Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (11): 116-122.doi: 10.19964/j.issn.1006-4990.2024-0049
• Research & Development • Previous Articles Next Articles
Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K
YANG Bo1( ), MA Zhen2, ZENG Ying1(
), MA Zhen2, ZENG Ying1( ), YAN Xiongzhong2, LI Qi1, HOU Yuansheng2, YU Xudong1
), YAN Xiongzhong2, LI Qi1, HOU Yuansheng2, YU Xudong1
- 1.College of Materials and Chemistry & Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
2.Qinghai Salt Lake Industrial Co. ,Ltd. ,Golmud 816000,China
-
Received:2024-01-24Online:2024-11-10Published:2024-11-27 -
Contact:ZENG Ying E-mail:yangboc413@126.com;zengyster@163.com
CLC Number:
Cite this article
YANG Bo, MA Zhen, ZENG Ying, YAN Xiongzhong, LI Qi, HOU Yuansheng, YU Xudong. Study on solid-liquid phase equilibria in ternary system of Li+(K+),Rb+//Cl--H2O at 288.2 K[J]. Inorganic Chemicals Industry, 2024, 56(11): 116-122.
share this article
Table 2
Solid-liquid phase equilibria data of ternary system of Li+,Rb+//Cl--H2O at 288.2 K"
编 号 | 折射 率/nD | 密度/ (g·cm-3) | 液相组成 | 湿固相组成 | 平衡 固相 | ||
|---|---|---|---|---|---|---|---|
w (RbCl)/ % | w (LiCl)/ % | w (RbCl)/ % | w (LiCl)/ % | ||||
| 1,a | 1.434 0 | 1.271 4 | 0.00 | 44.77 | LiCl·2H2O | ||
| 2 | 1.433 6 | 1.282 2 | 1.90 | 43.54 | LiCl·2H2O | ||
| 3 | 1.434 0 | 1.293 4 | 3.62 | 42.75 | LiCl·2H2O | ||
| 4 | 1.434 4 | 1.298 3 | 5.76 | 41.24 | LiCl·2H2O | ||
| 5 | 1.435 8 | 1.307 8 | 7.61 | 39.52 | LiCl·2H2O | ||
| 6 | 1.436 6 | 1.329 9 | 11.53 | 36.94 | LiCl·2H2O | ||
| 7 | 1.437 9 | 1.361 4 | 14.68 | 33.99 | LiCl·2H2O | ||
| 8,E | 1.438 2 | 1.401 7 | 19.18 | 26.92 | 23.98 | 45.52 | LiCl·2H2O+RbCl |
| 9 | 1.417 5 | 1.355 4 | 19.10 | 30.69 | 96.87 | 1.04 | RbCl |
| 10 | 1.414 5 | 1.345 6 | 19.71 | 25.71 | 74.72 | 8.02 | RbCl |
| 11 | 1.407 0 | 1.336 4 | 20.09 | 22.15 | 97.68 | 0.55 | RbCl |
| 12 | 1.409 6 | 1.333 0 | 20.31 | 23.21 | 78.64 | 6.12 | RbCl |
| 13 | 1.404 4 | 1.335 2 | 22.45 | 20.38 | 84.49 | 4.03 | RbCl |
| 14 | 1.400 0 | 1.328 8 | 25.16 | 17.17 | 82.16 | 4.01 | RbCl |
| 15 | 1.395 3 | 1.349 5 | 29.65 | 12.79 | 95.31 | 1.08 | RbCl |
| 16 | 1.391 6 | 1.367 6 | 33.72 | 9.13 | 95.92 | 0.41 | RbCl |
| 17 | 1.388 9 | 1.404 6 | 38.31 | 5.82 | 96.79 | 0.27 | RbCl |
| 18 | 1.388 0 | 1.435 8 | 41.28 | 3.30 | 93.93 | 0.32 | RbCl |
| 19,b | 1.388 0 | 1.481 4 | 46.46 | 0.00 | RbCl | ||
Table 3
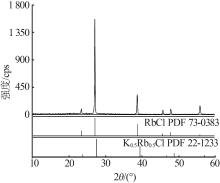
Solid-liquid phase equilibrium data of ternary system of K+,Rb+//Cl--H2O at 288.2 K"
| 编号 | 折射率/nD | 密度/ (g·cm-3) | 液相组成 | 湿固相组成 | 平衡固相 | |||
