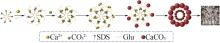
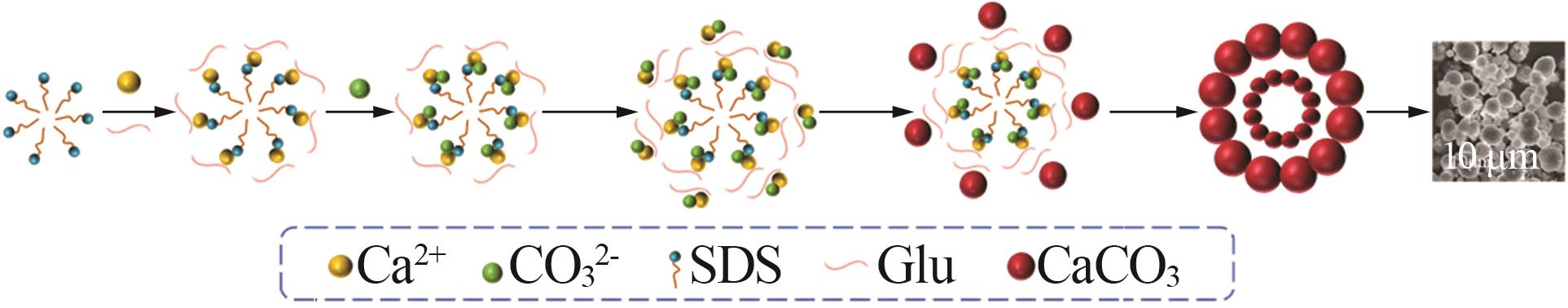
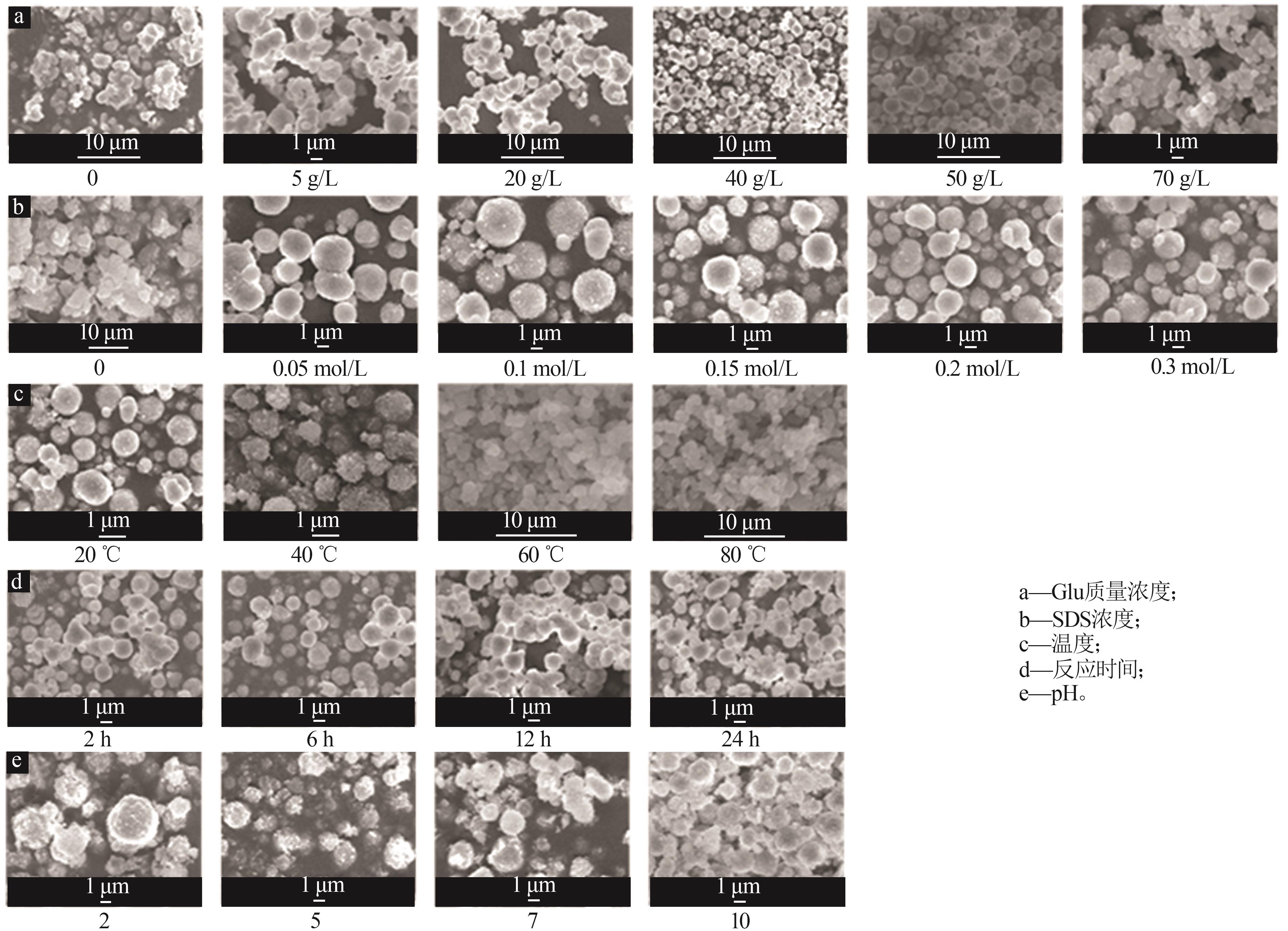
| 1 |
LU Hao, HUANG Y C, HUNGER J,et al.Role of water in CaCO3 biomineralization[J].Journal of the American Chemical Society,2021,143(4):1758-1762.
|
| 2 |
MOHAMMED A M, ABED A H.Enhancing asphalt binder performance through nano-SiO2 and nano-CaCO3 additives:Rheological and physical insights[J].Case Studies in Construction Materials,2023,19:e02492.
|
| 3 |
LONGKAEW K, TESSANAN W, DANIEL P,et al.Using sucrose to prepare submicrometric CaCO3 vaterite particles stable in natural rubber[J].Advanced Powder Technology,2023,34(1):103924.
|
| 4 |
LI Caili, LI Yingjie, ZHANG Chunxiao,et al.CaO/CaCO3 thermochemical energy storage performance of high-alumina granule stabilized papermaking soda residue[J].Fuel Processing Technology,2022,237:107444.
|
| 5 |
SANG Ruijuan, YANG Fei.Effect of TiO2@CaCO3 waterborne primer on the coloring performance of inkjet-printed wood product coatings[J].Coatings,2023,13(12):2071.
|
| 6 |
李洪江,卢泓冶,唐亮星,等.PVC/改性纳米CaCO3复合材料的力学性能和耐热性能研究[J].塑料科技,2024,52(1):40- 43.
|
|
