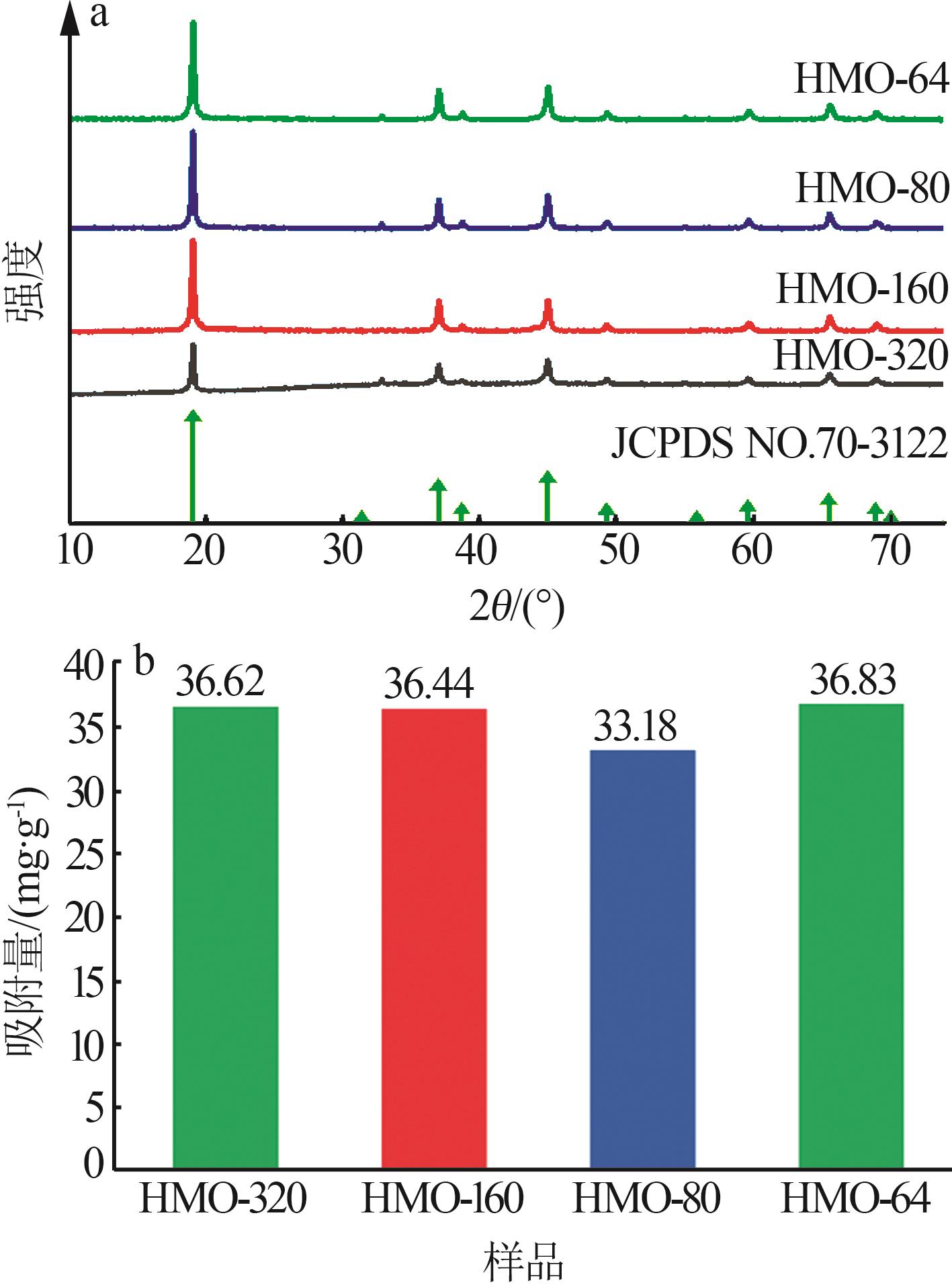
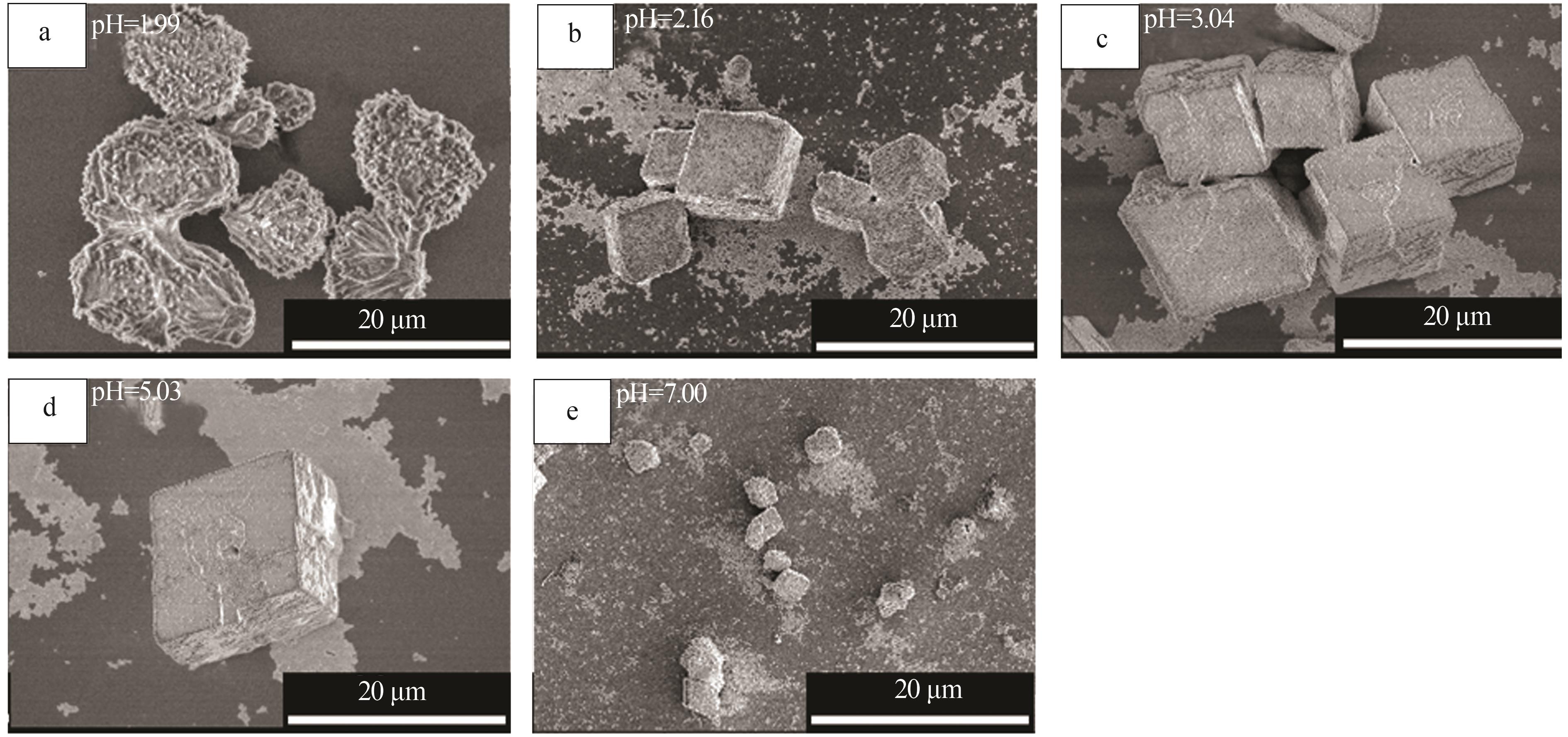
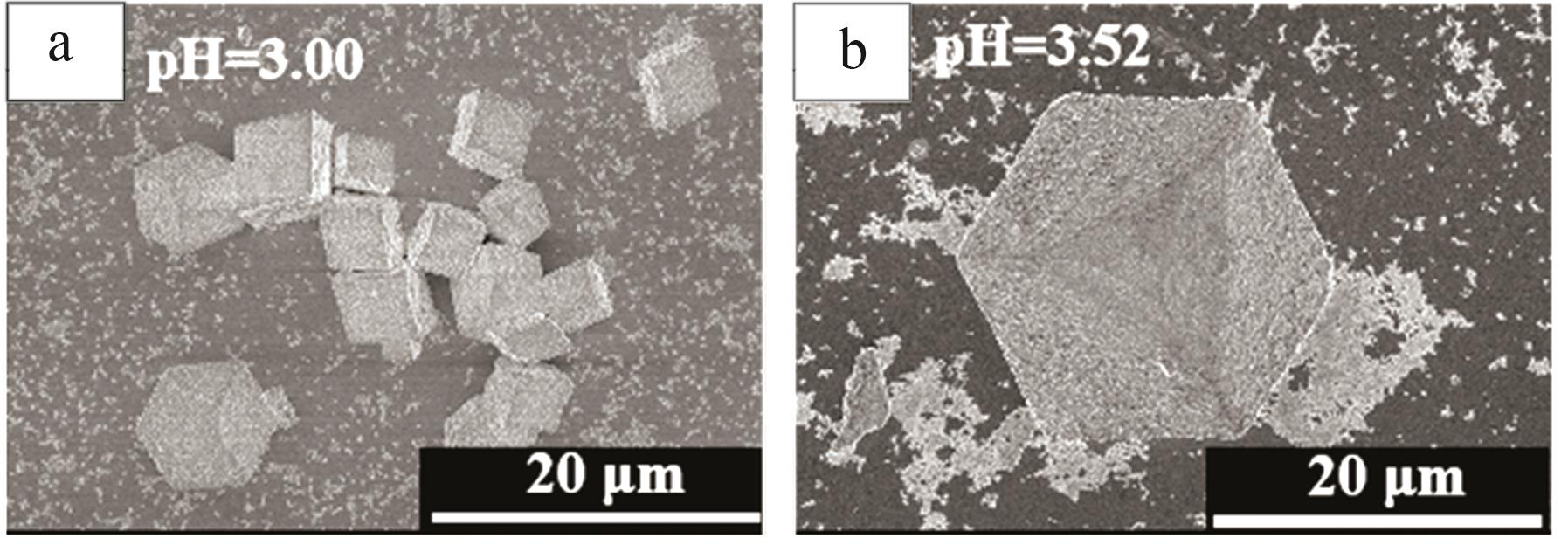
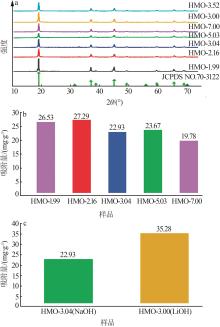
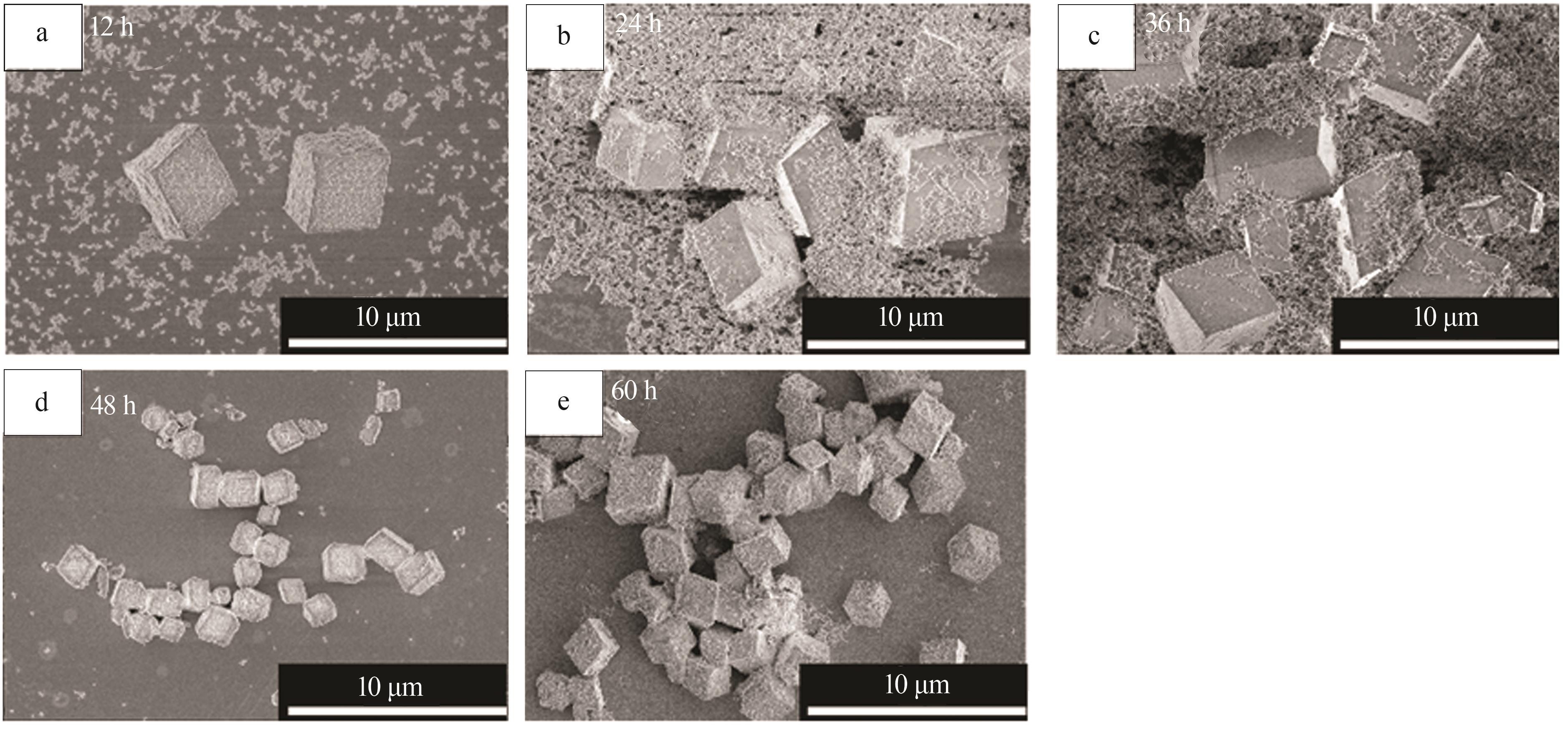
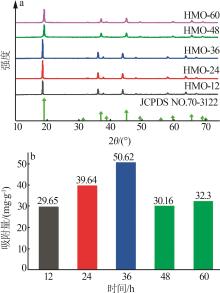
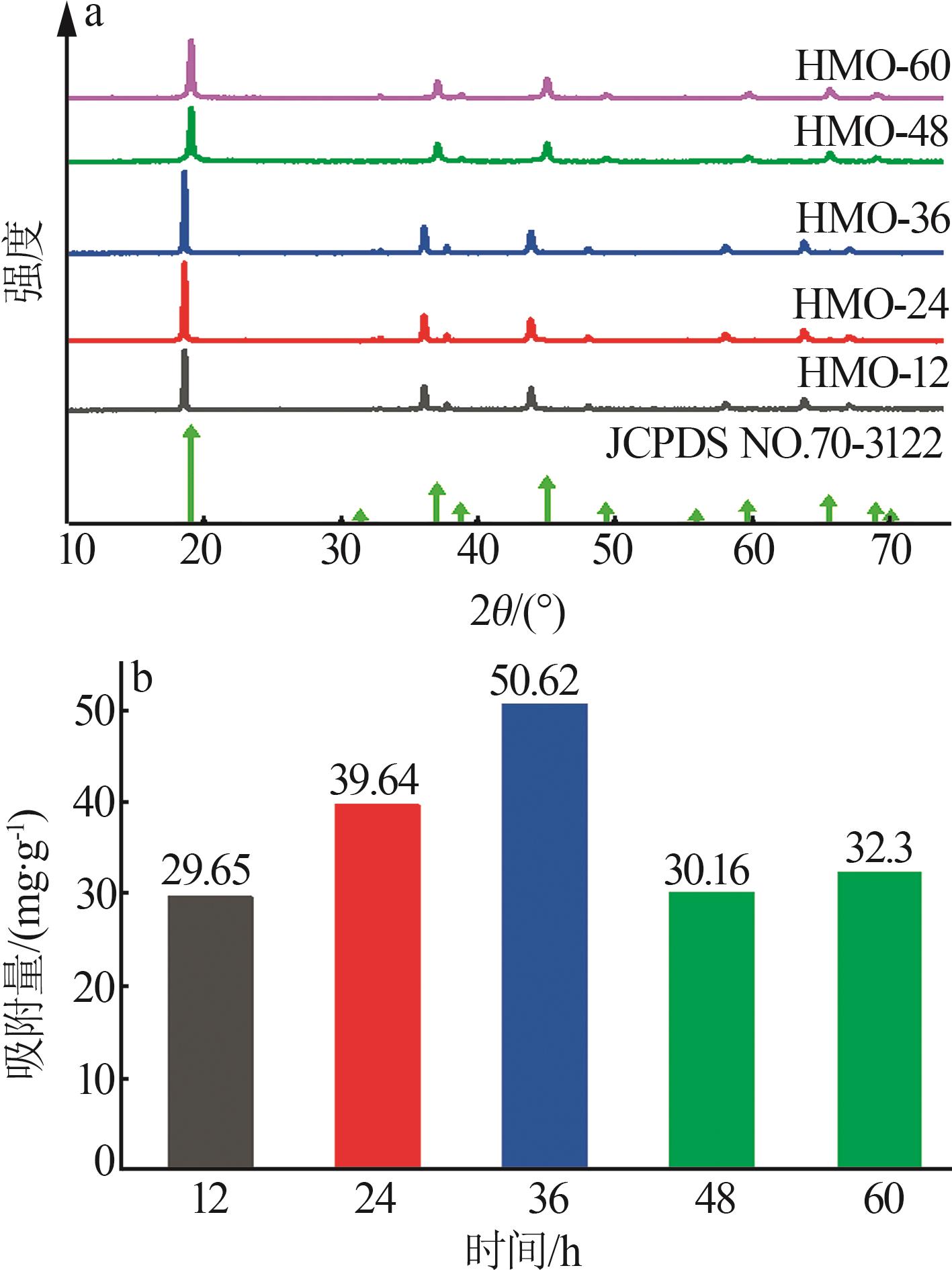
