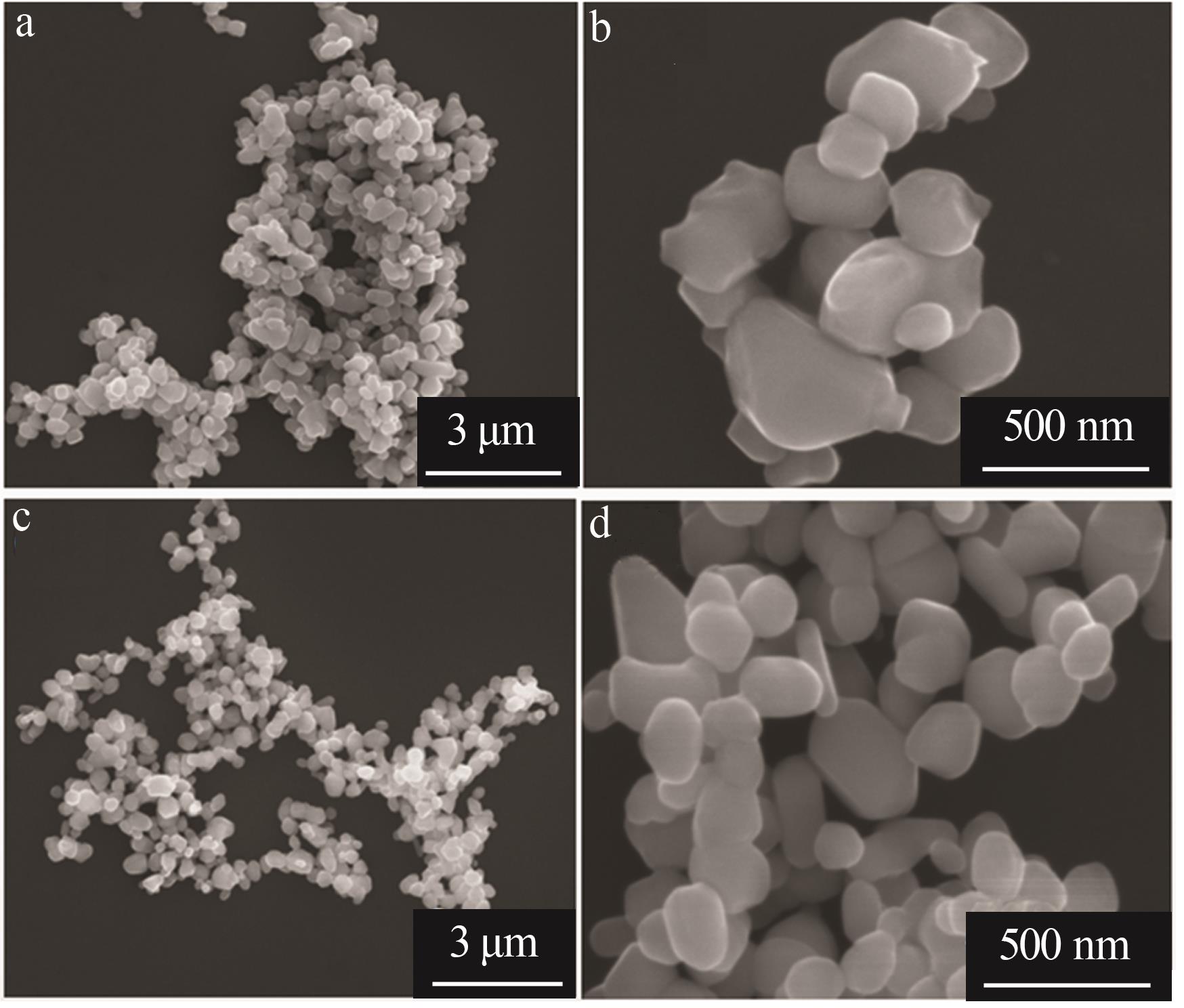
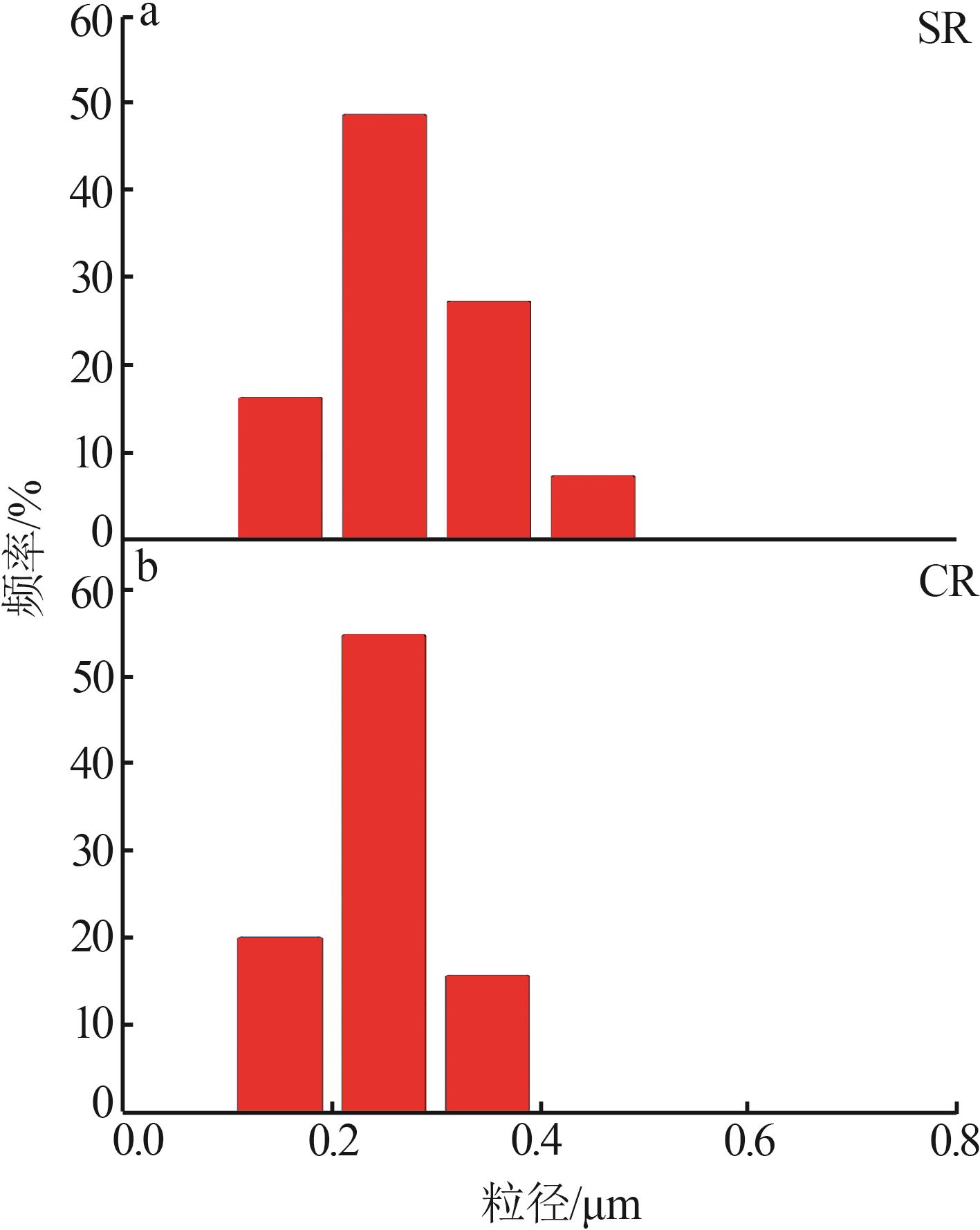
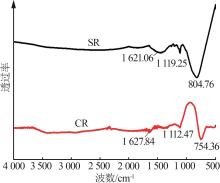
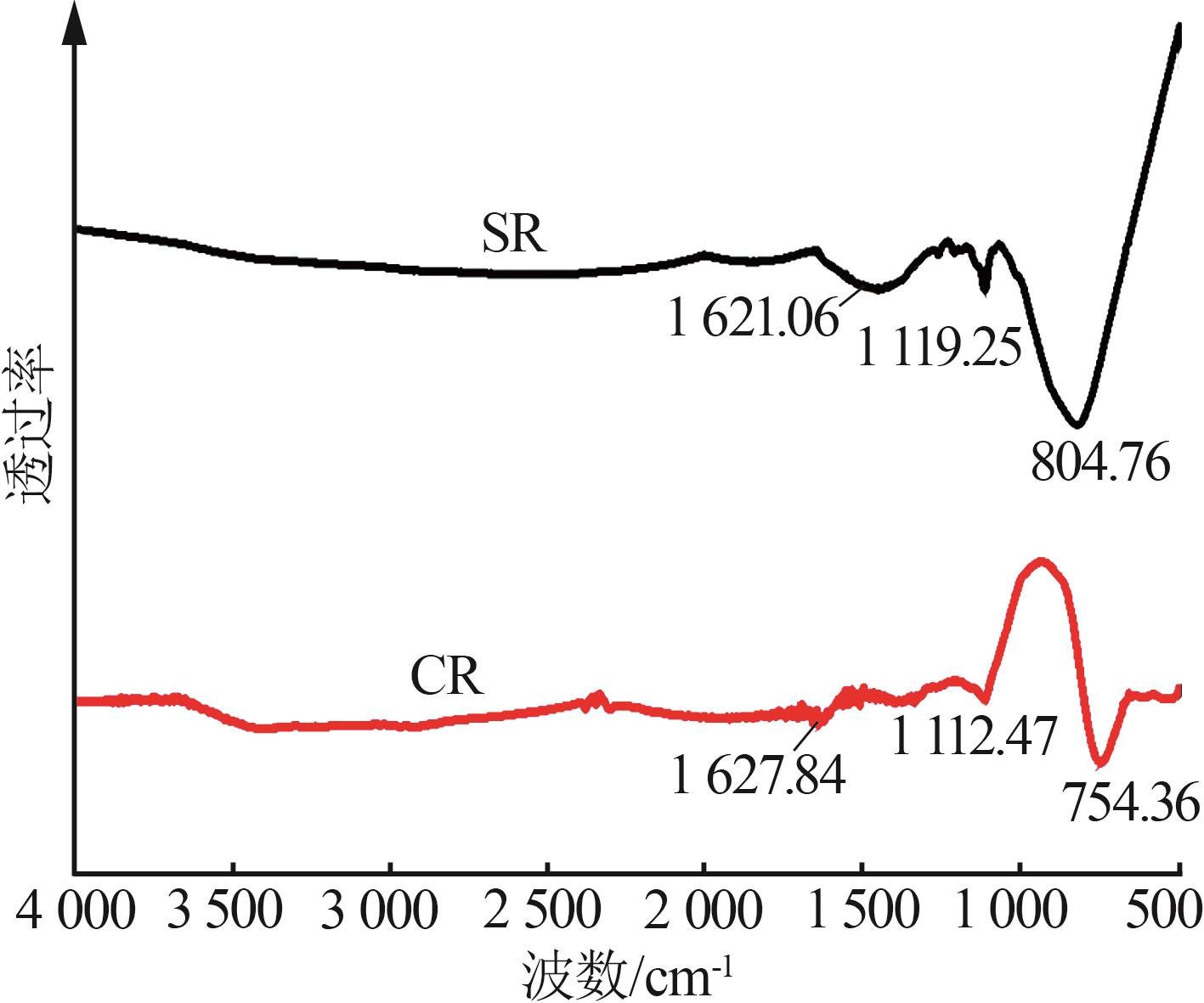
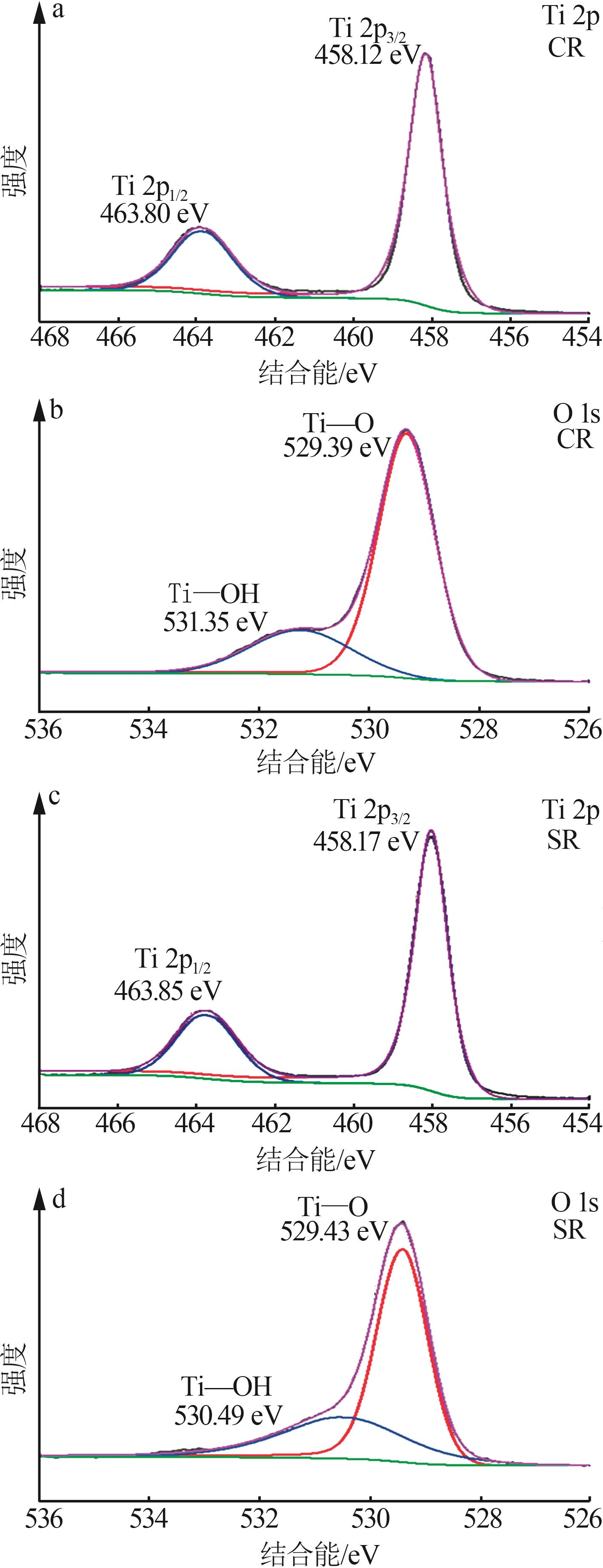
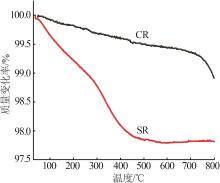