LI Hongjiang, LU Hongye, TANG Liangxing,et al.Study on mechanical properties and heat resistance of PVC/modified nano-CaCO3 composites[J].Plastics Science and Technology,2024,52(1):40-43.
|
| 7 |
曾令威,卢乐民,梁逸昊,等.重质碳酸钙应用于建筑涂料中的研究进展[J].当代化工,2023,52(11):2761-2766.
|
|
ZENG Lingwei, LU Lemin, LIANG Yihao,et al.Research progress of application of heavy calcium carbonate in architectural coatings[J].Contemporary Chemical Industry,2023,52(11):2761-2766.
|
| 8 |
YOO J H, LEE S.Cleavage of calcitic CaCO3 during dissolution in aqueous solution[J].ACS Omega,2021,6(36):23311-23316.
|
| 9 |
NÉMETH P, MUGNAIOLI E, GEMMI M,et al.A nanocrystalline monoclinic CaCO3 precursor of metastable aragonite[J].Science Advances,2018,4(12):eaau6178.
|
| 10 |
ZAFAR B, CAMPBELL J, COOKE J,et al.Modification of surfaces with vaterite CaCO3 particles[J].Micromachines,2022,13(3):473.
|
| 11 |
SHEIKHI A, MEJLSØE S L, LI Na,et al.Biomimetic dendrimers for mineralization:Rare fibrous amorphous calcium carbonate (ACC) and branch-and-bud ACC-vaterite polymorphs[J].Materials Chemistry Frontiers,2018,2(11):2081-2090.
|
| 12 |
卢秋影,覃沛,卓民权,等.球形纳米碳酸钙制备方法的研究进展[J].化工技术与开发,2024,53(7):39-42,92.