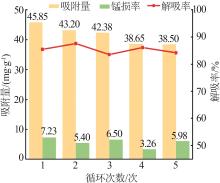
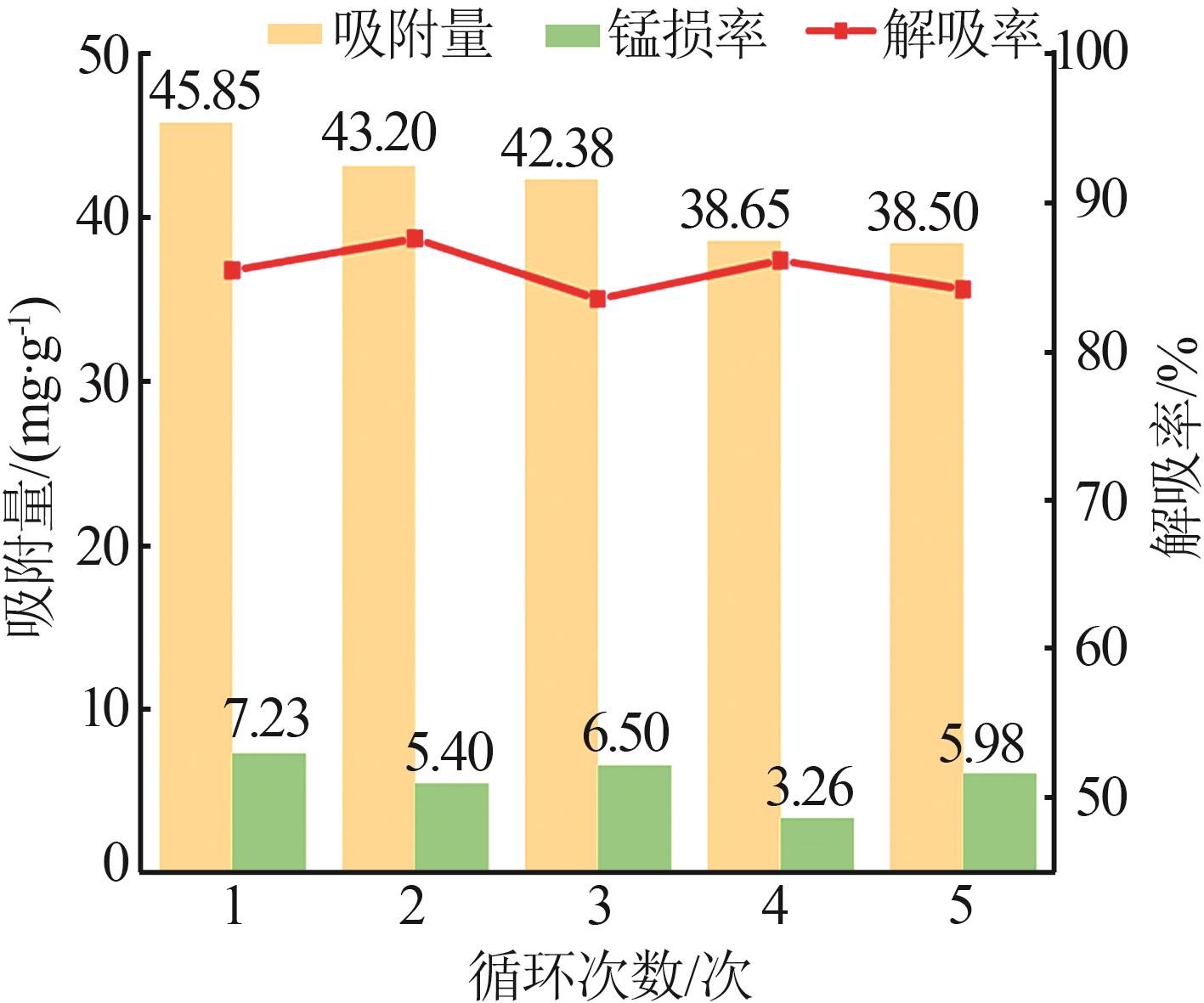
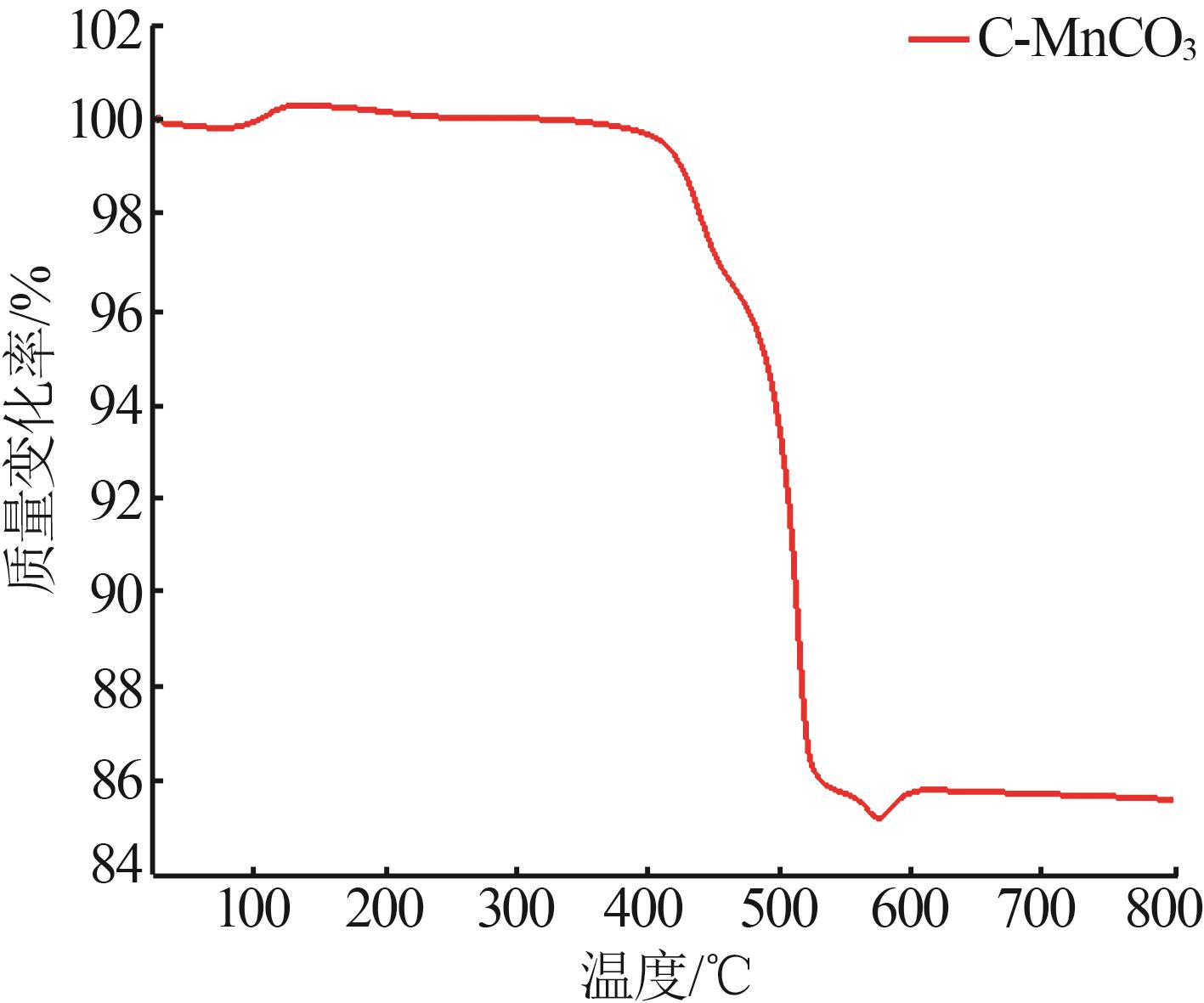
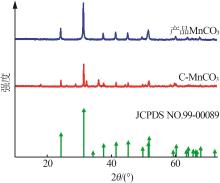
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (12): 94-103.doi: 10.19964/j.issn.1006-4990.2024-0090
• Research & Development • Previous Articles Next Articles