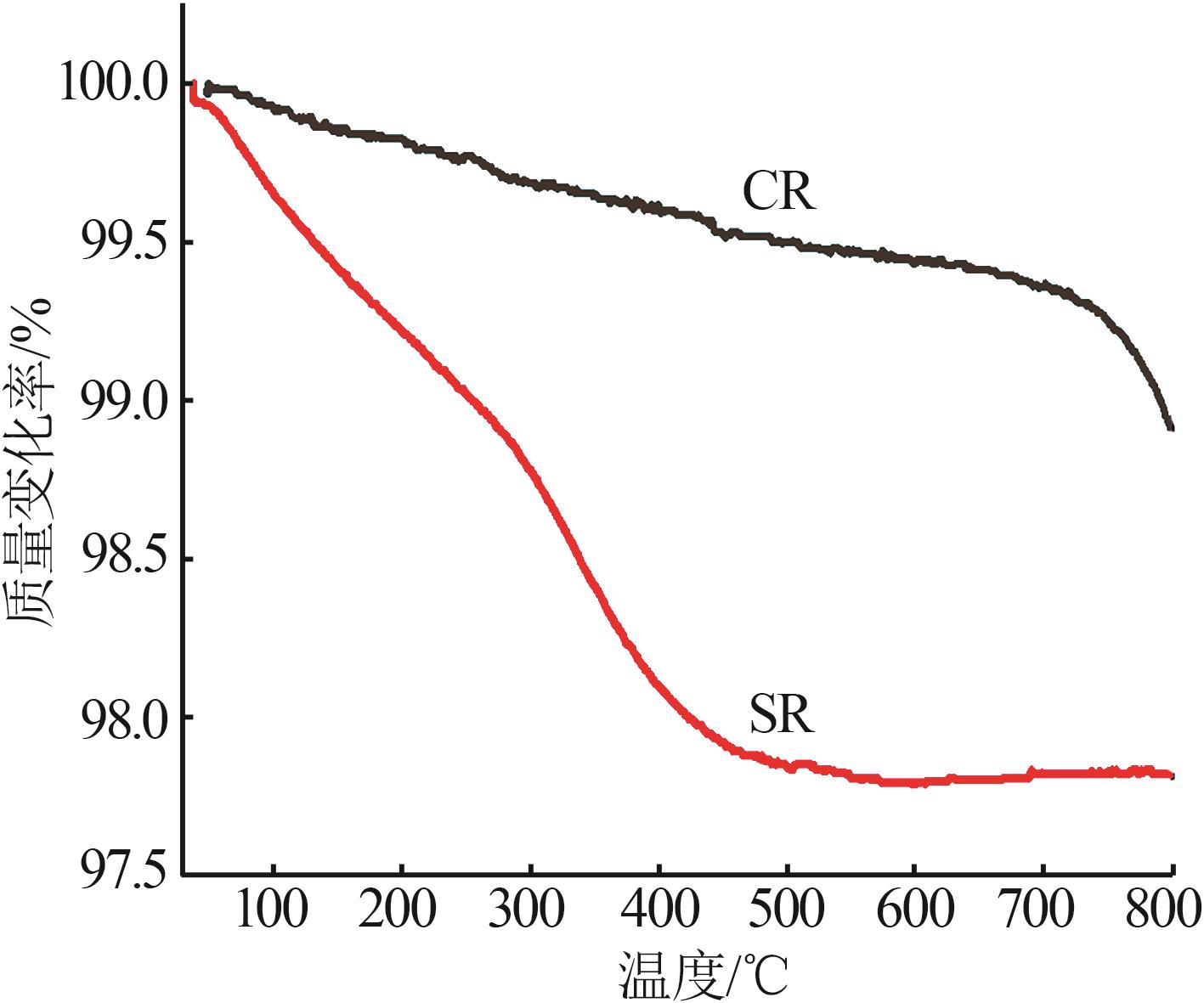
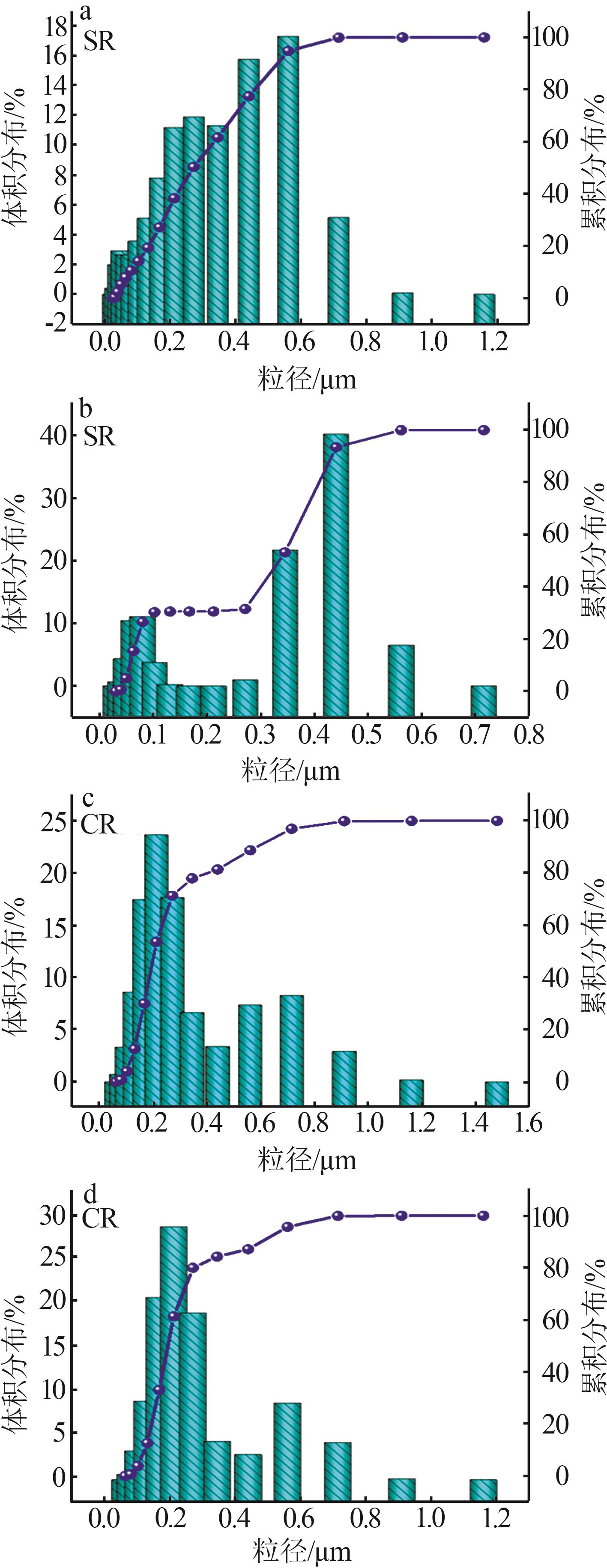
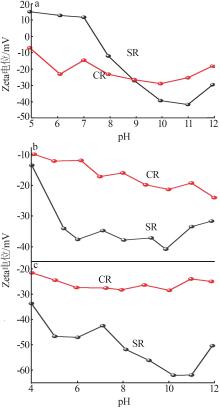
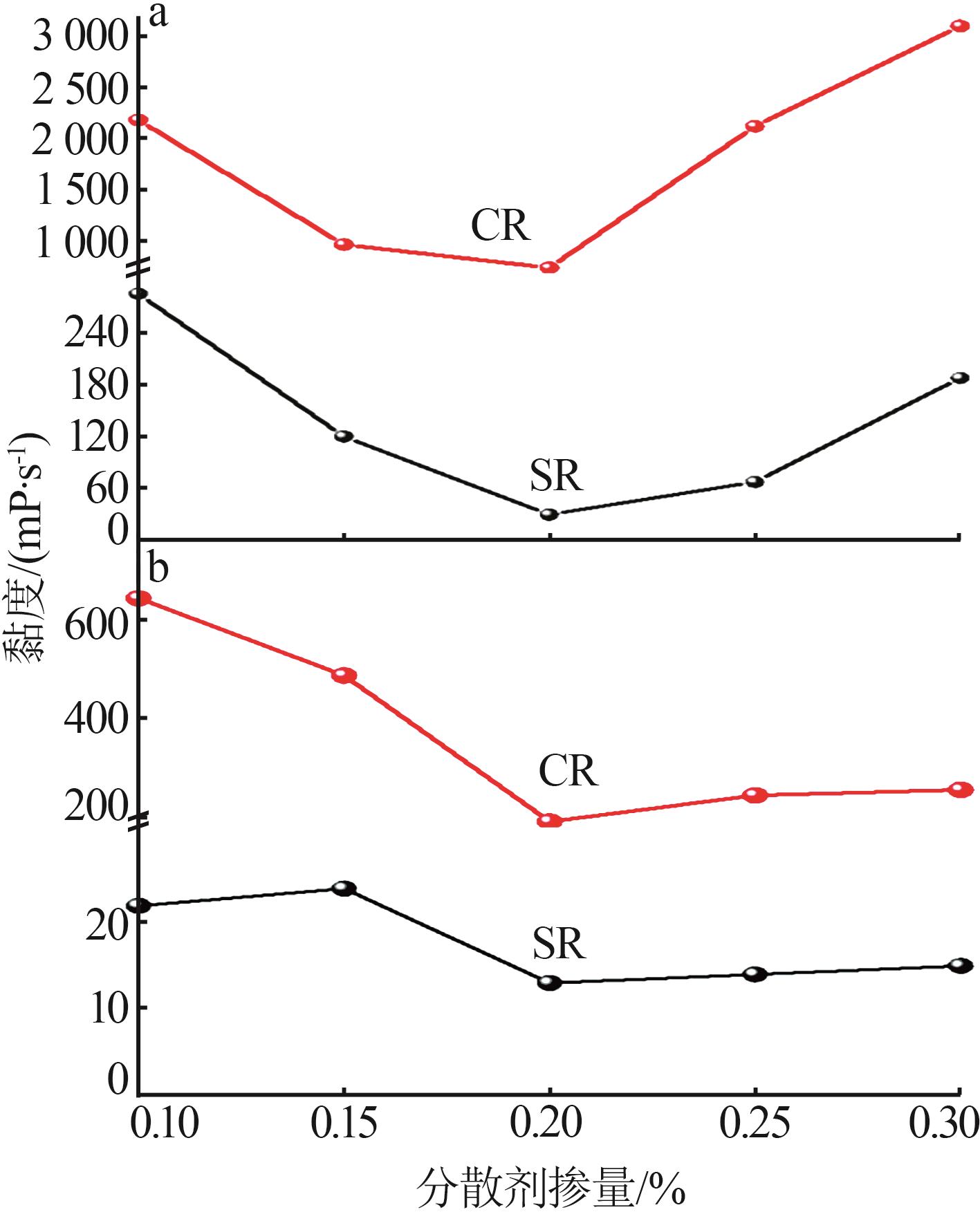
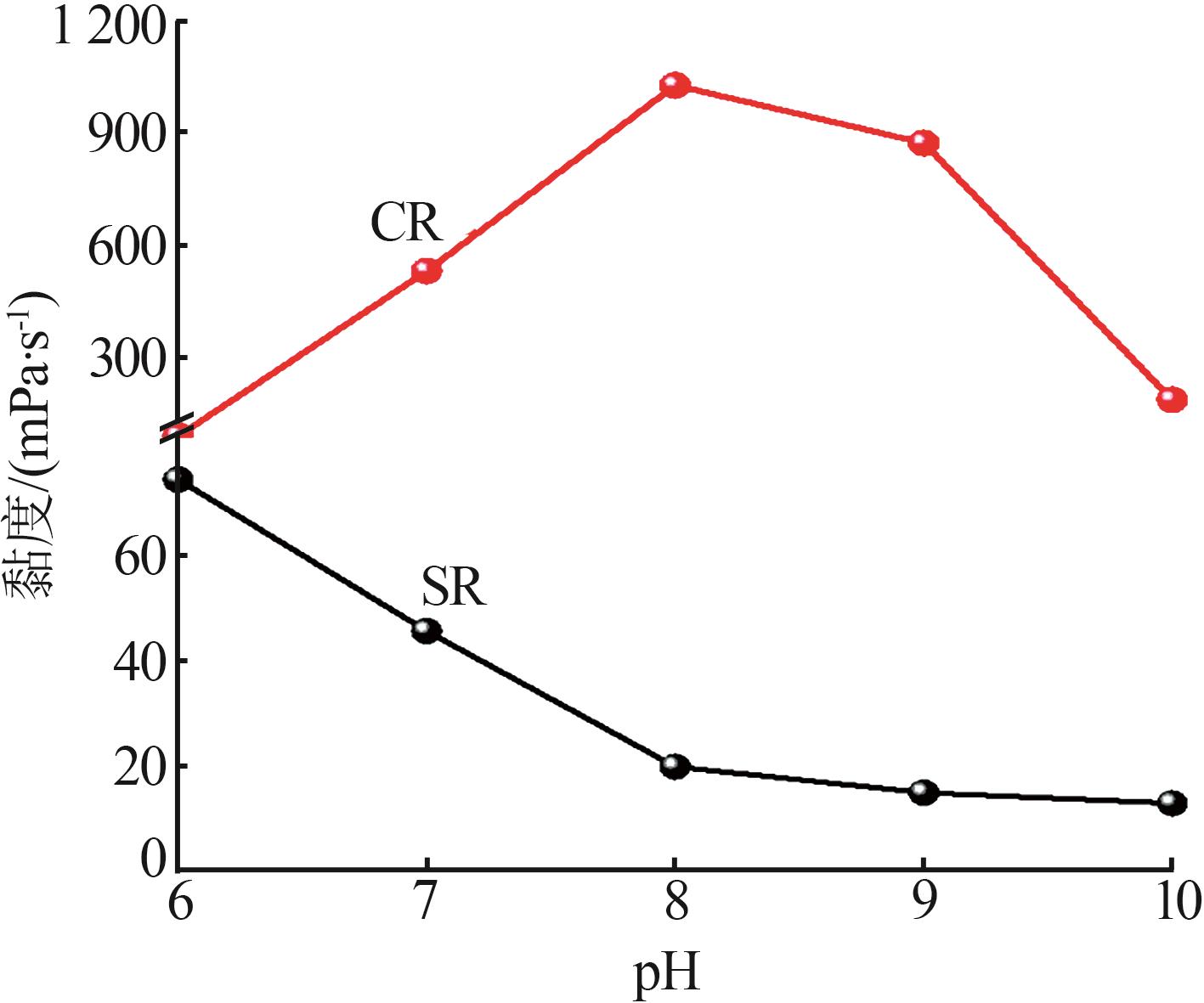
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (11): 