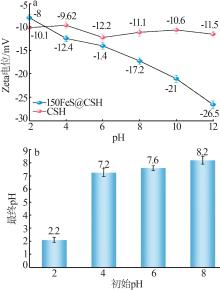
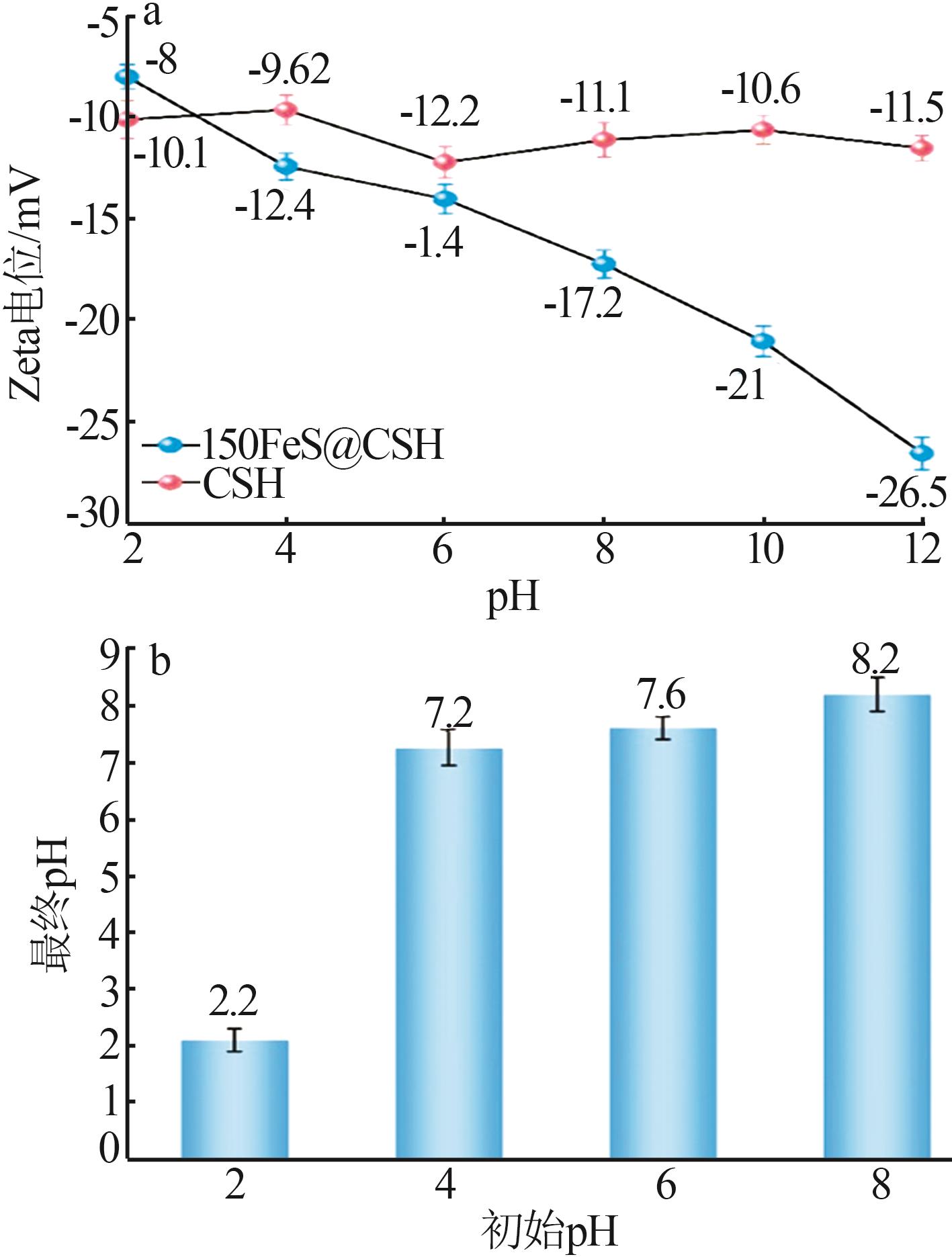
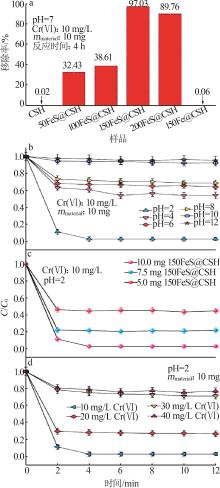
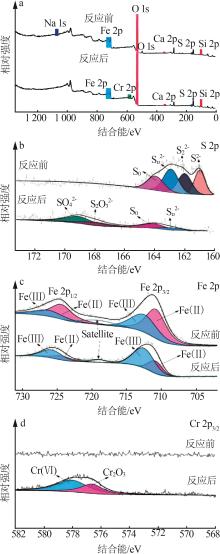
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (2): 57-67.doi: 10.19964/j.issn.1006-4990.2024-0407
• Research & Development • Previous Articles Next Articles
Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance
SHEN Xiaoqian1( ), ZHOU Fei2, LIU Wanchen2, XU Lu1, WU Junshu2
), ZHOU Fei2, LIU Wanchen2, XU Lu1, WU Junshu2
- 1.Hualu Engineering & Technology Co. ,Ltd. ,Xi′an 710065,China
2.Beijing University of Technology,Beijing 100124,China
-
Received:2024-07-15Online:2025-02-10Published:2024-08-09
CLC Number:
Cite this article
SHEN Xiaoqian, ZHOU Fei, LIU Wanchen, XU Lu, WU Junshu. Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance[J]. Inorganic Chemicals Industry, 2025, 57(2): 57-67.
