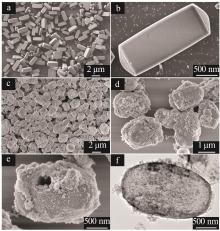
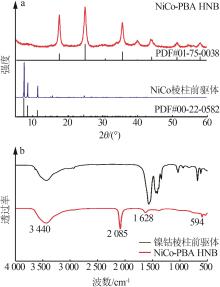
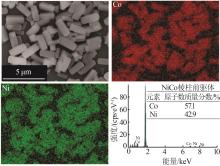
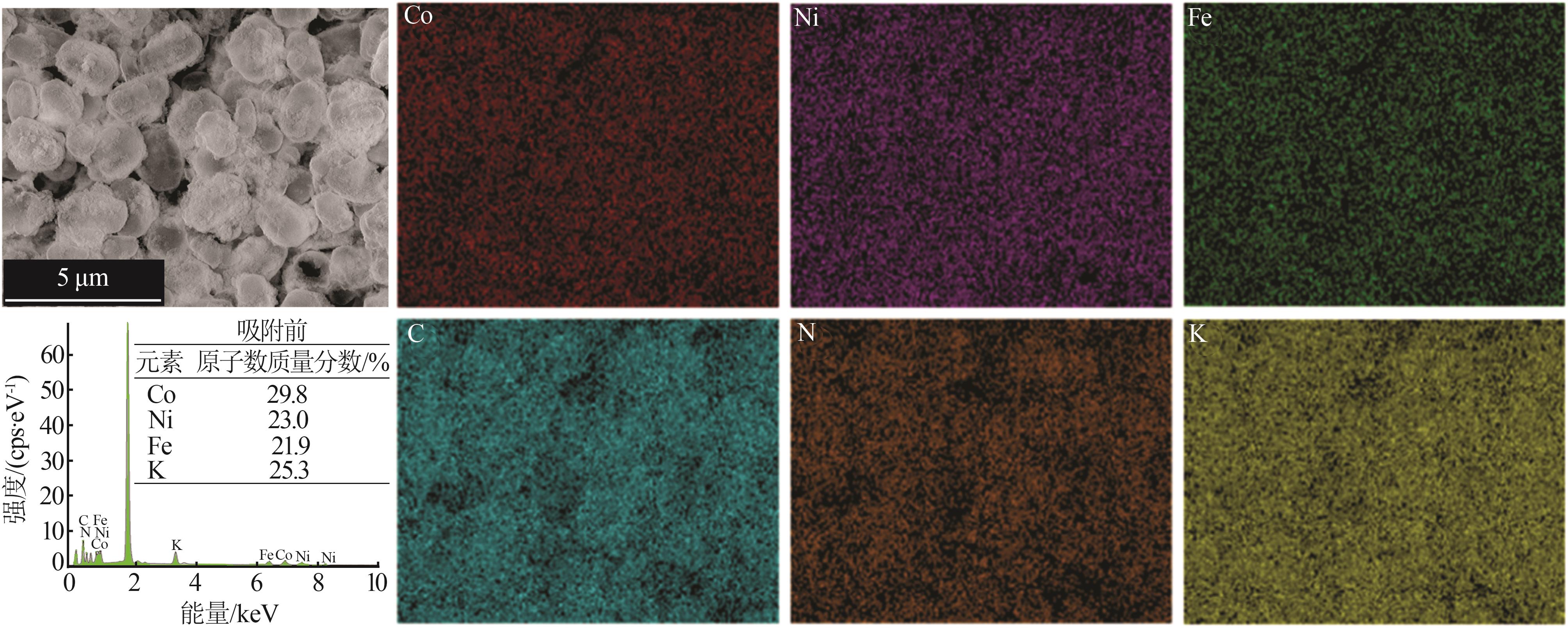
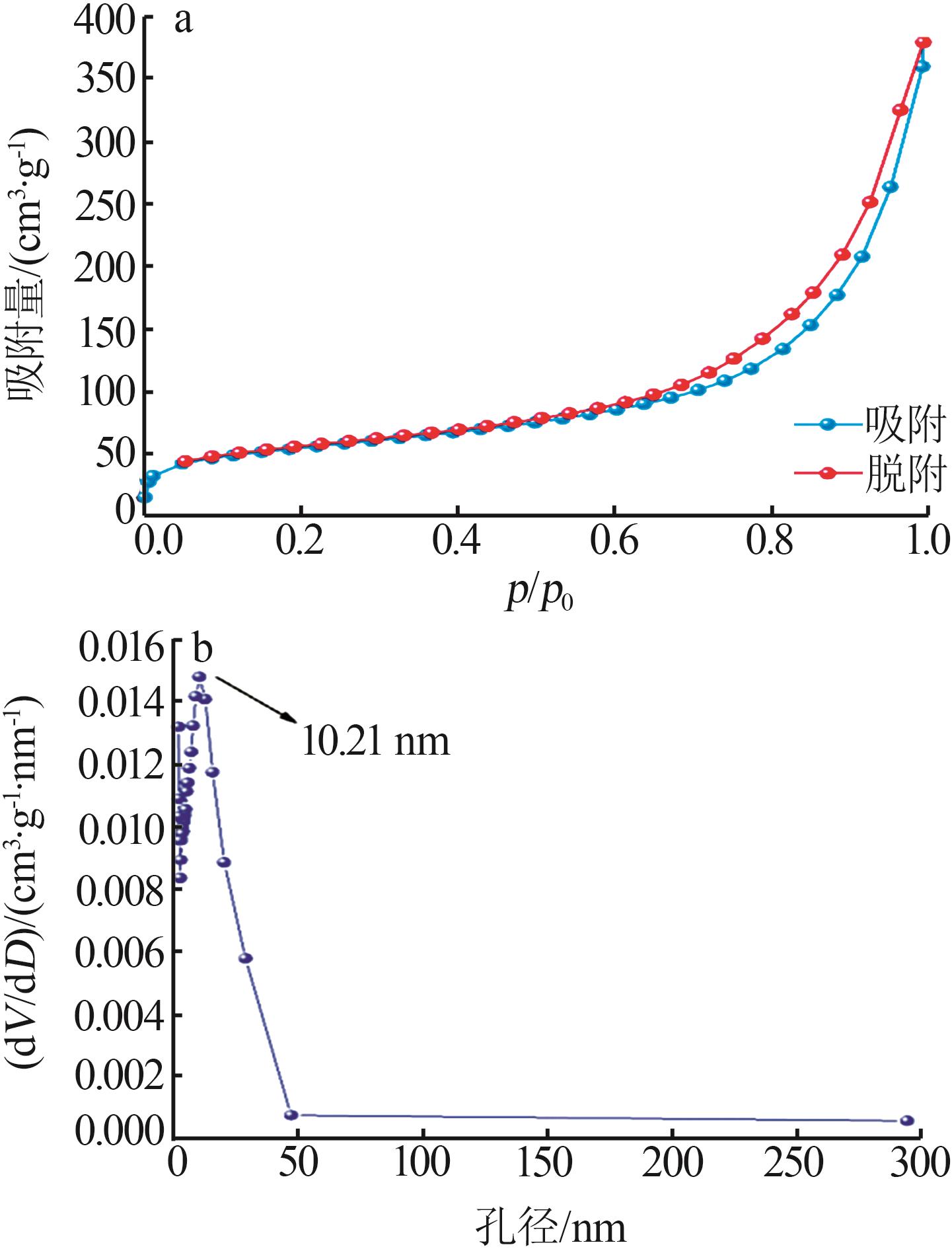
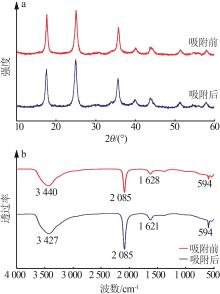
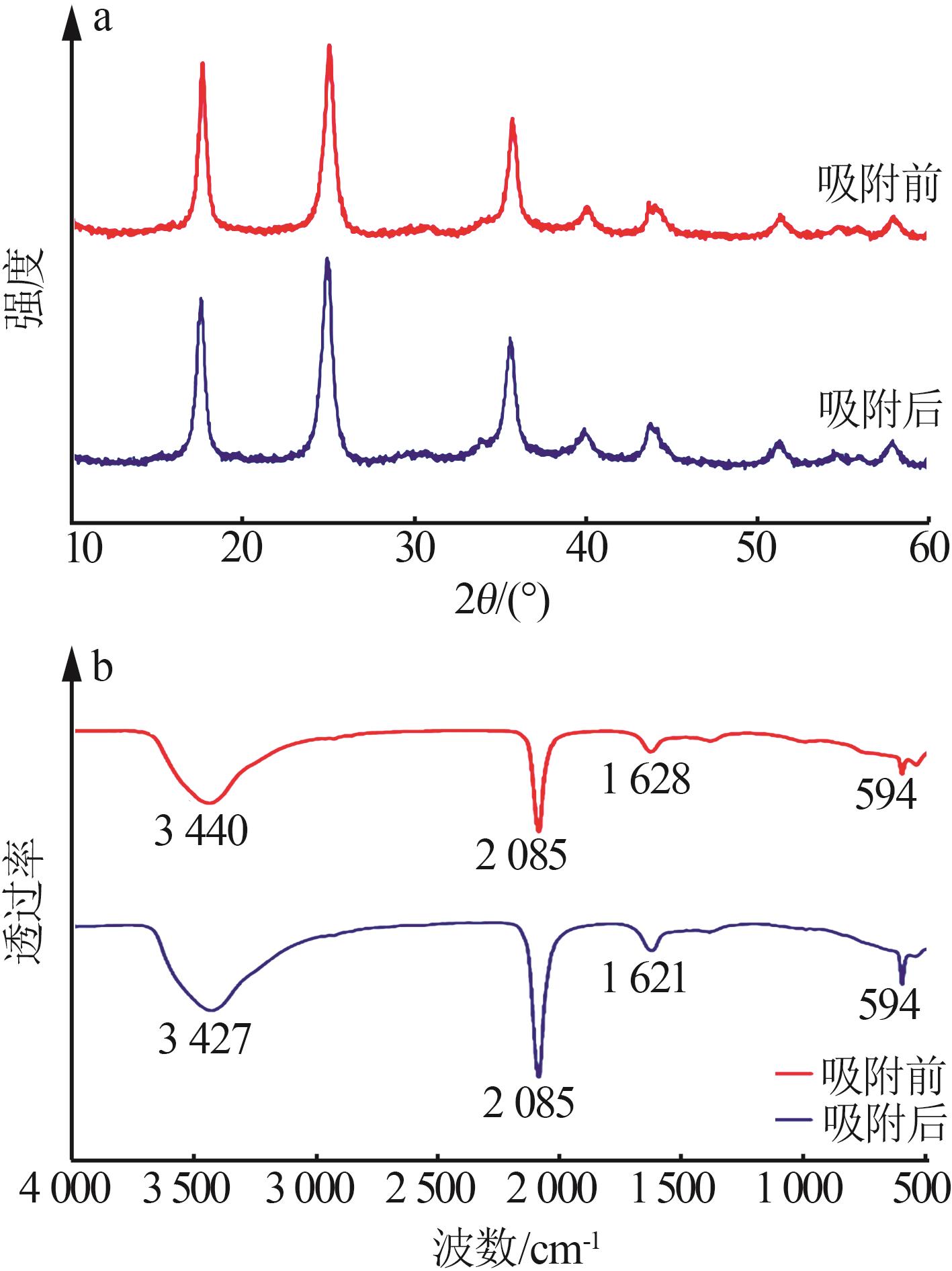