123-131.doi: 10.19964/j.issn.1006-4990.2023-0596
• Research & Development • Previous Articles Next Articles
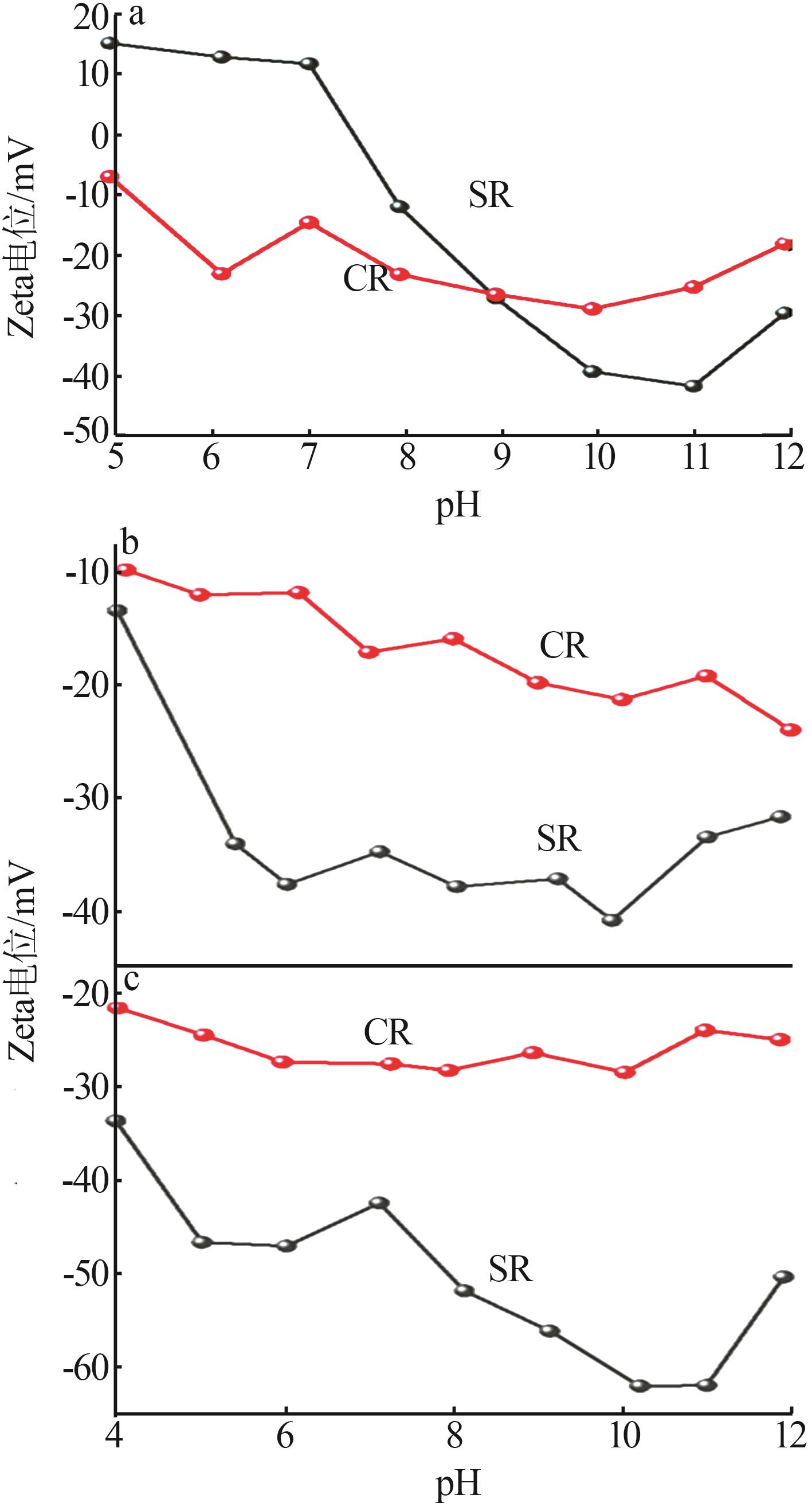
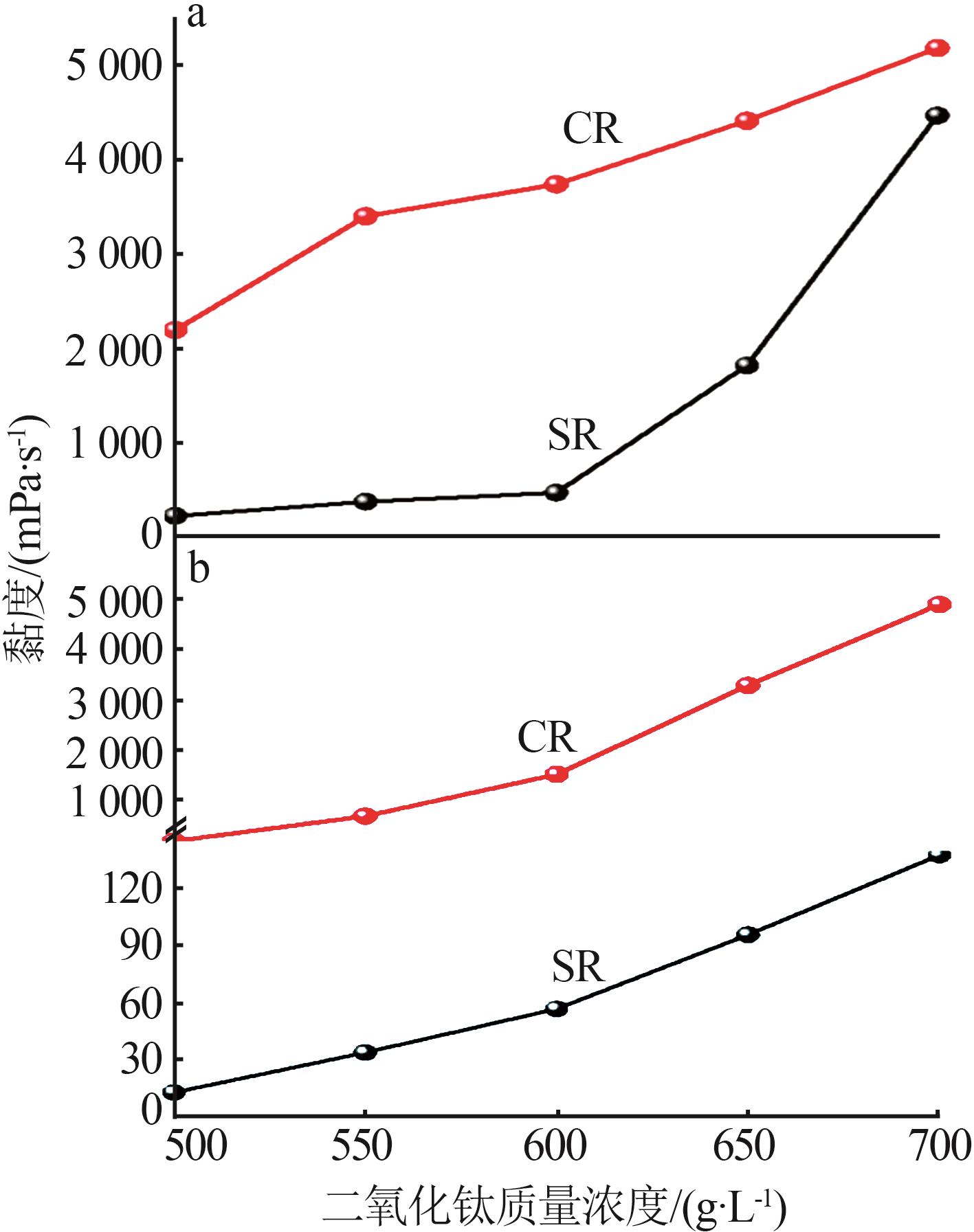
Study on surface characteristics and dispersion properties of titanium dioxide base particles by sulfate and chloride processes
QUAN Yuanxia1( ), QUAN Xuejun1, KE Lianghui2, LI Li1(
), QUAN Xuejun1, KE Lianghui2, LI Li1( )
)
- 1.College of Chemistry and Chemical Engineering,Chongqing University of Technology,Chongqing 400054,China
2.Pangang Group Vanadium & Titanium Resources Co. ,Ltd. ,Panzhihua 617000,China
-
Received:2023-12-12Online:2024-11-10Published:2024-01-26 -
Contact:LI Li E-mail:2407025687@qq.com;chenlili@cqut.edu.cn
CLC Number:
Cite this article
QUAN Yuanxia, QUAN Xuejun, KE Lianghui, LI Li. Study on surface characteristics and dispersion properties of titanium dioxide base particles by sulfate and chloride processes[J]. Inorganic Chemicals Industry, 2024, 56(11): 123-131.