share this article
Table 3
Performance comparison between this work and as-reported literatures"
| 材料 | 反应条件 | Cr(Ⅵ)还原去除率 | 总Cr移除率/% | 附加 条件 |
|---|---|---|---|---|
| Leonardite[ | [material]=0.25 g/L, [Cr(Ⅵ)]=10 mg/L,pH=2 | 100%, 2 h | 100 | 光辐照 |
| MXene[ | [material]=1.5 g/L, [Cr(Ⅵ)]=5 mg/L,pH=4 | 100%, 3 h | 100 | 光辐照 |
ZIF-8负载 的TiO2[ | [material]=1 g/L, [Cr(Ⅵ)]=20 mg/L,pH=7 | 93.1%, 2 h | — | 光辐照 |
酸改性 g-C3N4[ | [material]=1 g/L, [Cr(Ⅵ)]=40 mg/L,pH=2 | 99.7%, 1.25 h | — | 光辐照 |
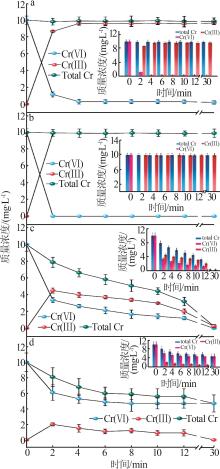
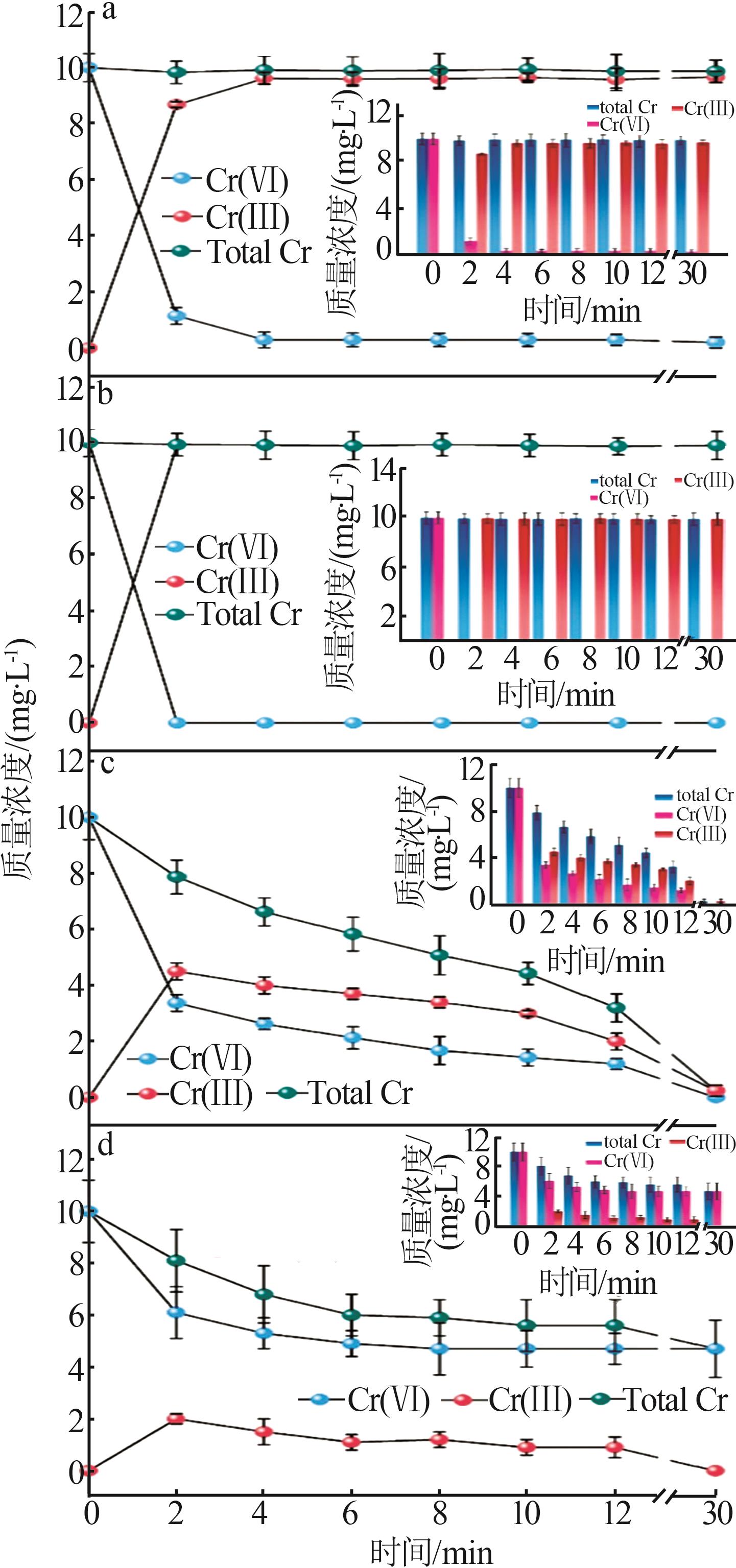
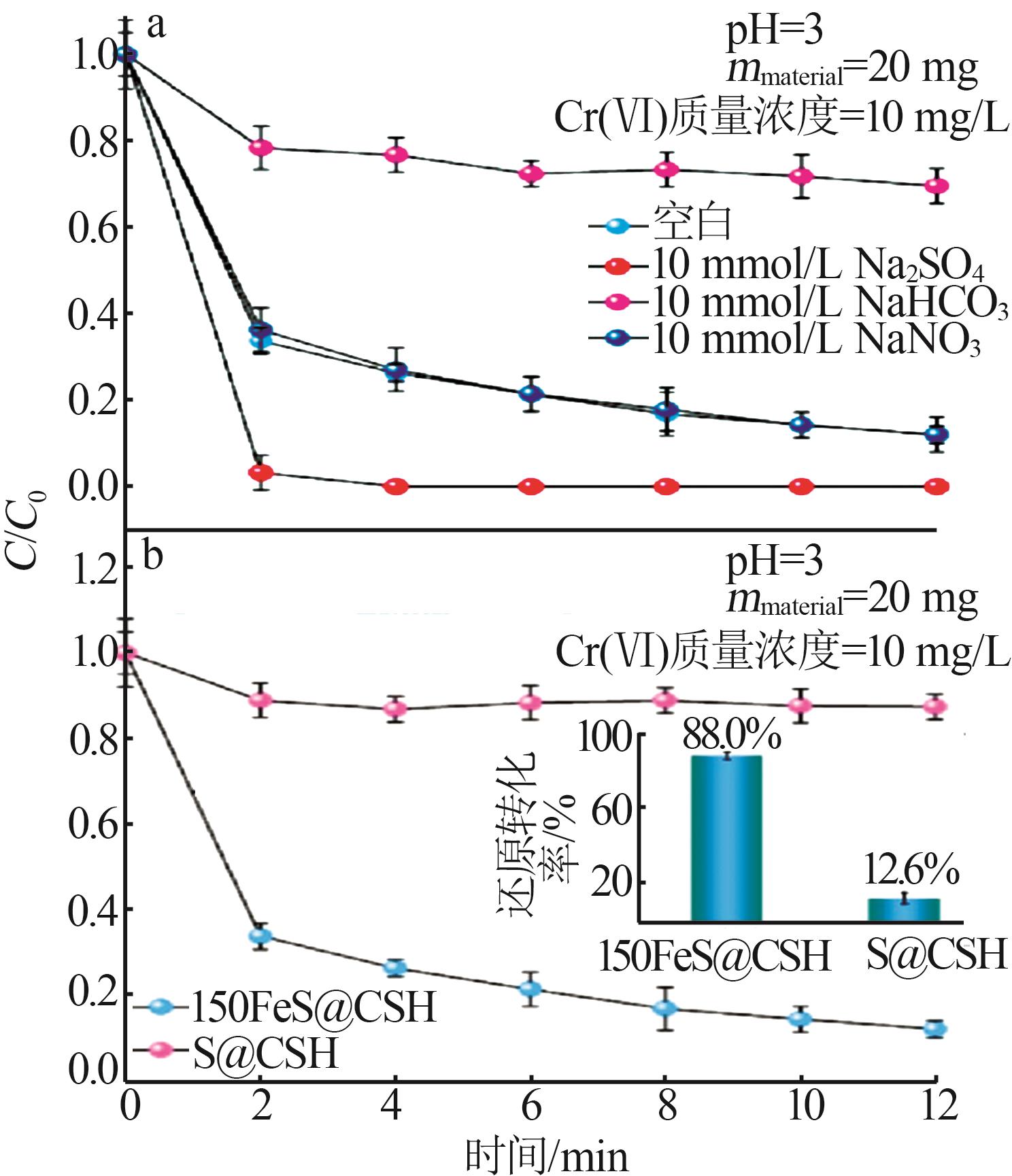
| FeS负载的CSH,本工作 | [material]=0.17 g/L, [Cr(Ⅵ)]=10 mg/L,pH=3 | 100%, 0.5 h | 100 | 无光辐照 |
| 1 | JAFARI A J, GOLBAZ S, KALANTARY R R.Treatment of hexavalent chromium by using a combined Fenton and chemical precipitation process[J].Journal of Water Reuse and Desalination,2013,3(4):373-380. |
| 2 | GOGOI S, CHAKRABORTY S, DUTTA SAIKIA M.Surface modified pineapple crown leaf for adsorption of Cr(Ⅵ) and Cr(Ⅲ) ions from aqueous solution[J].Journal of Environmental Chemical Engineering,2018,6(2):2492-2501. |
| 3 | AZARUDEEN R S, SUBHA R, JEYAKUMAR D,et al.Batch separation studies for the removal of heavy metal ions using a chelating terpolymer:Synthesis,characterization and isotherm models[J].Separation and Purification Technology,2013,116:366-377. |
| 4 | XU Qiupeng, LI Ruizhen, WANG Chengduan,et al.Visible-light photocatalytic reduction of Cr(Ⅵ) using nano-sized delafossite (CuFeO2) synthesized by hydrothermal method[J].Journal of Alloys and Compounds,2017,723:441-447. |
| 5 | GAO Jie, SUN Shipeng, ZHU Wenping,et al.Chelating polymer modified P84 nanofiltration(NF) hollow fiber membranes for high efficient heavy metal removal[J].Water Research,2014,63:252-261. |
| 6 | WANG Qi, WANG Longyang, ZHENG Shuzhen,et al.The strong interaction and confinement effect of Ag@NH2-MIL-88B for improving the conversion and durability of photocatalytic Cr(Ⅵ) reduction in the presence of a hole scavenger[J].Journal of Hazardous Materials,2023,451:131149. |