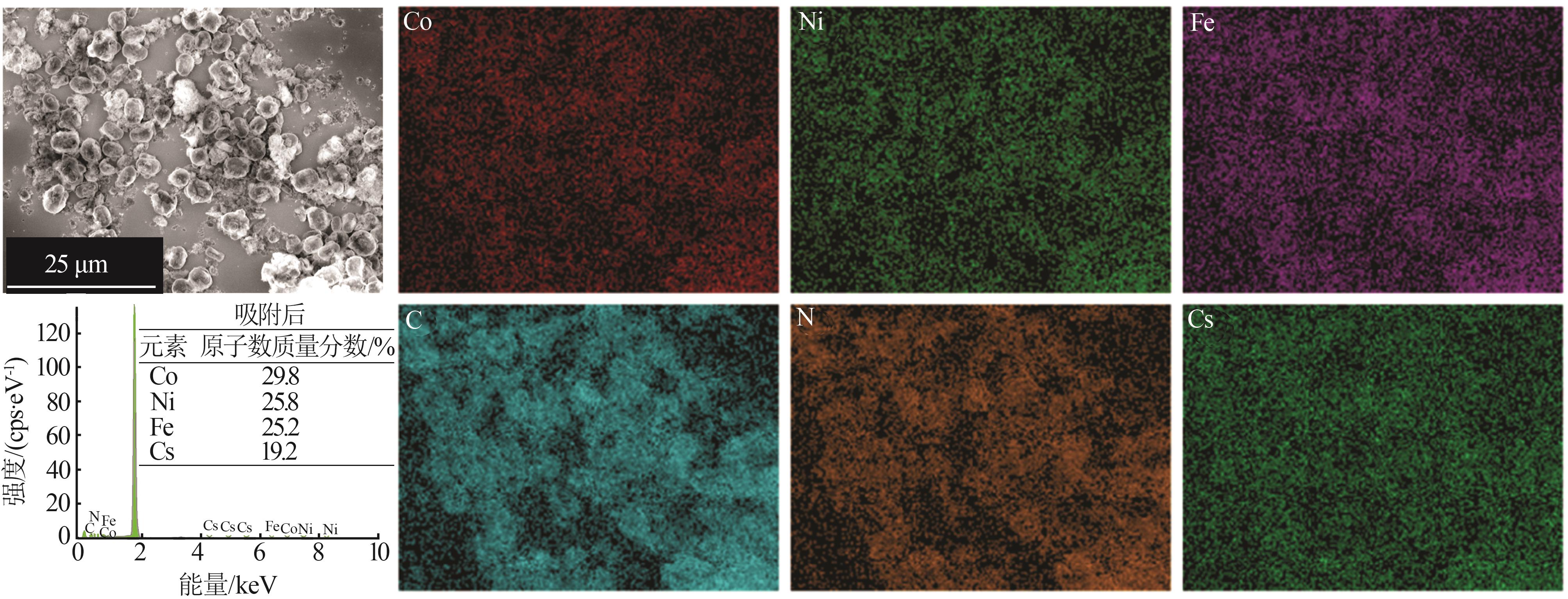
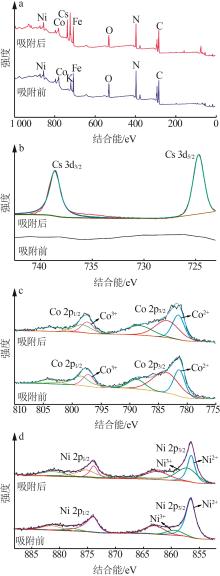
| 1 |
CHEN Shangqing, DONG Yanan, WANG Honghong,et al.Highly efficient and selective cesium recovery from natural brine resources using mesoporous Prussian blue analogs synthesized by ionic liquid-assisted strategy[J].Resources,Conservation and Recycling,2022,186:106542.
|
| 2 |
CHEN Shangqing, HU Jiayin, HAN Senjian,et al.A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade[J].Separation and Purification Technology,2020,251:117340.
|
| 3 |
ALBY D, CHARNAY C, HERAN M,et al.Recent developments in nanostructured inorganic materials for sorption of cesium and strontium:Synthesis and shaping,sorption capacity,mechanisms,and selectivity:A review[J].Journal of Hazardous Materials,2018,344:511-530.
|
| 4 |
姚初清,戴耀东,罗威,等.一维普鲁士蓝/硅藻土复合材料对Cs+的吸附[J].核化学与放射化学,2020,42(2):121-128.
