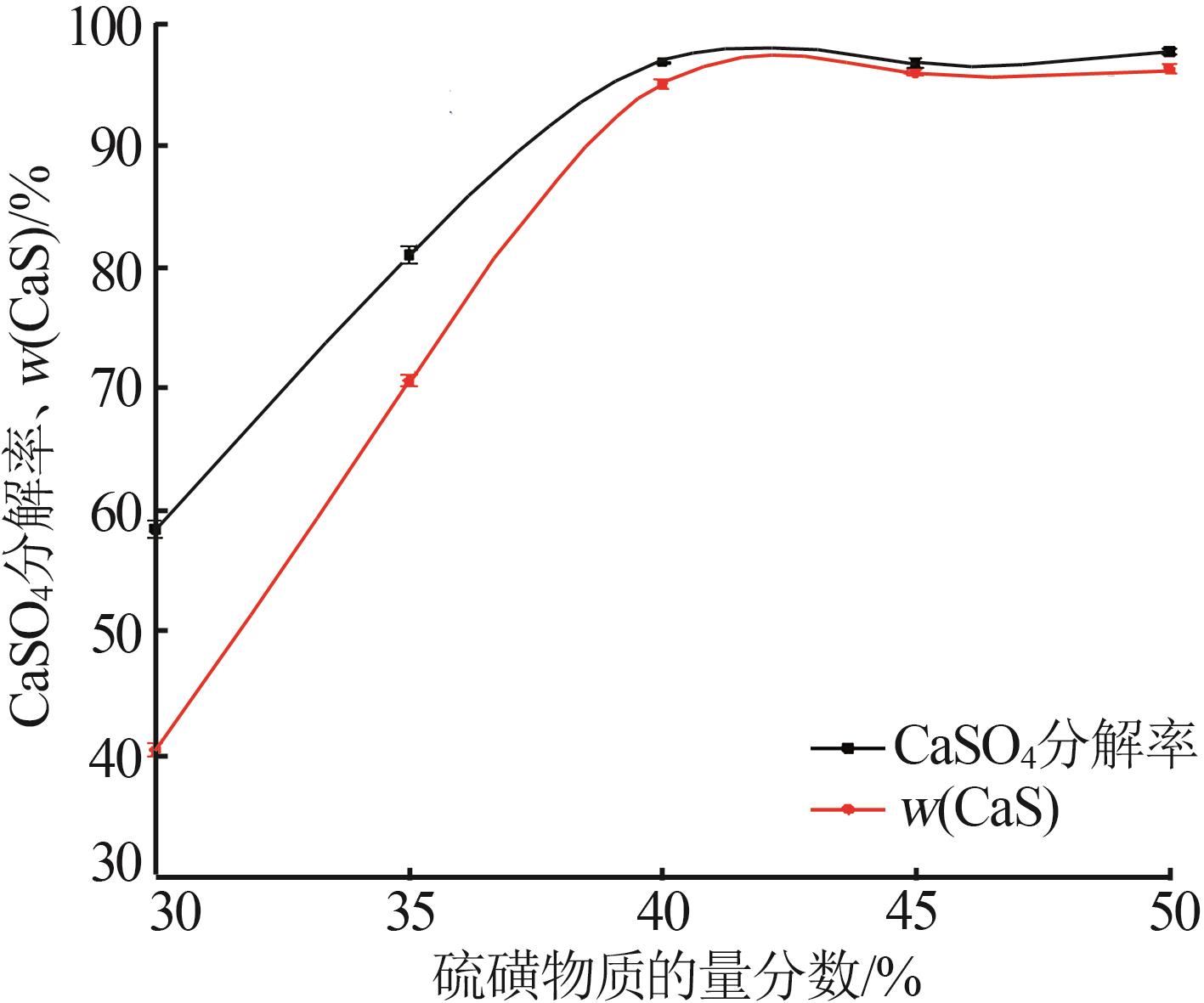
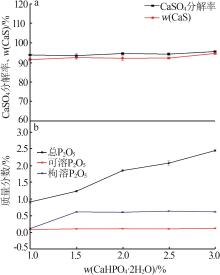
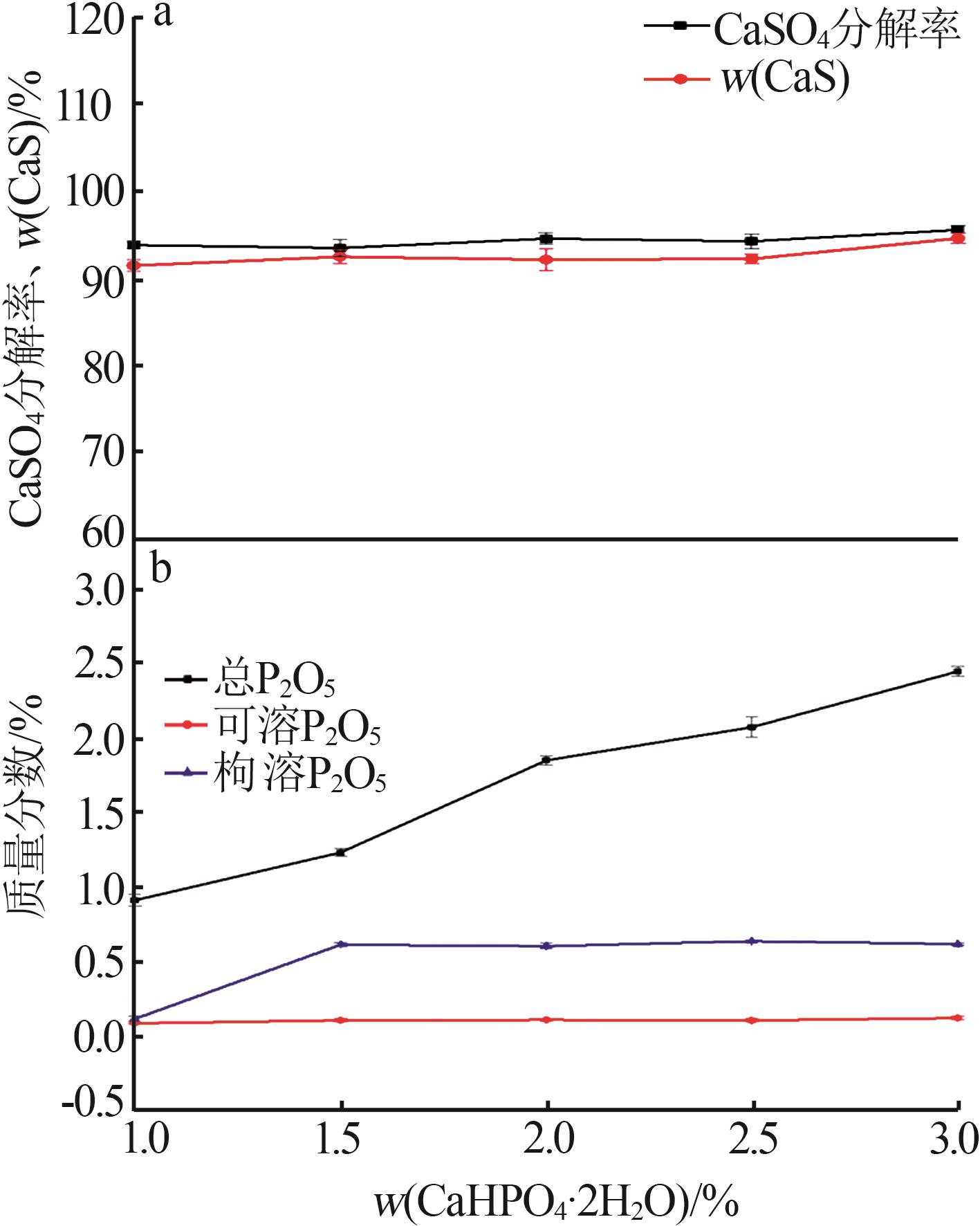
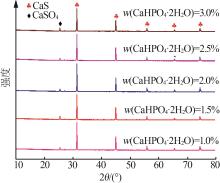
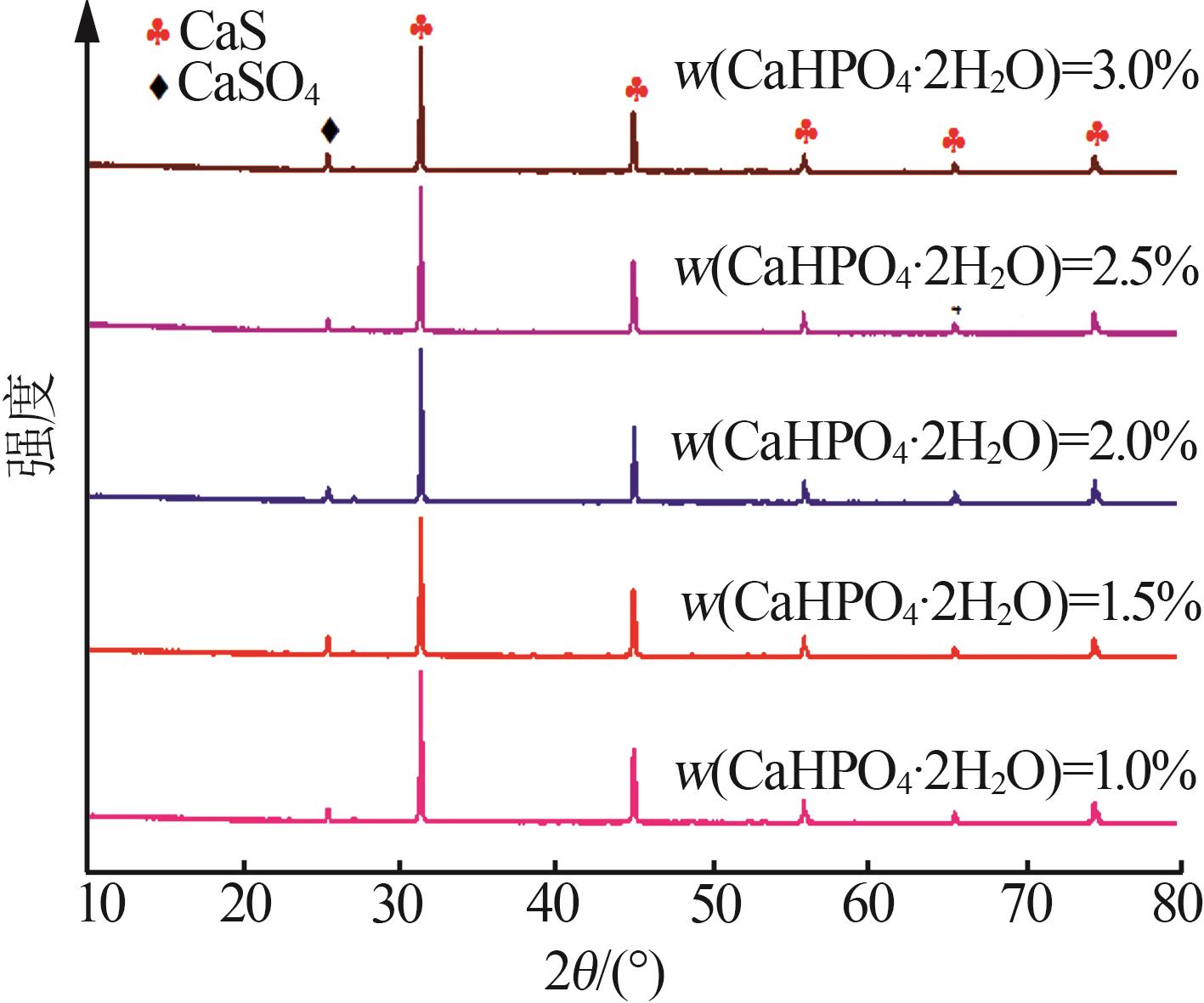
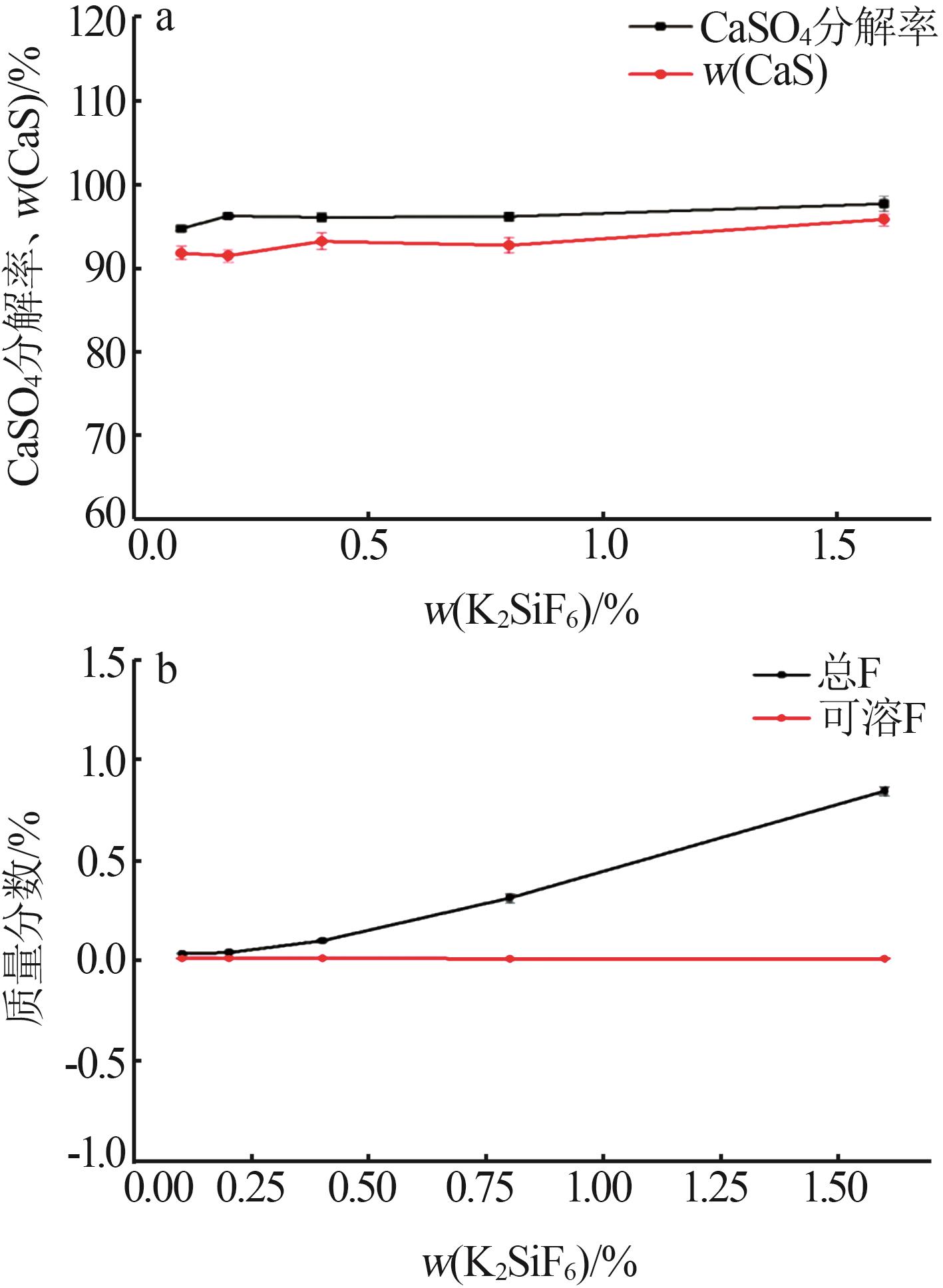
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (10): 110-117.doi: 10.19964/j.issn.1006-4990.2024-0092
• Environment·Health·Safety • Previous Articles Next Articles
Study on transfer laws of phosphorus and fluorine impurities during producing calcium sulfide through sulfur reduction decomposition of phosphogypsum
LI Shan( ), ZHANG Daping, YANG Xiushan(
), ZHANG Daping, YANG Xiushan( ), ZHAO Qiang, KONG Xingjian
), ZHAO Qiang, KONG Xingjian
- College of Chemical Engineering,Sichuan University,Ministry of Education Research Center for Comprehensive Utilization and Clean Processing Engineering of Phosphorus Resources,Chengdu 610065,China
-
Received:2024-02-21Online:2024-10-10Published:2024-11-05 -
Contact:YANG Xiushan E-mail:13795848281@163.com;yangxiushan3@163.com
CLC Number:
Cite this article
LI Shan, ZHANG Daping, YANG Xiushan, ZHAO Qiang, KONG Xingjian. Study on transfer laws of phosphorus and fluorine impurities during producing calcium sulfide through sulfur reduction decomposition of phosphogypsum[J]. Inorganic Chemicals Industry, 2024, 56(10): 110-117.