|---|---|---|---|---|---|---|---|---|
| w(RbCl)/% | w(KCl)/% | w(RbCl)/% | w(KCl)/% | |||||
| 1,c | 1.360 7 | 1.139 6 | 0.00 | 24.59 | KCl | |||
| 2 | 1.367 6 | 1.170 2 | 0.47 | 24.41 | 0.31 | 87.77 | KCl | |
| 3,F1 | 1.367 8 | 1.170 9 | 0.90 | 24.20 | 0.54 | 86.53 | KCl+(K,Rb)Cl | |
| 4 | 1.367 8 | 1.177 1 | 1.21 | 24.18 | 0.75 | 84.80 | (K,Rb)Cl | |
| 5 | 1.367 9 | 1.178 1 | 1.68 | 23.68 | 0.82 | 95.40 | (K,Rb)Cl | |
| 6 | 1.368 0 | 1.180 1 | 2.01 | 23.56 | 1.02 | 91.76 | (K,Rb)Cl | |
| 7 | 1.368 0 | 1.181 4 | 2.51 | 23.28 | 1.94 | 90.09 | (K,Rb)Cl | |
| 8 | 1.368 0 | 1.184 4 | 3.29 | 22.78 | 2.34 | 86.12 | (K,Rb)Cl | |
| 9 | 1.368 1 | 1.184 9 | 4.54 | 22.27 | 3.03 | 87.27 | (K,Rb)Cl | |
| 10 | 1.369 4 | 1.196 0 | 4.99 | 22.05 | 4.26 | 73.94 | (K,Rb)Cl | |
| 11 | 1.371 8 | 1.227 3 | 6.07 | 21.90 | 10.27 | 85.38 | (K,Rb)Cl | |
| 12 | 1.373 5 | 1.247 0 | 8.91 | 20.64 | 13.66 | 82.49 | (K,Rb)Cl | |
| 13 | 1.375 0 | 1.269 8 | 12.21 | 19.37 | 18.02 | 77.17 | (K,Rb)Cl | |
| 14 | 1.376 6 | 1.296 0 | 15.29 | 17.95 | 25.24 | 74.43 | (K,Rb)Cl | |
| 15 | 1.378 6 | 1.317 8 | 18.82 | 16.41 | 31.32 | 68.56 | (K,Rb)Cl | |
| 16 | 1.381 2 | 1.355 8 | 24.05 | 13.88 | 36.61 | 58.07 | (K,Rb)Cl | |
| 17 | 1.382 5 | 1.368 4 | 27.65 | 11.98 | 43.15 | 55.08 | (K,Rb)Cl | |
| 18 | 1.384 4 | 1.403 3 | 30.45 | 10.77 | 49.09 | 42.03 | (K,Rb)Cl | |
| 19 | 1.385 6 | 1.425 4 | 34.07 | 8.58 | 63.96 | 28.75 | (K,Rb)Cl | |
| 20 | 1.386 1 | 1.442 0 | 36.60 | 6.98 | 73.65 | 19.14 | (K,Rb)Cl | |
| 21 | 1.386 8 | 1.449 2 | 39.83 | 4.86 | 78.61 | 11.48 | (K,Rb)Cl | |
| 22 | 1.387 0 | 1.474 8 | 43.72 | 2.02 | 87.42 | 3.76 | (K,Rb)Cl | |
| 23 | 1.387 5 | 1.476 8 | 44.56 | 1.62 | 96.49 | 2.03 | (K,Rb)Cl | |
| 24,F2 | 1.387 4 | 1.478 8 | 45.95 | 0.70 | 94.23 | 1.54 | (K,Rb)Cl+RbCl | |
| 25 | 1.387 8 | 1.480 8 | 46.23 | 0.36 | 98.77 | 0.66 | RbCl | |
| 26,d | 1.388 0 | 1.481 4 | 46.46 | 0.00 | RbCl | |||
Table 4
Multi-temperature solid-liquid equilibrium data of ternary system of K+,Rb+//Cl--H2O"
| 温度/K | w(RbCl)/% | w(KCl)/% | w(H2O)/% | 平衡固相 |
|---|---|---|---|---|
| 288.2 | 0.90 | 24.20 | 74.90 | KCl+(K,Rb)Cl |
| 45.95 | 0.70 | 53.35 | RbCl+(K,Rb)Cl | |
| 298.2 | 1.15 | 26.34 | 72.51 | KCl+(K,Rb)Cl |
| 47.99 | 0.80 | 51.21 | RbCl+(K,Rb)Cl | |
| 348.2 | 1.92 | 30.27 | 67.81 | KCl+(K,Rb)Cl |
| 50.10 | 1.68 | 48.22 | RbCl+(K,Rb)Cl |
| 1 | 张苏江,张琳,姜爱玲,等.中国盐湖资源开发利用现状与发展建议[J].无机盐工业,2022,54(10):13-21. |
