Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (3): 66-70.doi: 10.19964/j.issn.1006-4990.2021-0240
• Research & Development • Previous Articles Next Articles
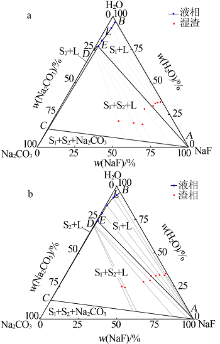
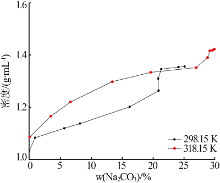
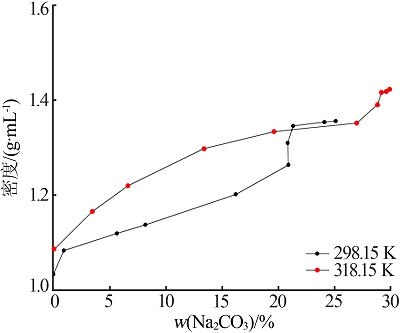
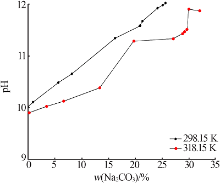
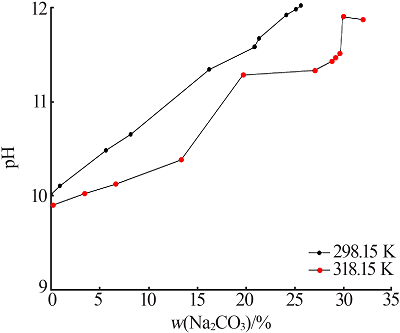
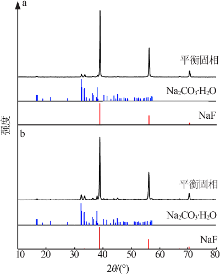
Study on phase equilibrium of ternary system of Na2CO3-NaF-H2O at 298.15 K and 318.15 K
LU Ziyu1,2,3( ),LIU Zhaogang1,2,3(
),LIU Zhaogang1,2,3( ),WU Jinxiu1,2,3,HU YanHong1,2,3,LIU Xingyu2,3,4
),WU Jinxiu1,2,3,HU YanHong1,2,3,LIU Xingyu2,3,4
- 1. School of Materials and Metallurgy,Inner Mongolia University of Science and Technology,Baotou 014010,China
2. Key Laboratory of the Ministry of Education for Green Extraction and Efficient Utilization of Light Rare Earth Resources
3. Key Laboratory of Rare Earth Hydrometallurgy and Light Rare Earth Application,Inner Mongolia Autonomous Region
4. Inner Mongolia University of Chemistry and Chemical Engineering