share this article
| 1 | 邓伏礼,夏志祥,龙秉文,等.磷石膏反浮选提纯工艺研究 [J].无机盐工业,2024,56(5):115-120. |
| DENG Fuli, XIA Zhixiang, LONG Bingwen,et al.Study on purification process of phosphogypsum by reverse flotation[J].Inorganic Chemicals Industry,2024,56(5):115-120. | |
| 2 | 张庆年,徐迅,严金生,等.磷石膏中可溶性氟对硅酸盐水泥性能的影响[J].新世纪水泥导报,2022,28(3):15-18. |
| ZHANG Qingnian, XU Xun, YAN Jinsheng,et al.Effect of soluble fluorine in phosphogypsum on properties of Portland cement[J].Cement Guide for New Epoch,2022,28(3):15-18. | |
| 3 | 高卫民,冉景,朱巧红.我国磷石膏资源化利用政策解读及研究进展刍议[J].化工矿物与加工,2022,51(7):48-53. |
| GAO Weimin, RAN Jing, ZHU Qiaohong.Policy interpretation and research progress of resource utilization of phosphogypsum in China[J].Industrial Minerals & Processing,2022,51(7):48-53. | |
| 4 | LI Xu, LV Xinfeng, XIANG Lan.Review of the state of impurity occurrences and impurity removal technology in phosphogypsum[J].Materials,2023,16(16):5630. |
| 5 | CAO Wenxiang, YI Wei, PENG Jiahui,et al.Preparation of anhydrite from phosphogypsum:Influence of phosphorus and fluorine impurities on the performances[J].Construction and Building Materials,2022,318:126021. |
| 6 | 陈凤,冯康,李铭,等.有机改性硫酸钙晶须在沥青改性中的应用[J].无机盐工业,2024,56(3):125-130. |
| CHEN Feng, FENG Kang, LI Ming,et al.Application of organically modified calcium sulfate whiskers in asphalt modification[J].Inorganic Chemicals Industry,2024,56(3):125-130. | |
| 7 | 吴浩,韩超南,汤昱.我国磷石膏资源化利用研究进展[J].现代化工,2023,43(3):18-21. |
| WU Hao, HAN Chaonan, TANG Yu.Research progress on reutilization of phosphogypsum in China[J].Modern Chemical Industry,2023,43(3):18-21. | |
| 8 | 王艳语,谷守玉,侯翠红,等.磷石膏配料碳热熔融还原硫逸出及熔渣物相分析[J].无机盐工业,2024,56(2):86-94. |
| WANG Yanyu, GU Shouyu, HOU Cuihong,et al.Sulfur escape and slag physical phase analysis by carbon thermal reduction melting based on phosphogypsum ingredients[J].Inorganic Chemicals Industry,2024,56(2):86-94. | |
| 9 | 胡成,刘梦,向玮衡,等.NaCl溶液浓度对磷石膏制备α-半水石膏转晶行为的影响[J].无机盐工业,2024,56(6):87-93. |
| HU Cheng, LIU Meng, XIANG Weiheng,et al.Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum[J].Inorganic Chemicals Industry,2024,56(6):87-93. | |
| 10 | 国务院办公厅.国务院办公厅关于加快构建废弃物循环利用体系的意见:国办发〔2024〕7号[EB/OL].(2024-02-09)[2024-02-21].https://www.gov.cn/zhengce/zhengceku/202402/content_6931080.htm. |
| 11 | 王辛龙,张志业,杨守明,等.硫磺分解磷石膏制硫酸技术进展及推广应用[J].硫酸工业,2018(1):45-49,53. |
| WANG Xinlong, ZHANG Zhiye, YANG Shouming,et al.Technical progress and application of sulphuric acid production by decomposing phosphogypsum with sulphur[J].Sulphuric Acid Industry,2018(1):45-49,53. | |
| 12 | 庞仁杰.以硫代碳化学分解石膏制硫酸的可行性分析[J].硫酸工业,2015(2):22-25. |
| PANG Renjie.Feasibility analysis of sulphuric acid production by gypsum decomposed by sulphur instead of carbon[J].Sulphuric Acid Industry,2015(2):22-25. | |
| 13 | 钟本和,王辛龙,张志业,等.硫磺还原分解磷石膏制硫酸节能减排新工艺[J].化肥工业,2014,41(2):7-10,27. |
| ZHONG Benhe, WANG Xinlong, ZHANG Zhiye,et al.New saving energy and reducing discharge process of producing sulfuric acid by phosphogypsum reduction and decomposition with sulf⁃ur[J].Chemical Fertilizer Industry,2014,41(2):7-10,27. | |