|
|
LU Qiuying, QIN Pei, ZHUO Minquan,et al.Research progress on preparation of spherical nanometer calcium carbonate[J].Technology & Development of Chemical Industry,2024,53(7):39-42,92.
|
| 13 |
李裕兴,张金才,程芳琴.多种晶型纳米碳酸钙制备及生长机理的研究进展[J].无机盐工业,2024,56(5):1-10.
|
|
LI Yuxing, ZHANG Jincai, CHENG Fangqin.Research progress of preparation and growth mechanism of various crystalline nano-calcium carbonate[J].Inorganic Chemicals Industry,2024,56(5):1-10.
|
| 14 |
陈彰旭,辛梅华,李明春,等.模板法合成碳酸钙研究进展[J].化工进展,2014,33(10):2687-2692.
|
|
CHEN Zhangxu, XIN Meihua, LI Mingchun,et al.Progress of preparation of calcium carbonate with template method[J].Che-mical Industry and Engineering Progress,2014,33(10):2687-2692.
|
| 15 |
LIU Xun, LI Kangxin, WU Chaoqun,et al.Influence of copper(Ⅱ) on biomineralization of CaCO3 and preparation of micron pearl-like biomimetic CaCO3 [J].Ceramics International,2019,45(11):14354-14359.
|
| 16 |
POLAT S.Experimental investigations on the effects of asparagine and serine on the polymorphism of calcium carbonate[J].Advanced Powder Technology,2020,31(10):4282-4291.
|
| 17 |
ABEYWARDENA M R, ELKADUWE R K W H M K, KARUNARATHNE D G G P,et al.Surfactant assisted synthesis of precipitated calcium carbonate nanoparticles using dolomite:Effect of pH on morphology and particle size[J].Advanced Powder Technology,2020,31(1):269-278.
|
| 18 |
谭婷婷,仲剑初.球形碳酸钙的控制合成研究[J].无机盐工业,2019,51(12):30-34.
|
|
TAN Tingting, ZHONG Jianchu.Study on controllable synthesis of spherical calcium carbonate[J].Inorganic Chemicals Industry,2019,51(12):30-34.
|
| 19 |
CHEN Tao, SHI Peiheng, LI Yi,et al.Crystallization of calcium carbonate mineral with hierarchical structures regulated by silk fibroin in microbial mineralization system[J].Journal of Crystal Growth,2018,493:51-57.
|
| 20 |
VIDALLON M L P, YU F, TEO B M.Controlling the size and polymorphism of calcium carbonate hybrid particles using natural biopolymers[J].Crystal Growth & Design,2020,20(2):645-652.
|
| 21 |
胡彩霞,胡辰鑫,彭剑龙,等.以可溶性淀粉为晶型调控剂制备多孔球形碳酸钙[J].无机盐工业,2021,53(5):51-55.
|
|
HU Caixia, HU Chenxin, PENG Jianlong,et al.Preparation of porous spherical calcium carbonate with soluble starch as crystallization controller[J].Inorganic Chemicals Industry,2021,53(5):51-55.
|
| 22 |
邓伟林,黄文柯,黄崇秋,等.聚丙烯酸和聚苯乙烯磺酸钠调控制备多孔碳酸钙[J].无机盐工业,2023,55(2):67-72.
|
|
DENG Weilin, HUANG Wenke, HUANG Chongqiu,et al.Regulatory preparation of porous calcium carbonate by polyacrylic acid and polystyrene sulfonate[J].Inorganic Chemicals Industry,2023,55(2):67-72.