| ZHANG Sujiang, ZHANG Lin, JIANG Ailing,et al.Current situation and development suggestions of development and utilization of salt lake resources in China[J].Inorganic Chemicals Industry,2022,54(10):13-21. | |
| 2 | 郑绵平.中国盐湖资源与环境研究[M].北京:科学出版社,2022. |
| 3 | 于建国,孙庆,裘晟波,等.支撑国家新能源战略发展的锂资源开发[J].无机盐工业,2023,55(1):1-14. |
| YU Jianguo, SUN Qing, QIU Shengbo,et al.Lithium resources development supporting national new energy strategy developme-nt[J].Inorganic Chemicals Industry,2023,55(1):1-14. | |
| 4 | MA Fangtong, ZENG Ying, YU Xudong,et al.The leaching behavior of potassium extraction from polyhalite ore in water[J].ACS Omega,2023,8(40):37162-37175. |
| 5 | 高芯蕊,贾宏翔,李天骄,等.中国铷铯资源需求展望[J].地球学报,2023,44(2):279-285. |
| GAO Xinrui, JIA Hongxiang, LI Tianjiao,et al.Perspective of rubidium and caesium resource demand in China[J].Acta Geoscientica Sinica,2023,44(2):279-285. | |
| 6 | 杨游胜,姚智豪,赵志星,等.富锂硫酸盐型盐湖卤水蒸发实验研究进展[J].无机盐工业,2024,56(4):1-7. |
| YANG Yousheng, YAO Zhihao, ZHAO Zhixing,et al.Research progress of lithium-rich sulfate type salt lake brine evaporation experiment[J].Inorganic Chemicals Industry,2024,56(4):1-7. | |
| 7 | 刘佳,葛飞,钟永恒,等.建设世界级盐湖产业基地的战略思 考[J].无机盐工业,2022,54(10):30-36. |
| LIU Jia, GE Fei, ZHONG Yongheng,et al.Strategic thinking on constructing world-class salt lake industrial base[J].Inorganic Che-Industry micals,2022,54(10):30-36. | |
| 8 | 熊增华,张建伟,王石军.察尔汗盐湖资源开发的生态环境保护对策[J].中国矿业,2021,30(3):113-117. |
| XIONG Zenghua, ZHANG Jianwei, WANG Shijun.Countermeasures of ecological and environmental protection for qarhan salt lake resource exploitation[J].China Mining Magazine,2021,30(3):113-117. | |
| 9 | 王振东,时贞,童海奎,等.青海柴达木盆地盐湖资源保障能力分析与对策研究[J].中国矿业,2023,32(2):38-42. |
| WANG Zhendong, SHI Zhen, TONG Haikui,et al.Analysis and countermeasures study of guarantee capability of salt lake resourc-es in Qinghai Qaidam Basin[J].China Mining Magazine,2023,32(2):38-42. | |
| 10 | 王超,孙小虹,丁晓姜,等.察尔汗盐湖晒卤过程中铷和铯元素富集规律研究[J].岩石矿物学杂志,2023,42(5):701-710. |
| WANG Chao, SUN Xiaohong, DING Xiaojiang,et al.Research on the enrichment regularity of rubidium and cesium in Qarhan Salt Lake[J].Acta Petrologica Mineralogica,2023,42(5):701-710. | |
| 11 | 高丹丹,李东东,樊燕飞,等.察尔汗盐湖铷资源利用:从基础认知到技术创新[J].盐湖研究,2022,30(3):1-11,41. |
| GAO Dandan, LI Dongdong, FAN Yanfei,et al.Economically utilization of the rubidium resources in Qarhan Salt Lake:From fundamental study to technology innovation[J].Journal of Salt Lake Research,2022,30(3):1-11,41. | |
| 12 | 刘敏,诸葛福瑜,王林,等.三元体系KCl+RbCl+H2O 298 K稳定相平衡研究[J].化学工程,2018,46(11):21-24,50. |
| LIU Min, ZHUGE Fuyu, WANG Lin,et al.Stable phase equilibrium for ternary system KCl+RbCl+H2O at 298 K[J].Chemical Engineering(China),2018,46(11):21-24,50. | |