|
|
YAO Chuqing, DAI Yaodong, LUO Wei,et al.Adsorption of Cs+ on one⁃dimensional Prussian blue/diatomite[J].Journal of Nuclear and Radiochemistry,2020,42(2):121-128.
|
| 5 |
ABDEL RAHMAN R O, IBRAHIUM H A, HUNG Y T.Liquid radioactive wastes treatment:A review[J].Water,2011,3(2):551- 565.
|
| 6 |
WANG Jianlong, ZHUANG Shuting.Removal of cesium ions from aqueous solutions using various separation technologies[J].Reviews in Environmental Science and Bio/Technology,2019,18(2):231-269.
|
| 7 |
ZHANG Ruihong, AI Yuejie, LU Zhanhui.Application of multifunctional layered double hydroxides for removing environmental pollutants:Recent experimental and theoretical progress[J].Journal of Environmental Chemical Engineering,2020,8(4):103908.
|
| 8 |
郭雪琴,邓小川,朱朝梁,等.改性硅藻土负载磷钼酸铵复合吸附剂的制备及其吸附Cs+研究[J].无机盐工业,2023,55(11):19-26,36.
|
|
GUO Xueqin, DENG Xiaochuan, ZHU Chaoliang,et al.Study on preparation of modified diatomite loaded ammonium phosphomolybdate composite adsorbent and its adsorption performance ofCs+ [J].Inorganic Chemicals Industry,2023,55(11):19-26,36.
|
| 9 |
FIGUEIREDO B R, CARDOSO S P, PORTUGAL I,et al.Inorganic ion exchangers for cesium removal from radioactive wastewater[J].Separation & Purification Reviews,2018,47(4):306-336.
|
| 10 |
BIKASH BARUAH J.Coordination polymers in adsorptive remediation of environmental contaminants[J].Coordination Chemistry Reviews,2022,470:214694.
|
| 11 |
YANG Hongjun, YU Hongwen, SUN Jingkuan,et al.Facile synthesis of mesoporous magnetic AMP polyhedric composites for rapid and highly efficient separation of Cs+ from water[J].Chemical Engineering Journal,2017,317:533-543.
|
| 12 |
KRAFT A.Some considerations on the structure,composition,and properties of Prussian blue:A contribution to the current discussion[J].Ionics,2021,27(6):2289-2305.
|
| 13 |
WU Xinyue, RU Yue, BAI Yang,et al.PBA composites and their derivatives in energy and environmental applications[J].Coordination Chemistry Reviews,2022,451:214260.
|
| 14 |
刘霂宇,邱杨率,张凌燕.膨胀石墨负载铁氰化铜吸附Cs+特性研究[J].环境污染与防治,2023,45(11):1554-1558.
|
|
LIU Muyu, QIU Yangshuai, ZHANG Lingyan.Adsorption characteristics of Cs+ by expanded graphite loaded with copper ferricyanide[J].Environmental Pollution & Control,2023,45(11):1554-1558.
|
| 15 |
BOSTRÖM H L B, COLLINGS I E, CAIRNS A B,et al.High⁃pressure behaviour of Prussian blue analogues:Interplay of hydration,Jahn-Teller distortions and vacancies[J].Dalton Transactions,2019,48(5):1647-1655.
|
| 16 |
ZENG Yinxiang, LU Xuefeng, ZHANG Songlin,et al.Construction of Co-Mn Prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries[J].Angewandte Chemie,2021,60(41):22189-22194.
|
| 17 |
MORITOMO Y, URASE S, SHIBATA T.Enhanced battery performance in manganese hexacyanoferrate by partial substitution[J].Electrochimica Acta,2016,210:963-969.
|
| 18 |
YIN Xuemin, LI Hejun, WANG Haiqi,et al.Self⁃templating synthesis of cobalt hexacyanoferrate hollow structures with superior performance for Na-ion hybrid supercapacitors[J].ACS Applied Materials & Interfaces,2018,10(35):29496-29504.