|
| 23 |
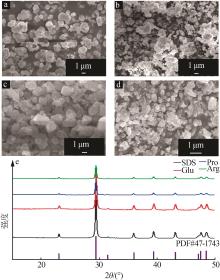
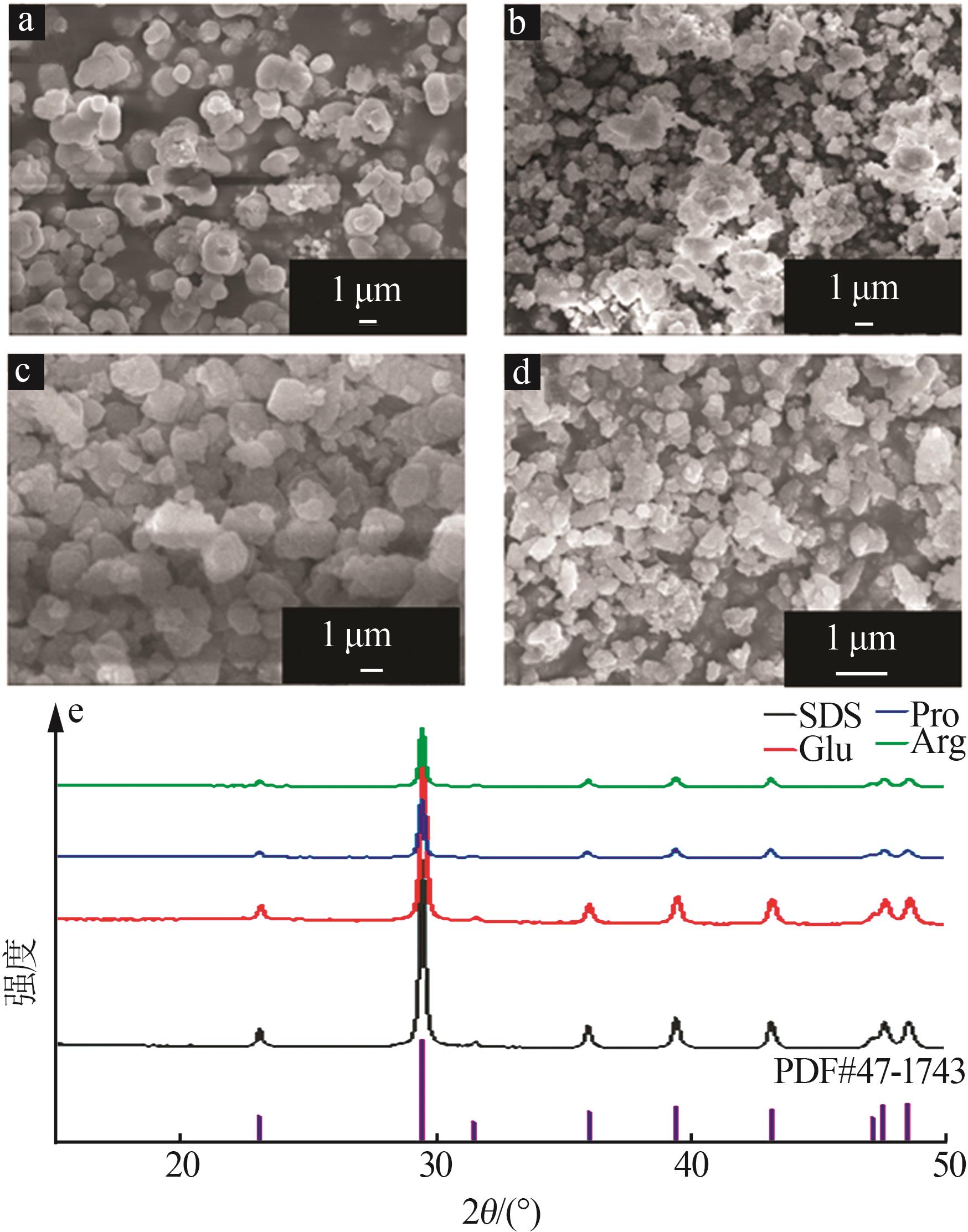
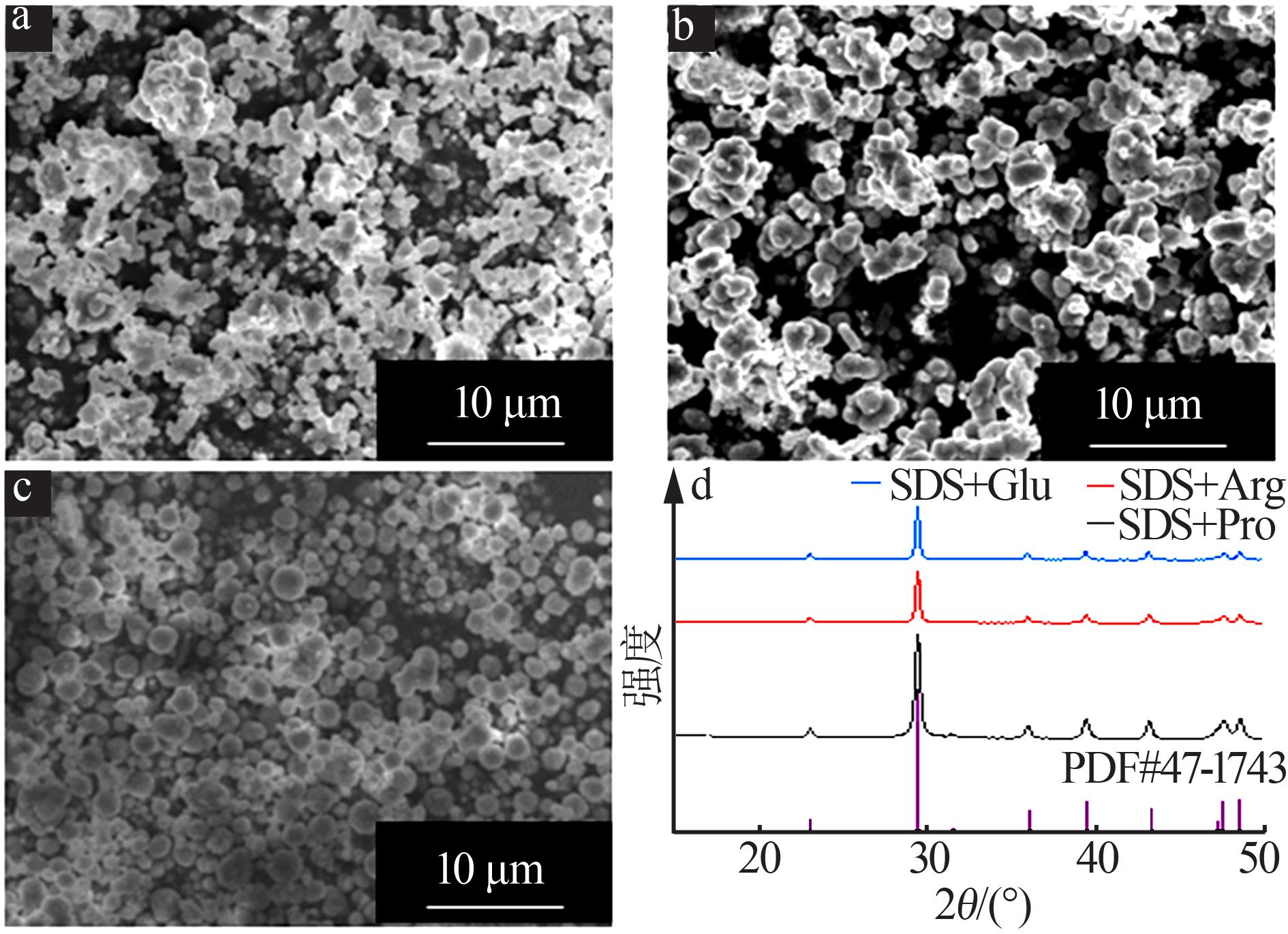
CHEN Zhiying, LI Caifen, YANG Qianqian,et al.Transformation of novel morphologies and polymorphs of CaCO3 crystals induced by the anionic surfactant SDS[J].Materials Chemistry and Physics,2010,123(2/3):534-539.
|
| 24 |
CHEN Zhiying, Zhaodong NAN.Effects of 1,3,5-trimethylbenzene on morphology and polymorph of CaCO3 crystals in the presence of SDS[J].Materials Research Bulletin,2013,48(10):4319-4328.
|
| 25 |
YAN Jiawei, TAN Xiao, QI Suitao.High-temperature-resistant sc-ale inhibitor polyaspartic acid-prolineamide for inhibiting CaCO3 scale in geothermal water and speculation of scale inhibition mechanism[J].Water,2023,15(8):1457.
|
| 26 |
ALI A, ANSARI N H.Studies on the effect of amino acids/peptide on micellization of SDS at different temperatures[J].Journal of Surfactants and Detergents,2010,13(4):441-449.
|
 ), CAO Yapeng(
), CAO Yapeng( ), ZU Minghua, ZHANG Zhikun, LIU Yumin, HAN Jilong(
), ZU Minghua, ZHANG Zhikun, LIU Yumin, HAN Jilong( )
)