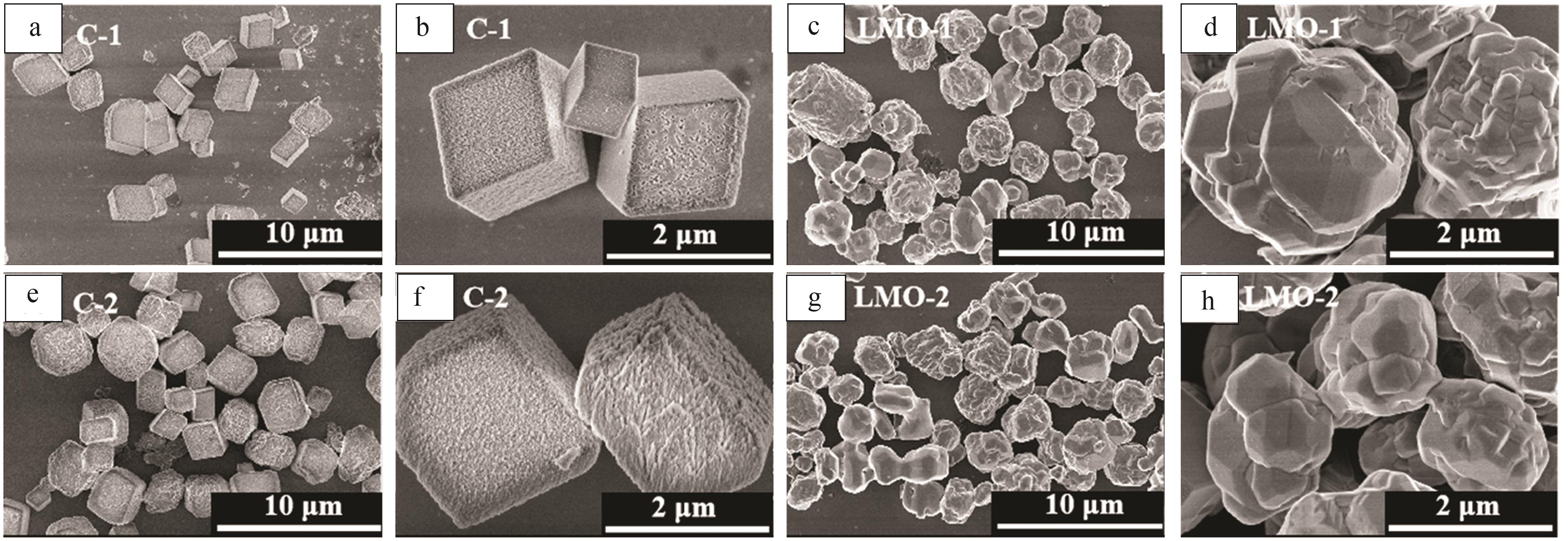
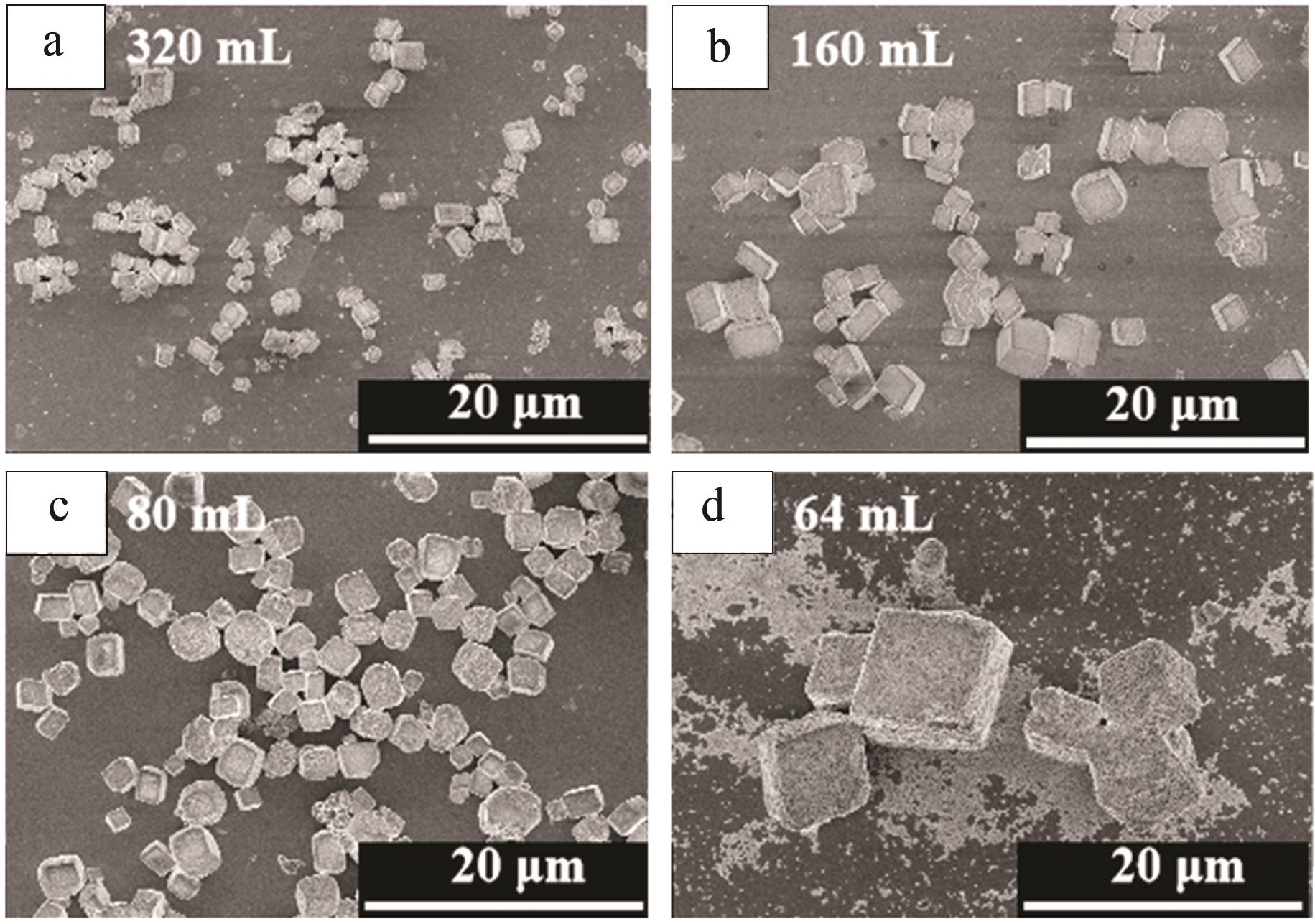
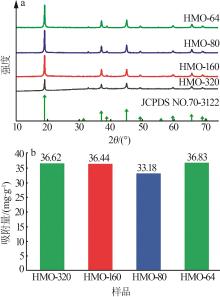
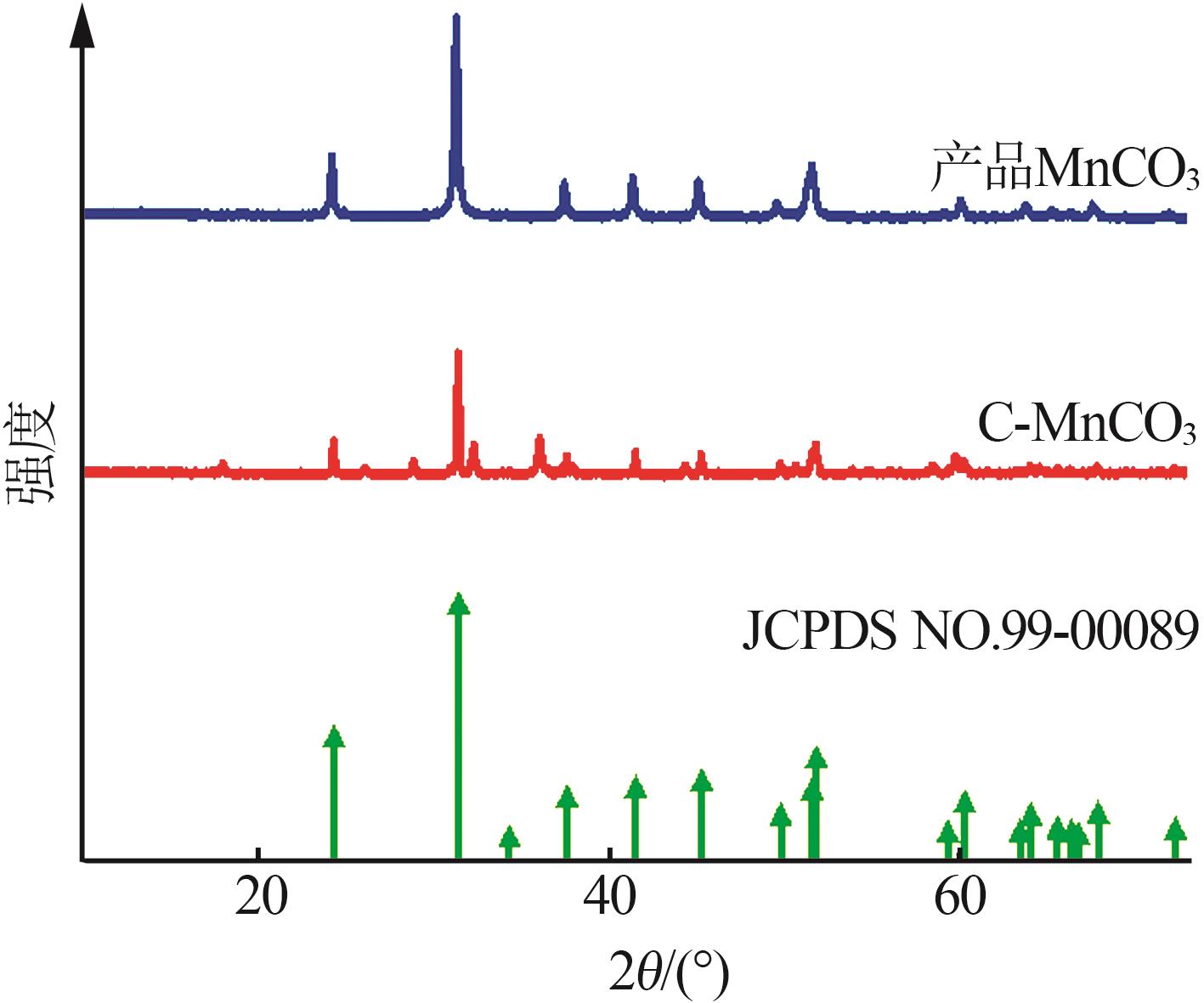
Preparation of cubic manganese carbonate by hydrothermal method and its application in extraction of lithium
WANG Ruirui1,2,3( ), ZHU Chaoliang1,2, MU Bing1,2,3, MA Wanxia1,2, FAN Jie1,2, XU Guowang1,2, SHI Yifei1,2, DENG Xiaochuan1,2(
), ZHU Chaoliang1,2, MU Bing1,2,3, MA Wanxia1,2, FAN Jie1,2, XU Guowang1,2, SHI Yifei1,2, DENG Xiaochuan1,2( ), QING Binju1,2(
), QING Binju1,2( )
)
- 1.Key Laboratory of Green and High-end Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2.Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources,Xining 810008,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2024-02-21Online:2024-12-10Published:2024-03-20 -
Contact:DENG Xiaochuan, QING Binju E-mail:wangruirui21@mails.ucas.ac.cn;dengxch@isl.ac.cn;qbj@isl.ac.cn
CLC Number:
Cite this article
WANG Ruirui, ZHU Chaoliang, MU Bing, MA Wanxia, FAN Jie, XU Guowang, SHI Yifei, DENG Xiaochuan, QING Binju. Preparation of cubic manganese carbonate by hydrothermal method and its application in extraction of lithium[J]. Inorganic Chemicals Industry, 2024, 56(12): 94-103.