| 7 | FU Yangjie, TAN Meng, GUO Zhonglu,et al.Fabrication of wide-spectra-responsive NA/NH2-MIL-125(Ti) with boosted activity for Cr(Ⅵ) reduction and antibacterial effects[J].Chemical Engineering Journal,2023,452:139417. |
| 8 | DU Hao, LI Ningyi, YANG Lingxuan,et al.Plasmonic Ag modified Ag3VO4/AgPMo S-scheme heterojunction photocatalyst for boosted Cr(Ⅵ) reduction under visible light:Performance and mechanism[J].Separation and Purification Technology,2023,304:122204. |
| 9 | 刘新,冯攀,沈叙言,等.水泥水化产物——水化硅酸钙(C-S-H)的研究进展[J].材料导报,2021,35(9):9157-9167. |
| LIU Xin, FENG Pan, SHEN Xuyan,et al.Advances in the understanding of cement hydrate-hydrate calcium silicate(C-S-H)[J].Materials Reports,2021,35(9):9157-9167. | |
| 10 | LE Q T N, VIVAS E L, CHO K.Calcium oxalate/calcium silicate hydrate(Ca-O x /C-S-H) from blast furnace slag for the highly efficient removal of Pb2+ and Cd2+ from water[J].Journal of Environmental Chemical Engineering,2021,9(5):106287. |
| 11 | LI Y, LI S, PAN X,et al.Eco-utilization of steel slag Preparation of Fe-based calcium silicate hydrate and its application in As(V) removal[J].Applied Surface Science,2022,597:153763. |
| 12 | LIU Shijie, CUI Suping, GUO Hongxia,et al.Adsorption of lead ion from wastewater using non-crystal hydrated calcium silicate gel[J].Materials,2021,14(4):842. |
| 13 | SUN Lingmin, WU Junshu, WANG Jinshu,et al.CO2-assisted ‘weathering’ of steel slag-derived calcium silicate hydrate:A generalized strategy for recycling noble metals and constructing SiO2-based nanocomposites[J].Journal of Colloid and Interface Science,2022,622:1008-1019. |
| 14 | XU Huichao, REN Liming, QIN Chuanyu,et al.New insights on zero-valent iron permeable reactive barrier for Cr(Ⅵ) removal:The function of FeS reaction zone downstream in-situ generated by sulfate-reducing bacteria[J].Journal of Hazardous Materials,2024,480:136282. |
| 15 | GONG Yanyan, GAI Longshuang, TANG Jingchun,et al.Reduction of Cr(Ⅵ) in simulated groundwater by FeS-coated iron magnetic nanoparticles[J].Science of the Total Environment,2017,595:743-751. |