| 13 | YU Xudong, ZENG Ying, YIN Qinghong,et al.Solubilities,densities,and refractive indices of the ternary systems KCl+RbCl+H2O and KCl+MgCl2+H2O at 348.15 K[J].Journal of Chemical & Engineering Data,2012,57(12):3658-3663. |
| 14 | LI Zhongquan, YU Xudong, YIN Qinghong,et al.Thermodynamics metastable phase equilibria of aqueous quaternary system LiCl+KCl+RbCl+H2O at 323.15 K[J].Fluid Phase Equilibria,2013,358:131-136. |
| 15 | 宋彭生.湿渣法在水盐体系相平衡研究中的应用[J].盐湖研究,1991(1):15-23,7. |
| SONG Pengsheng.Application of wet slag method in phase equilibrium study of water-salt system [J].Journal of Salt Lake Research,1991(1):15-23,7. | |
| 16 | 钟远,李海军,王涛,等.四苯硼钠—季铵盐质量滴定法分析常量铷[J].盐湖研究,2014,22(4):11-16. |
| ZHONG Yuan, LI Haijun, WANG Tao,et al.Determination of rubidium in high concentrations by the mass titration with sodium tetraphenylboron-quaternary ammonium salt[J].Journal of Salt Lake Research,2014,22(4):11-16. | |
| 17 | HE Chunxia, ZHANG Hanzhong, SANG Shihua,et al.Studies on phase equilibria in ternary system LiCl-SrCl2-H2O at 288.15 K and quaternary system LiCl-KCl-SrCl2-H2O at 288.15 and 323.15 K[J].Journal of Chemical & Engineering Data,2021, 66(9):3386-3396. |
| 18 | HUANG Qin, LI Maolan, WANG Lin,et al.Solid-liquid equilibria and thermodynamic correlation for the ternary system(KCl+PEG10000/20000+H2O) at 288.2 K and 298.2 K[J].The Journal of Chemical Thermodynamics,2020,150:106221. |
| 19 | LI Maolan, YU Xudong, HUANG Qin,et al.Phase equilibria measurements and correlation of aqueous solvent of PEG4000 with rubidium chloride at(288.15,298.15,and 308.15) K[J].Journal of Chemical Thermodynamics,2020,149:106151. |
| 20 | 宋彭生,杜宪惠,许恒存.三元体系Li2B4O7-Li2SO4-H2O 25 ℃相关系和溶液物化性质的研究[J].科学通报,1983,28(2):106-110. |
| SONG Pengsheng, DU Xianhui, XU Hengcun.Study on the phase relationship and physicochemical properties of the ternary system Li2B4O7-Li2SO4-H2O at 25 ℃[J].Chinese Science Bulletin,1983,28(2):106-110. | |
| 21 | 赵珊茸.结晶学及矿物学[M].3版.北京:高等教育出版社,2017. |
| 22 | 冯珊,于旭东,罗军,等.298.2 K四元体系NH4Cl-CaCl2-SrCl2-H2O稳定相平衡实验[J].高校化学工程学报,2023,37(2):194-201. |
| FENG Shan, YU Xudong, LUO Jun,et al.Stable phase equilibria of quaternary system NH4Cl-CaCl-SrCl2-H2O at 298.2 K[J].Journal of Chemical Engineering of Chinese Universities,2023,37(2):194-201. |
| [1] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [2] | GUO Kaihua, FAN Yuxin, YANG Jing, ZHAO Wenli, JIA Yuanyuan, WANG Yanfei. Analysis of effect of carnallite raw ore grade on its cold decomposition and crystallization of potassium chloride [J]. Inorganic Chemicals Industry, 2024, 56(8): 9-18. |