share this article
| [1] | 刘景,温兆银,吴梅梅,等.锂离子电池正极材料的研究进展[J].无机材料学报,2002,(1):1-9. |
| LIU Jing, WEN Zhaoyin, WU Meimei,et al.Progress on studies of the cathode materials for Li-ion batteries[J].Journal of Inorganic Materials,2002,(1):1-9. | |
| [2] | 吴国华,孙江伟,张亮,等.铝锂合金材料研究应用现状与展望[J].有色金属科学与工程,2019,10(2):31-46. |
| WU Guohua, SUN Jiangwei, ZHANG Liang,et al.Current status and prospects of research and application of aluminum-lithium alloy[J].Nonferrous Metals Science and Engineering,2019,10(2):31-46. | |
| [3] | 栗志彬,康军,吴宝杰.锂基润滑脂的微观结构[J].石油炼制与化工,2019,50(9):75-80. |
| LI Zhibin, KANG Jun, WU Baojie.Microstructure of lithium grease[J].Petroleum Processing and Petrochemicals,2019,50(9):75-80. | |
| [4] | 陈海霞,严红,孙云龙,等.锂资源提取技术研究进展[J].无机盐工业,2024,56(1):9-22. |
| CHEN Haixia, YAN Hong, SUN Yunlong,et al.Research progress of lithium resource extraction technology[J].Inorganic Chemicals Industry,2024,56(1):9-22. | |
| [5] | 郑绵平,刘喜方.中国的锂资源[J].新材料产业,2007(8):13-16. |
| ZHENG Mianping, LIU Xifang.Lithium resources in China[J].Advanced Materials Industry,2007(8):13-16. | |
| [6] | 朱瑞松,曹靖,刘陶然,等.全球非常规卤水的提锂技术及产业化研究进展[J].无机盐工业,2023,55(11):1-11. |
| ZHU Ruisong, CAO Jing, LIU Taoran,et al.Research progress of lithium extraction technology and industrialization of unconventional brines in global[J].Inorganic Chemicals Industry,2023,55(11):1-11. | |
| [7] | 乜贞,伍倩,丁涛,等.中国盐湖卤水提锂产业化技术研究进展[J].无机盐工业,2022,54(10):1-12. |
| NIE Zhen, WU Qian, DING Tao,et al.Research progress on industrialization technology of lithium extraction from salt lake brine in China[J].Inorganic Chemicals Industry,2022,54(10):1-12. | |
| [8] | 刘向磊,钟辉,唐中杰.盐湖卤水提锂工艺技术现状及存在的问题[J].无机盐工业,2009,41(6):4-6,16. |
| LIU Xianglei, ZHONG Hui, TANG Zhongjie.Current status and existing problems of lithium extraction technology from salt lake[J].Inorganic Chemicals Industry,2009,41(6):4-6,16. | |
| [9] | DUAN Xiaowei, ZHU Wenkun, RUAN Zhongkui,et al.Recycling of lithium batteries:A review[J].Energies,2022,15(5):1611. |
| [10] | 罗清龙,董明哲,李军,等.吸附法分离盐湖卤水中锂的研究进展[J].盐湖研究,2023,31(1):106-115. |
| LUO Qinglong, DONG Mingzhe, LI Jun,et al.Research progress of lithium separation from salt lake brine by adsorption meth-od[J].Journal of Salt Lake Research,2023,31(1):106-115. | |
| [11] | 赵元元,陈海峰,刘云云,等.锰系锂离子筛的制备与改性的研究进展[J].无机盐工业,2022,54(2):21-29. |
| ZHAO Yuanyuan, CHEN Haifeng, LIU Yunyun,et al.Research progress on preparation and modification of manganese based lithium ion sieve[J].Inorganic Chemicals Industry,2022,54(2):21-29. | |
| [12] | LI Xu, WANG Jianchuan, ZHANG Shiwei,et al.Intrinsic defects in LiMn2O4:First-principles calculations[J].ACS Omega,2021,6(33):21255-21264. |
| [13] | ZANDEVAKILI S, RANJBAR M, EHTESHAMZADEH M.Recovery of lithium from Urmia Lake by a nanostructure MnO2 ion sieve[J].Hydrometallurgy,2014,149:148-152. |
| [14] | ZHAO Bing, QIAN Zhiqiang, QIAO Yinjun,et al.The Li(H2O)n dehydration behavior influences the Li+ ion adsorption on H4Ti5O12 with different facets exposed[J].Chemical Engineering Journal,2023,451:138870. |
| [15] | LI Xiulei, TAO Baifu, JIA Qingyuan,et al.Preparation and adsorption performance of multi-morphology H1.6Mn1.6O4 for lithium extraction[J].Chinese Journal of Chemical Engineering,2021,34:68-76. |
| [16] | 王龙,李艳,陈多福.纳米氧化锰离子筛提取卤水溶液中锂的实验研究[J].地球化学,2017,46(4):373-379. |
| WANG Long, LI Yan, CHEN Duofu.Experimental study on the extraction of lithium from brine solution by a manganese oxide nanometer ion sieve[J].Geochimica,2017,46(4):373-379. | |
| [17] | 张果泰,漆贵财,海春喜,等.球形MnO2·0.5H2O提锂性能及其机理研究(英文) [J].盐湖研究,2022,30(3):21-33. |
| ZHANG Guotai, QI Guicai, Chunxi HAI,et al.Performance and mechanism of recovering lithium from aqueous solution by spherical MnO2·0.5H2O[J].Journal of Salt Lake Research,2022,30(3):21-33. | |
| [18] | 漆贵财.锂离子筛复合材料的制备及性能研究[D].西宁:中国科学院大学(中国科学院青海盐湖研究所),2019. |
| QI Guicai.Preparation and properties of lithium ion screen composites[D].Xining:Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,2019. | |
| [19] | 韩红静,张竞择,拉毛卓玛,等.铝钴共掺杂锂锰氧化物的制备及吸附提锂性能[J].无机盐工业,2023,55(7):38-44, 50. |