| 16 | YANG Yan, ZHANG Yuhao, WANG Guiyin,et al.Adsorption and reduction of Cr(Ⅵ) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution[J].Journal of Environmental Chemical Engineering,2021,9(4):105407. |
| 17 | SAHOO S K, PANIGRAHI G K, SAHU M K,et al.Biological synthesis of GO-MgO nanomaterial using Azadirachta indica leaf extract:A potential bio-adsorbent for removing Cr(Ⅵ) ions from aqueous media[J].Biochemical Engineering Journal,2022,177:108272. |
| 18 | YU Yangyang, CHENG Qianwen, SHA Chong,et al.Size-controlled biosynthesis of FeS nanoparticles for efficient removal of aqueous Cr(Ⅵ)[J].Chemical Engineering Journal,2020,379:122404. |
| 19 | LV Dan, ZHOU Jiasheng, CAO Zhen,et al.Mechanism and influence factors of chromium(Ⅵ) removal by sulfide-modified nanoscale zerovalent iron[J].Chemosphere,2019,224:306-315. |
| 20 | FU Saiou, DI Junzhen, GUO Xuying,et al.Preparation of lignite-loaded nano-FeS and its performance for treating acid Cr(Ⅵ)-containing wastewater[J].Environmental Science and Pollution Research,2023,30(2):3351-3366. |
| 21 | LI Meng, TANG Chunxia, FU Shaohai,et al.Cellulose-based aerogel beads for efficient adsorption-reduction-sequestration of Cr(Ⅵ)[J].International Journal of Biological Macromolecules,2022,216:860-870. |
| 22 | ZENG Qiang, HUANG Yongji, WANG Haibei,et al.A novel composite of almandine supported humboldtine nanospheres,in situ synthesized from natural almandine,possesses high removal efficiency of Cr(Ⅵ) over a wide pH range[J].Journal of Hazardous Materials,2020,383:121199. |
| 23 | ARSLAN H, ESKIKAYA O, BILICI Z,et al.Comparison of Cr(Ⅵ) adsorption and photocatalytic reduction efficiency using leonardite powder[J].Chemosphere,2022,300:134492. |
| 24 | JAMALUDDIN N S, ALIAS N H, SAMITSU S,et al.Efficient chromium(Ⅵ) removal from wastewater by adsorption-assisted photocatalysis using MXene[J].Journal of Environmental Chemical Engineering,2022,10(6):108665. |
| 25 | SONG Yu, LU Xi, LIU Zhibao,et al.Efficient removal of Cr(Ⅵ) by TiO2 based micro-nano reactor via the synergy of adsorption and photocatalysis[J].Nanomaterials,2022,12(2):291. |