| 14 | 资泽城,马丽萍,马俊,等.杂质对磷石膏碳粉还原分解的影响研究[J].硅酸盐通报,2014,33(11):2772-2777. |
| ZI Zecheng, MA Liping, MA Jun,et al.Effect of impurities on the reductive decomposition of phosphogypsum by powdered carb⁃on[J].Bulletin of the Chinese Ceramic Society,2014,33(11):2772-2777. | |
| 15 | XIAO Junhui, LU Tao, ZHUANG Yuanfa,et al.A novel process to recover gypsum from phosphogypsum[J].Materials,2022, 15(5):1944. |
| 16 | 唐明珠,王志英,王云山,等.EBSD-XPS法分析磷石膏中杂质物相[J].光谱学与光谱分析,2022,42(1):136-140. |
| TANG Mingzhu, WANG Zhiying, WANG Yunshan,et al.Characterization of the impurity phases in phosphogypsum by the EBSD-XPS method[J].Spectroscopy and Spectral Analysis,2022,42(1):136-140. | |
| 17 | 杨皓奇,武发德,朱干宇,等.天然陈化对磷石膏理化性质的影响及作用机理研究[J].新型建筑材料,2023,50(5):99-105. |
| YANG Haoqi, WU Fade, ZHU Ganyu,et al.The effect and mechanism of natural aging procedure on the physicochemical properties of phosphogypsum[J].New Building Materials,2023,50(5):99-105. | |
| 18 | 李恒,郭旭东,钟晋,等.磷石膏杂质及净化研究现状[J].磷肥与复肥,2022,37(5):22-26. |
| LI Heng, GUO Xudong, ZHONG Jin,et al.Research status of phosphogypsum impurities and purification[J].Phosphate & Compound Fertilizer,2022,37(5):22-26. | |
| 19 | WANG Mingshun, YUAN Xing, DONG Wenyan,et al.Gradient removal of Si and P impurities from phosphogypsum and preparation of anhydrous calcium sulfate[J].Journal of Environmental Chemical Engineering,2023,11(3):110312. |
| 20 | 唐明珠.磷石膏杂质组分赋存及净化研究[D].天津:河北工业大学,2021. |
| TANG Mingzhu.Study on the occurrence and purification of impurity components in phosphogypsum[D].Tianjin:Hebei University of Technology,2021. | |
| 21 | YANG Xiushan, ZHANG Zhiye, WANG Xinlong,et al.Thermodynamic study of phosphogypsum decomposition by sulfur[J].The Journal of Chemical Thermodynamics,2013,57:39-45. |
| 22 | 周丽娜,周亮亮.CaSO4热分解反应机理的研究进展[J].化工中间体,2013(5):29-32. |
| ZHOU Lina, ZHOU Liangliang.Study process of calcium sulfate thermal decomposition reaction mechanism[J].Chemical Intermediates,2013(5):29-32. | |
| 23 | 郑绍聪,宁平,成飞翔,等.磷石膏分解制备硫化钙的试验分析[J].土木建筑与环境工程,2013(S2):160-163. |
| ZHENG Shaocong, NING Ping, CHENG Feixiang,et al.Experimental study of preparation of calcium sulfide from thermal decomposition of phosphogypsum[J].Journal of Civil,Architectural & Environment Engineering,2013(S2):160-163. | |
| 24 | 朱昌宇.磷石膏工业化应用的无害化处理研究[J].天津化工,2023,37(3):1-3. |
| ZHU Changyu.Study on harmless treatment of phosphogypsum for industrial application[J].Tianjin Chemical Industry,2023,37(3):1-3. | |
| 25 | CAO Wenxiang, YI Wei, PENG Jiahui,et al.Recycling of phosphogypsum to prepare gypsum plaster:Effect of calcination temperature[J].Journal of Building Engineering,2022,45:103511. |
| [1] | LENG Manxi, ZHU Yu, YAN Jikang, XIE Ke, ZOU Yongjie. Study on crystallization characteristics and mineral flotation performance of industrial waste phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(3): 108-115. |