| HAN Hongjing, ZHANG Jingze, Maozhuoma LA,et al.Preparation of Al-Co Co-doped lithium manganese oxide and its adsorption performance of lithium[J].Inorganic Chemicals Industry,2023,55(7):38-44,50. | |
| [20] | 袁明亮,邱冠周.高纯微晶碳酸锰制备中的粒度和质量控制[J].中南工业大学学报(自然科学版),2001,32(5):473-476. |
| YUAN Mingliang, QIU Guanzhou.Controlling of the particle size in the preparation process of manganese carbonate and its effect on the product quality[J].Journal of Central South University of Technology (Natural Science),2001,32(5):473-476. | |
| [21] | 粟海锋,屈雄,文衍宣,等.球形碳酸锰微晶制备过程中的形貌控制[J].过程工程学报,2008,8(2):280-284. |
| SU Haifeng, QU Xiong, WEN Yanxuan,et al.Morphology control in preparation process of spherical manganese carbonate particles[J].The Chinese Journal of Process Engineering,2008,8(2):280-284. | |
| [22] | 杜泽伟,曹秀华.水热晶化法制备超细MnCO3的研究[J].电子元件与材料,2006,25(3):12-14. |
| DU Zewei, CAO Xiuhua.Study on ultrafine MnCO3 prepared by hydrothermal-crystal method[J].Electronic Components and Materials,2006,25(3):12-14. | |
| [23] | HUANG Sisi, WU Hao, CHEN Penghui,et al.Facile pH-mediated synthesis of morphology-tunable MnCO3 and their trans-formation to truncated octahedral spinel LiMn 2O4 cathode materials for superior lithium storage[J].Journal of Materials Chemistry A,2015,3(7):3633-3640. |
| [24] | NGUYEN DINH M T, NGUYEN C C, TRUONG VU T L,et al.Tailoring porous structure,reducibility and Mn4+ fraction of ε-MnO2 microcubes for the complete oxidation of toluene[J].Applied Catalysis A:General,2020,595:117473. |
| [1] | WANG Minrui, TIAN Guiying, ZHANG Ao, GE Junjie, ZHANG Lei, XIANG Jun, TANG Na. Study on granulation optimization for Al-based lithium adsorbent and its lithium recovery performance from brine [J]. Inorganic Chemicals Industry, 2025, 57(3): 36-42. |
| [2] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [3] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [4] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [5] | CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun. Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance [J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139. |
| [6] | JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun. Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate [J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81. |
| [7] | CHEN Jun,ZHONG Jing,LIN Sen,YU Jianguo. Study on lithium separation from brine by aluminum-based adsorbent in fixed bed [J]. Inorganic Chemicals Industry, 2023, 55(1): 64-73. |
| [8] | LU Yang,WANG Jing,HE Lijie,WANG Fudong,ZHANG Jiaming. Research progress on preparation of magnesium aluminate spinel powders and it in field of luminescent material [J]. Inorganic Chemicals Industry, 2022, 54(9): 39-46. |
| [9] | LI Xiao,ZHAO Yuxiang,LI Bo,ZOU Xingwu,WANG Shuxuan. Study on controllable synthesis of spherical strontium fluoride [J]. Inorganic Chemicals Industry, 2022, 54(5): 67-71. |
| [10] | LU Zheng,CHEN Kunfeng,XUE Dongfeng. Study on large-scale preparation and electrochemical properties of high thermal stabilized α-Fe2O3 [J]. Inorganic Chemicals Industry, 2022, 54(3): 45-50. |
| [11] | PEI Yinchang,MO Shengpeng,XIE Qinglin,CHEN Nanchun. Study on mechanism of zeolite A synthesized from stellerite zeolite by two-step hydrothermal method [J]. Inorganic Chemicals Industry, 2022, 54(11): 59-64. |
| [12] | QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin. Preparation and characterization of anhydrous calcium sulfate whisker [J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115. |
| [13] | Cheng Penggao,Huang Chuanfeng,Gan Shantian,Gong Jingkuan,Xiang Jun,Tang Na. Preparation of aluminum-based lithium adsorbent and its application in extracting lithium from Taihe underground brine [J]. Inorganic Chemicals Industry, 2021, 53(6): 140-144. |
| [14] | ZHU Danchen,LI Mingjie,CHEN Zhangxu,LIN Qian. Study on hydrothermal synthesis of MnO2 and its decolorization performance on dyes [J]. Inorganic Chemicals Industry, 2021, 53(11): 60-65. |
| [15] | Li Xiangguo,Guo Miao,Yao Huimin,Lü Jing. Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2 [J]. Inorganic Chemicals Industry, 2020, 52(6): 30-35. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||