| 26 | SUN Huimin, WANG Le, LIU Yue,et al.Photocatalytic reduction of Cr(Ⅵ) via surface modified g-C3N4 by acid-base regulation[J].Journal of Environmental Management,2022,324:116431. |
| 27 | SETSHEDI K Z, BHAUMIK M, SONGWANE S,et al.Exfoliated polypyrrole-organically modified montmorillonite clay nanocomposite as a potential adsorbent for Cr(Ⅵ) removal[J].Chemical Engineering Journal,2013,222:186-197. |
| 28 | XU Meng, WU Junshu, WANG Jinshu,et al.Covalent organic framework modified vermiculite for total Cr removal and subsequent recycling for efficient ciprofloxacin and NO photooxidation[J].Journal of Colloid and Interface Science,2023,652:218- 230. |
| 29 | SASS B M,RAI D.Solubility of amorphous chromium(Ⅲ)-iron(Ⅲ) hydroxide solid solutions[J].Inorganic Chemistry,1987,26(14):2228-2232. |
| 30 | LUO Hao, FU Fenglian, TANG Bing.Ferrous sulfide supported on modified diatomite for the removal of Cr(Ⅵ):Performance and mechanism[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2023,670:131538. |
| [1] | WANG Minrui, TIAN Guiying, ZHANG Ao, GE Junjie, ZHANG Lei, XIANG Jun, TANG Na. Study on granulation optimization for Al-based lithium adsorbent and its lithium recovery performance from brine [J]. Inorganic Chemicals Industry, 2025, 57(3): 36-42. |
| [2] | PEI Xiaogang, ZHANG Peng, DONG Shanshan, GE Shanshan, ZHAO Yuelong. Hydroxyapatite composite humic acid and its removal of Mn(Ⅱ) [J]. Inorganic Chemicals Industry, 2024, 56(5): 70-77. |
| [3] | ZHAO Chuang, CHEN Zihao, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation performance of molecular sieve adsorbents for different types of diesel [J]. Inorganic Chemicals Industry, 2024, 56(3): 80-85. |
| [4] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [5] | LIU Fujie, HE Qian, SU Long, JIANG Caiyun. Adsorption properties of methylene blue by surface functionalized magnetic biochar with sodium alginate [J]. Inorganic Chemicals Industry, 2024, 56(2): 65-73. |
| [6] | WANG Ruirui, ZHU Chaoliang, MU Bing, MA Wanxia, FAN Jie, XU Guowang, SHI Yifei, DENG Xiaochuan, QING Binju. Preparation of cubic manganese carbonate by hydrothermal method and its application in extraction of lithium [J]. Inorganic Chemicals Industry, 2024, 56(12): 94-103. |