| [3] | CHEN Junhui, LIU Xiang, HU Qingxi, TIAN Bangxin, CHEN Jiale. Study on leaching extraction of high purity potassium chloride from sintering machine head ash [J]. Inorganic Chemicals Industry, 2024, 56(6): 102-108. |
| [4] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [5] | ZHU Zenghu, WANG Min, PENG Zhengjun, JIA Guofeng, LI Yan. Study on adsorption of potassium ions in lithium chloride solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 61-66. |
| [6] | YANG Shuiyan, YANG Mingxia, XIN Wanwan. Study on preparation and properties of high⁃purity lithium 2-trifluoromethyl-4,5-dicyanoimidazole [J]. Inorganic Chemicals Industry, 2024, 56(2): 74-79. |
| [7] | DING Xiaojiang, WU Yanni, LI Boyun, HUANG Youliang. Research on preparation of carnallite concentrate by pretreatment combined with reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(12): 113-119. |
| [8] | WANG Yanfei, YANG Chaofan, CAO Di, XU Shijie. Study on solid-liquid phase equilibrium of ternary system of Li+, Na+∥Cl--H2O at different temperatures [J]. Inorganic Chemicals Industry, 2024, 56(1): 33-39. |
| [9] | LI Yan, MA Zhen, SONG Xingfu. New development advance and industrial development proposals of chloride-type salt lake potassium resources in Qinghai [J]. Inorganic Chemicals Industry, 2023, 55(8): 84-90. |
| [10] | GONG Xuemin, ZHANG Rongyu, LI Na, HAO Ya′nan, ZHANG Qian. Study on crystallization process of Na2CO3 hydrate in seawater system and its application [J]. Inorganic Chemicals Industry, 2023, 55(3): 55-59. |
| [11] | YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K [J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66. |
| [12] | YANG Hongjun, WANG Min, GE Haiwen, QIAO Youmin, QIAO Ziyang. Study on recycling process of potassium from calcium aluminate fly ash [J]. Inorganic Chemicals Industry, 2023, 55(10): 121-127. |
| [13] | JIN Yinghao, WANG Siyuan, YU Xuefeng, WANG Jinjing, WEI Zhengying, LIU Liwu, TIAN Haifeng, ZHA Fei. Recombination and application on mixed amine collector(cationic/anionic) for KCl flotation [J]. Inorganic Chemicals Industry, 2023, 55(10): 93-99. |
| [14] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [15] | DU Bingxuan,LI Haichao,LIN Zezhong. Study on preparation of food?grade potassium chloride by one?step adsorption and purification [J]. Inorganic Chemicals Industry, 2022, 54(9): 96-101. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||