|
| 19 |
GUAN Guoxiang, HUANG Jie, CHEN Ming,et al.WS2-doped CuCo2S4 hollow nano⁃prisms as high⁃efficiency Pt-free bifunctional electrocatalysts for dye⁃sensitized solar cell and acid hydrogen evolution reaction[J].Applied Surface Science,2022,599:153989.
|
| 20 |
YU Le, ZHANG Lei, WU Haobin,et al.Formation of Ni x Co3- x S4 hollow nanoprisms with enhanced pseudocapacitive properties[J].Angewandte Chemie,2014,53(14):3711-3714.
|
| 21 |
DU Wei, LIU Rongmei, JIANG Yuanwen,et al.Facile synthesis of hollow Co3O4 boxes for high capacity supercapacitor[J].Journal of Power Sources,2013,227:101-105.
|
| 22 |
CHEN Shangqing, YANG Xiaonan, WANG Zheng,et al.Prussian blue analogs⁃based layered double hydroxides for highly efficient Cs+ removal from wastewater[J].Journal of Hazardous Materials,2021,410:124608.
|
| 23 |
YANG H M, PARK C W, KIM I,et al.Hollow flower⁃like titanium ferrocyanide structure for the highly efficient removal of radioactive cesium from water[J].Chemical Engineering Journal,2020,392:123713.
|
| 24 |
GAO Chao, HE Jiaying, HAN Senjian,et al.A highly efficient metal ferrocyanide adsorbent based on zinc phytate for cesium removal[J].Applied Surface Science,2023,614:156231.
|
| 25 |
HAN Weixing, HUANG Ying, SU Minhua,et al.Highly selective adsorption and lattice process of cesium by cubic cyanide⁃based functional materials[J].Environmental Research,2022,214:114085.
|
| 26 |
JIANG Chuanyang, NI Jiahui, JIN Guanping.Magnetic potassium cobalt hexacyanoferrate nanocomposites for efficient adsorption of rubidium in solution[J].Separation and Purification Technology,2022,296:121383.
|
| 27 |
郭雪琴,邓小川,朱朝梁,等.改性硅藻土负载亚铁氰化铜复合物的制备及其对Cs+的吸附性能[J].无机化学学报,2023,39(5):815-829.
|
|
GUO Xueqin, DENG Xiaochuan, ZHU Chaoliang,et al.Preparation and adsorption properties for Cs+ of modified diatomite⁃supported copper hexacyanoferrate composite[J].Chinese Journal of Inorganic Chemistry,2023,39(5):815-829.
|
| 28 |
ZHANG Tongrui, HUANG Haifu, HAN Junxing,et al.Manganese⁃doped hollow layered double(Ni,Co) hydroxide microcuboids as an efficient electrocatalyst for the oxygen evolution reaction[J].ChemElectroChem,2020,7(18):3852-3858.
|
| 29 |
YANG Chenyang, CHO K.Rapid and selective removal of Cs+ from water by layered potassium antimony thiostannate[J].Journal of Hazardous Materials,2021,403:124105.
|
| 30 |
TRUNG N D, PING Ning, LAN L T H,et al.Synthesis,characterization,and caesium adsorbent application of trigonal zinc hexacyanoferrate(Ⅱ) nanoparticles[J].Journal of Environmental Che⁃ mical Engineering,2021,9(6):106772.
|
| 31 |
ZHANG Lihong, LI Yun, LIN Nana,et al.Significantly enhanced alkaline stability and cyanide suppression of Prussian blue analogues using montmorillonite for high⁃performance cesium remo⁃val[J].Separation and Purification Technology,2023,325:124662.
|
| 32 |
YAN Jie, ZHANG Bo, LI Jun,et al.Rapid and selective uptake of radioactive cesium from water by a microporous zeolitic⁃like sulfide[J].Inorganic Chemistry,2023,62(32):12843-12850.
|
 ), DU Yifa3, GUO Xia4, NA Yuxuan4, WAN Macuo4, ZHOU Yongquan1(
), DU Yifa3, GUO Xia4, NA Yuxuan4, WAN Macuo4, ZHOU Yongquan1( )
)