| [7] | WANG Hangyu, DU Yifa, GUO Xia, NA Yuxuan, WAN Macuo, ZHOU Yongquan. Study on preparation of NiCo Prussian blue analogue hollow nanobubbles and their Cs+ adsorption properties [J]. Inorganic Chemicals Industry, 2024, 56(10): 55-63. |
| [8] | WANG Mengdi, LUO Jin, WU Wei, ZHOU Jinghui, WANG Jing, SUN Yanmin, YU Haibin. Study on preparation of γ-Al2O3 by microreaction and its properties for methyl orange adsorption [J]. Inorganic Chemicals Industry, 2023, 55(9): 66-74. |
| [9] | GUI Changqing, WANG Yajing, LING Changjian, WANG Huaiyou, TANG Zhongfeng. Research progress of preparation and modification of MgO-based CO2 adsorbents [J]. Inorganic Chemicals Industry, 2023, 55(8): 77-83. |
| [10] | GAO Fei, DONG Liangfei, GE Yulong, TANG Yuanyuan, LI Fen. Study on preparation of sludge biochar/attapulgite and its adsorption properties [J]. Inorganic Chemicals Industry, 2023, 55(5): 91-99. |
| [11] | CUI Xiangmei, PAN Tongtong, LUO Qinglong, BIAN Fuxuan, YE Xiushen. Preparation of amino alcohol modified GO/CNTs composite aerogel and boron adsorption from salt lake brines [J]. Inorganic Chemicals Industry, 2023, 55(12): 59-65. |
| [12] | JI Ying, ZHANG Ying, HOU Xuechao, ZHU Xiaofeng, JIANG Run, PENG Wenjuan, SUN Guangdong, LÜ Long. Application research of titanium adsorbent of carbonate-type salt lake in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 70-77. |
| [13] | LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao. Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92. |
| [14] | CHEN Jun,ZHONG Jing,LIN Sen,YU Jianguo. Study on lithium separation from brine by aluminum-based adsorbent in fixed bed [J]. Inorganic Chemicals Industry, 2023, 55(1): 64-73. |
| [15] | ZHANG Xiaoya,LI Jiali,FENG Lijuan,WANG Ji. Research progress on preparation technology and application of municipal sludge ceramsite [J]. Inorganic Chemicals Industry, 2022, 54(9): 28-38. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||