| [2] | TAN Shanyi, WEN Huizi, HE Shuyu, ZHANG Liwen, CHEN Shaohua, XI Benjun. Study on leaching behavior and kinetics of phosphorus from phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(2): 105-112. |
| [3] | TANG Kaijing, LIU Chuanbei, LI Yingding, JIANG Yong, WU Junnan, ZHANG Tao. Research on preparation and mechanism of superhydrophobic phosphogypsum products [J]. Inorganic Chemicals Industry, 2025, 57(1): 97-102. |
| [4] | LI Xuelian, TANG Zihan, XU Jie, LI Xiong. Study on performance of calcium sulfate whisker/SBS composite modified asphalt [J]. Inorganic Chemicals Industry, 2024, 56(9): 82-89. |
| [5] | TANG Xuemei, WANG Meibo, XU Li, ZHANG Xujie, TAI Shijun, YI Xianmei, LIU Hongjuan, PAN Linyi. Study on whitening of phosphogypsum by low⁃temperature calcination [J]. Inorganic Chemicals Industry, 2024, 56(8): 110-115. |
| [6] | SUN Lan, CHEN Shiying, YANG Liuxu, NIU Yiming, ZHAO Aonan. Basic study on fixing soluble phosphorus in phosphogypsum with magnesium slag [J]. Inorganic Chemicals Industry, 2024, 56(8): 92-98. |
| [7] | HU Cheng, LIU Meng, XIANG Weiheng, CHEN Ping, WANG Neng, LU Guanju, ZHOU Jinlan. Preparation of α-hemihydrous gypsum from CaCl2 and MgCl2 and their composite solution [J]. Inorganic Chemicals Industry, 2024, 56(7): 112-117. |
| [8] | HU Dian, GUO Ze, ZHANG Hanquan, LU Manman. Research on effects of roasting process and typical impurities on reduction and decomposition process of phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(7): 88-95. |
| [9] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [10] | TU Yanping, BAI Dengxian, CHENG Shukai, XIE Junjie, HUANG Zhiliang, CHEN Guofu. Effect of high temperature modification of mineral powder and quicklime on properties of phosphogypsum cement based materials [J]. Inorganic Chemicals Industry, 2024, 56(6): 94-101. |
| [11] | WANG Mingshun, AO Xianquan, YUAN Xing, DONG Wenyan, CHEN Qianlin. Study on effect of crystallizer on preparation of anhydrous calcium sulfate from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(5): 101-107. |
| [12] | WANG Luwei, WANG Jie, LI Keke, FENG Chunhua, ZHANG Wenyan, ZHU Jianping. Study on leaching treatment of titanium gypsum with oxalic acid [J]. Inorganic Chemicals Industry, 2024, 56(5): 108-114. |
| [13] | DENG Fuli, XIA Zhixiang, LONG Bingwen, ZHANG Yi, DAI Yafen, WANG Bin, DING Yigang. Study on purification process of phosphogypsum by reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(5): 115-120. |
| [14] | WANG Peixiong, GONG Xiaomei, DING Jiaqi, CAO Hong. Effect of crystal modifier on preparation of α-hemihydrates gypsum from industrial gypsum [J]. Inorganic Chemicals Industry, 2024, 56(4): 112-117. |
| [15] | CHEN Feng, FENG Kang, LI Ming, SHEN Haojie, TIAN Chengtao, TANG Yuan, LI Zhili, HE Dongsheng. Application of organically modified calcium sulfate whiskers in asphalt modification [J]. Inorganic Chemicals Industry, 2024, 56(3): 125-130. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||