share this article
| 1 | MATHAI S, SHAJI P S.Different coating methods of titanium dioxide on metal substrates for orthopedic and dental applications:A review[J].Asian Journal of Chemistry,2021,34(1):9-17. |
| 2 | LIANG Ying, HUANG Guohe, XIN Xiaying,et al.Black titanium dioxide nanomaterials for photocatalytic removal of pollutants:A review[J].Journal of Materials Science & Technology,2022,112:239-262. |
| 3 | GUAN Rengui, HE Zhijuan, LIU Shanshan,et al.A novel photoelectrochemical approach for efficient assessment of TiO2 pigments weatherability[J].Powder Technology,2021,380:334- 340. |
| 4 | VYBOISHCHIK A V, KOSTIUNINA I L.Contemporary methods of production and application of pigments obtained from titanium dioxide[J].IOP Conference Series:Materials Science and Engineering,2018,451:012004. |
| 5 | LUO Ke, YOON Y L, PARK H,et al.Effect of organic acids on the morphology and particle size of titanium dioxide(E171) in processed food[J].Journal of Hazardous Materials,2022,432:128666. |
| 6 | TIAN Congxue.Calcination intensity on rutile white pigment production via short sulfate process[J].Dyes and Pigments,2016,133:60-64. |
| 7 | TAYLOR M L, MORRIS G E, SMART R ST C.Influence of aluminum doping on titania pigment structural and dispersion properties[J].Journal of Colloid and Interface Science,2003,262(1):81-88. |
| 8 | 王海波,陈新红,吴小平,等.砂磨次数对钛白初品质量的影 响[J].钢铁钒钛,2017,38(3):34-37. |
| WANG Haibo, CHEN Xinhong, WU Xiaoping,et al.Effect of sand milling times on the quality of TiO2 initial product[J].Iron Steel Vanadium Titanium,2017,38(3):34-37. | |
| 9 | 陈新红,王荣凯,李礼.升温速率对金红石型二氧化钛颜料性能的影响研究[J].钢铁钒钛,2020,41(2):20-23,28. |
| CHEN Xinhong, WANG Rongkai, LI Li.Effects of heating rate on pigment properties of rutile TiO2 [J].Iron Steel Vanadium Titanium,2020,41(2):20-23,28. | |
| 10 | ŠTENGL V, BAKARDJIEVA S, ŠUBRT J,et al.Titania aerogel prepared by low temperature supercritical drying[J].Microporous and Mesoporous Materials,2006,91(1/2/3):1-6. |
| 11 | KHUSNUN N F, JALIL A A, ABDULLAH T A T,et al.Influence of TiO2 dispersion on silica support toward enhanced amine assisted CO2 photoconversion to methanol[J].Journal of CO2 Utilization,2022,58:101901. |
| 12 | ZHANG Yunsheng, YIN Hengbo, WANG Aili,et al.Deposition and characterization of binary Al2O3/SiO2 coating layers on the surfaces of rutile TiO2 and the pigmentary properties[J].Applied Surface Science,2010,257(4):1351-1360. |
| 13 | LIU Yumin, ZHANG Yunsheng, GE Chen,et al.Evolution mecha-nism of alumina coating layer on rutile TiO2 powders and the pigmentary properties[J].Applied Surface Science,2009,255(16):7427-7433. |
| 14 | LIU Jiaqi, ZHANG Fengmei, DOU Shengping,et al.Adsorption of serine at the anatase TiO2/water interface:A combined ATR-FT-IR and DFT study[J].The Science of the Total Environment,2022,807:150839. |
| 15 | ERDEM B, HUNSICKER R A, SIMMONS G W,et al.XPS and FT-IR surface characterization of TiO2 particles used in polymer encapsulation[J].Langmuir,2001,17(9):2664-2669. |
| 16 | MANSO M, VALADAS S, PESSANHA S,et al.Characterization of Japanese color sticks by energy dispersive X-ray fluorescence,X-ray diffraction and Fourier transform infrared analysis[J].Spectrochimica Acta Part B:Atomic Spectroscopy,2010,65(4):321-327. |
| 17 | KREBS F, HÖFFT O, ENDRES F.Investigations on the electrochemistry and reactivity of tantalum species in 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide using X-ray photoelectron spectroscopy(in situ and ex-situ XPS)[J].Applied Surface Science,2023,608:155130. |
| 18 | ROBERT B, FLAUD V, ESCALIER R,et al.XPS study of Ge-Se-Te surfaces functionalized with organosilanes[J].Applied Surface Science,2023,607:154921. |
| 19 | GONZÁLEZ V J, VÁZQUEZ E, VILLAJOS B,et al.Eco-friendly mechanochemical synthesis of titania-graphene nanocomposites for pesticide photodegradation[J].Separation and Purification Technology,2022,289:120638. |
| 20 | MA Dan, TAO E, YANG Shuyi.Efficient removal of Cu(Ⅱ) with graphene oxide-titanium dioxide/sodium alginate composite beads:Preparation,characterization,and adsorption mechanism[J].Journal of Environmental Chemical Engineering,2021,9(6):106501. |
| 21 | DONG Xiongbo, SUN Zhiming, JIANG Lei,et al.Investigation on the film-coating mechanism of alumina-coated rutile TiO2 and its dispersion stability[J].Advanced Powder Technology,2017, 28(8):1982-1988. |
| 22 | LAMPIMÄKI M, SCHREIBER S, ZELENAY V,et al.Exploring the environmental photochemistry on the TiO2(110) surface in situ by near ambient pressure X-ray photoelectron spectrosco-py[J].The Journal of Physical Chemistry C,2015,119(13):7076-7085. |
| 23 | ZHAO Jie, MILANOVA M, WARMOESKERKEN M M C G,et al.Surface modification of TiO2 nanoparticles with silane coupling agents[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2012,413:273-279. |
| 24 | JIN Xiaotong, ZHANG Tianqi, YUAN Kangkang,et al.Unveiling temperature-modified electrospun TiO2 nanofibers with size-driven microstructure evolution and enhanced photocatalytic effect[J].Optical Materials,2022,123:111840. |
| 25 | SAEED A M EL, FATTAH M A E, DARDIR M M.Synthesis and characterization of titanium oxide nanotubes and its performance in epoxy nanocomposite coating[J].Progress in Organic Coatings,2015,78:83-89. |
| 26 | DUAN Xiaoli, JIN Guoliang, ZHANG Luyao,et al.Insight into the pyrolysis of 3,7-dinitro-1,3,5,7-tetraazabicyclo[3,3,1]nonan(DPT) based on ReaxFF MD simulations and TG-FT-IR-MS techniques[J].Fuel,2023,331:125860. |
| 27 | BLAKE T D, FERNÁNDEZ-TOLEDANO J C, DE CONINCK J.A possible way to extract the dynamic contact angle at the molecular scale from that measured experimentally[J].Journal of Colloid and Interface Science,2023,629:660-669. |
| 28 | HUMINIC A, HUMINIC G, FLEACA C,et al.Influence of solid surface,temperature and concentration on contact angle of water-FeC nanofluid[J].International Communications in Heat and Mass Transfer,2021,128:105650. |
| 29 | TANAKA T, TAKAYANAGI T.Quantum reactive scattering calculations of H+F2 and Mu+F2 reactions on a new ab initio potential energy surface[J].Chemical Physics Letters,2010,496(4/5/6):248-253. |
| 30 | WEI Xiaoyu, JIANG Shidong, KLÖCKNER A,et al.An integral equation method for the Cahn-Hilliard equation in the wetting problem[J].Journal of Computational Physics,2020,419:109521. |
| 31 | COSTELLO M J, JOHNSEN S, GILLILAND K O,et al.Predicted light scattering from particles observed in human age-related nuclear cataracts using Mie scattering theory[J].Investigative Ophthalmology & Visual Science,2007,48(1):303-312. |
| 32 | PAZOKIFARD S, MIRABEDINI S M, ESFANDEH M,et al.Silane grafting of TiO2 nanoparticles:Dispersibility and photoactivity in aqueous solutions[J].Surface and Interface Analysis,2012, 44(1):41-47. |
| 33 | XU Q, ZHAO J, XU H,et al.Dispersion of TiO2 particles and preparation of SiO2 coating layers on the surfaces of TiO2 [J].Materials Research Innovations,2015,19(sup5):S5-142-S5-145.Doi:10.1179/1432891715Z.0000000001351. |
| 34 | MATHAN KUMAR P, PARAMASIVAM V, BEEMARAJ R K,et al.Investigate the characterization and synthesis process of titanium dioxide nanoparticles[J].Materials Today:Proceedings,2022,52:1140-1142. |
| 35 | PANDEY F P, SINGH S.Time resolved fluorescence and Raman properties,and zeta potential of zinc ferrite nanoparticles dispersed nematic liquid crystal 4′-heptyl-4-biphenylcarbonitrile (7CB)[J].Journal of Molecular Liquids,2020,315:113820. |
| 36 | FORTUNATO G, TENNICHE A, GOTTARDO L,et al.Development of poly-(ethylene terephthalate) masterbatches incorporating highly dispersed TiO2 nanoparticles:Investigation of morphologies by optical and rheological procedures[J].European Polymer Journal,2014,57:75-82. |
| 37 | KOSMULSKI M, MĄCZKA E.Zeta potential and particle size in dispersions of alumina in 50-50 w/w ethylene glycol-water mixture[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2022,654:130168. |
| [1] | XIANG Quanjin, QUAN Xuejun, LI Li, WANG Haibo, CHEN Xinhong, LI Ping. Formation rules and emission reduction method of sublimed sulfur in acidolysis exhaust gas of titanium concentrate [J]. Inorganic Chemicals Industry, 2024, 56(7): 96-103. |
| [2] | LI Huaquan, QIU Guibao, LÜ Xuewei. Research progress of titanium dioxide preparation technology [J]. Inorganic Chemicals Industry, 2024, 56(10): 20-27. |
| [3] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [4] | WEI Kang, ZHANG Hao, GAN Shunpeng. Research on mechanical drive enhancement crystallization process for titanium gypsum [J]. Inorganic Chemicals Industry, 2023, 55(11): 78-85. |
| [5] | HU Luoxing,HUANG Qimao,QU Hongyou. Preparation of Fe3O4 from FeCl2 by-product of new process of titanium dioxide by hydrochloric acid [J]. Inorganic Chemicals Industry, 2023, 55(1): 118-123. |
| [6] | ZHU Wanye,TANG Ding,CHI Heting,LIAO Xianghui,ZHUANG Rongchuan,WANG Qiankun,SHEN Qingfeng. Study on impurity removal rule of ferrous sulfate from by-product of titanium dioxide by crystallization purification [J]. Inorganic Chemicals Industry, 2022, 54(7): 105-109. |
| [7] | MA Lei,SHENG Yu,ZHOU Junhong,YANG Yujun,LUO Hui,PAN Chunying,WANG Guo. Study on new process of comprehensive utilization and separation of sulfur and calcium of titanium gypsum [J]. Inorganic Chemicals Industry, 2022, 54(7): 124-128. |
| [8] | TANG Shuyang,GUO Yufeng,ZHENG Fuqiang,CHEN Feng,WANG Shuai,YANG Lingzhi. Present situation and prospects of preparation methods of titanium dioxide [J]. Inorganic Chemicals Industry, 2022, 54(7): 27-34. |
| [9] | CHEN Feng,WEN Yuekai,GUO Yufeng,ZHENG Fuqiang,WANG Shuai,YANG Lingzhi,ZHENG Yu,LI Dongyue,REN Yuqiao. Research status of viscosity characteristics of chlorinated molten salt system [J]. Inorganic Chemicals Industry, 2022, 54(6): 1-5. |
| [10] | ZHANG Jingchun,LIAO Xianyan,MENG Xue,LUO Teng,HUI Zeyou,HOU Junwei. Study on effect of alkali on performance of chemical flooding system of wastewater [J]. Inorganic Chemicals Industry, 2022, 54(2): 101-105. |
| [11] | CHEN Wenting,LIU Guangsheng,CHEN Xiaorong,LI Zhen,ZENG Minli,WANG Nannan. Study on tribological properties of inorganic fullerene-like tungsten disulfide as lubricant additive of PAO6 [J]. Inorganic Chemicals Industry, 2022, 54(1): 45-50. |
| [12] | Hou Yifei,Bai Ni,Sun Jian,Jiao Lina,Ju Dianchun. Effects of ZrO2 on the physicochemical properties of NaCl-KCl molten salt system for the purification of crude ZrCl4 [J]. Inorganic Chemicals Industry, 2021, 53(7): 63-67. |
| [13] | Chen Wen,Long Xiang,Jin Yaqiu,Mao Duan. Determination of major component in inorganic coated titanium pigment by X-ray fluorescence spectrometry with pressed powder [J]. Inorganic Chemicals Industry, 2021, 53(5): 93-95. |
| [14] | Shu Yirui,Zhang Pan,Wang Wei,Xiang Hengli,Ren Genkuan,Xu Dehua,Zhang Zhiye,Yang Xiushan. Titanium white by-product ferrous sulfate photofenton oxidation degradation of methyl orange in wastewater [J]. Inorganic Chemicals Industry, 2021, 53(3): 68-72. |
| [15] | Wu Yali,Wu Yihan,Zhang Ruimin,Shi Xiaoyu,Dong Xin. Preparation and formation mechanism of hollow barium titanate [J]. Inorganic Chemicals Industry, 2021, 53(2): 51-54. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||