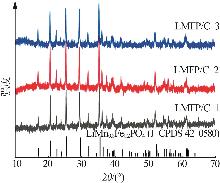
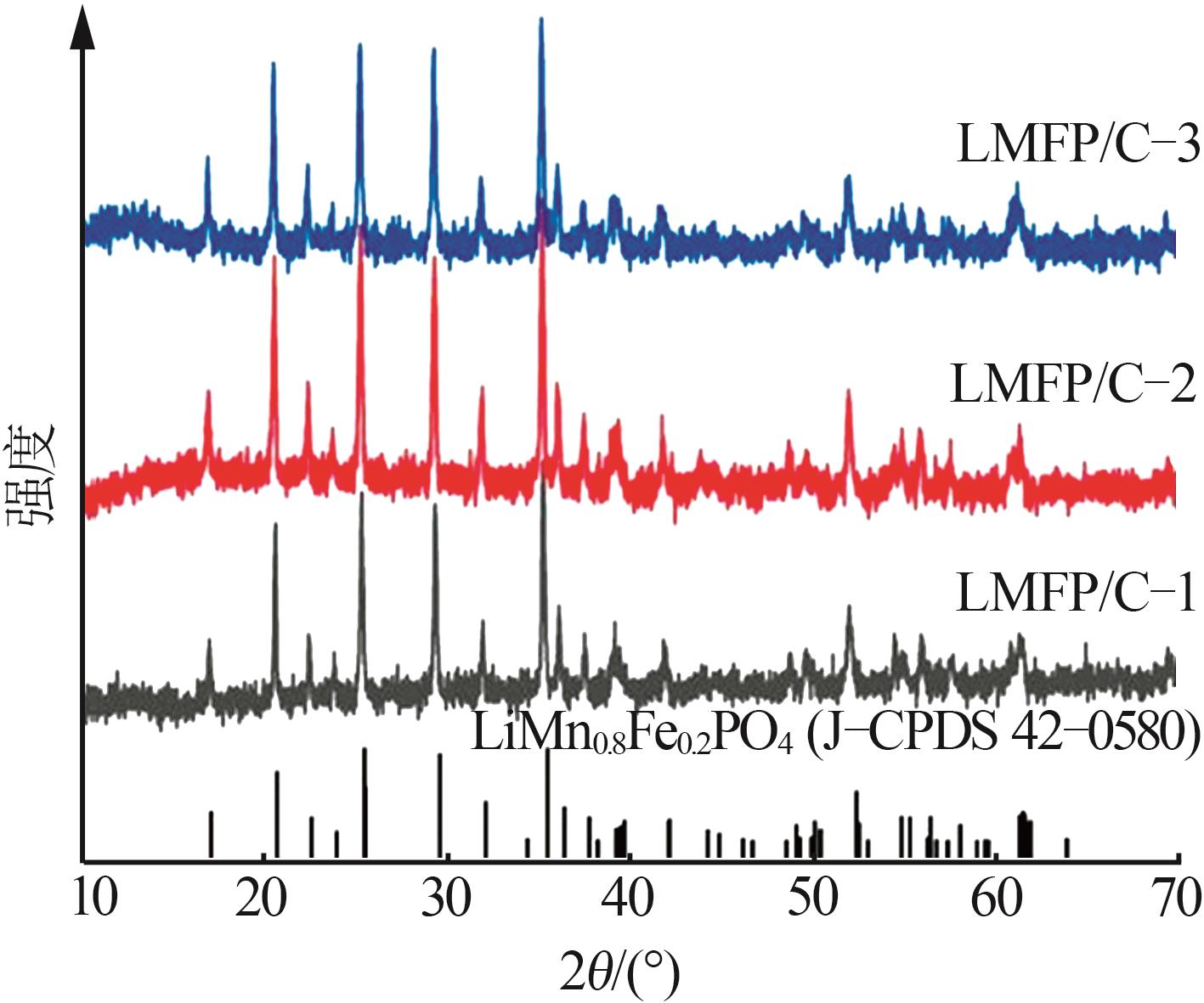
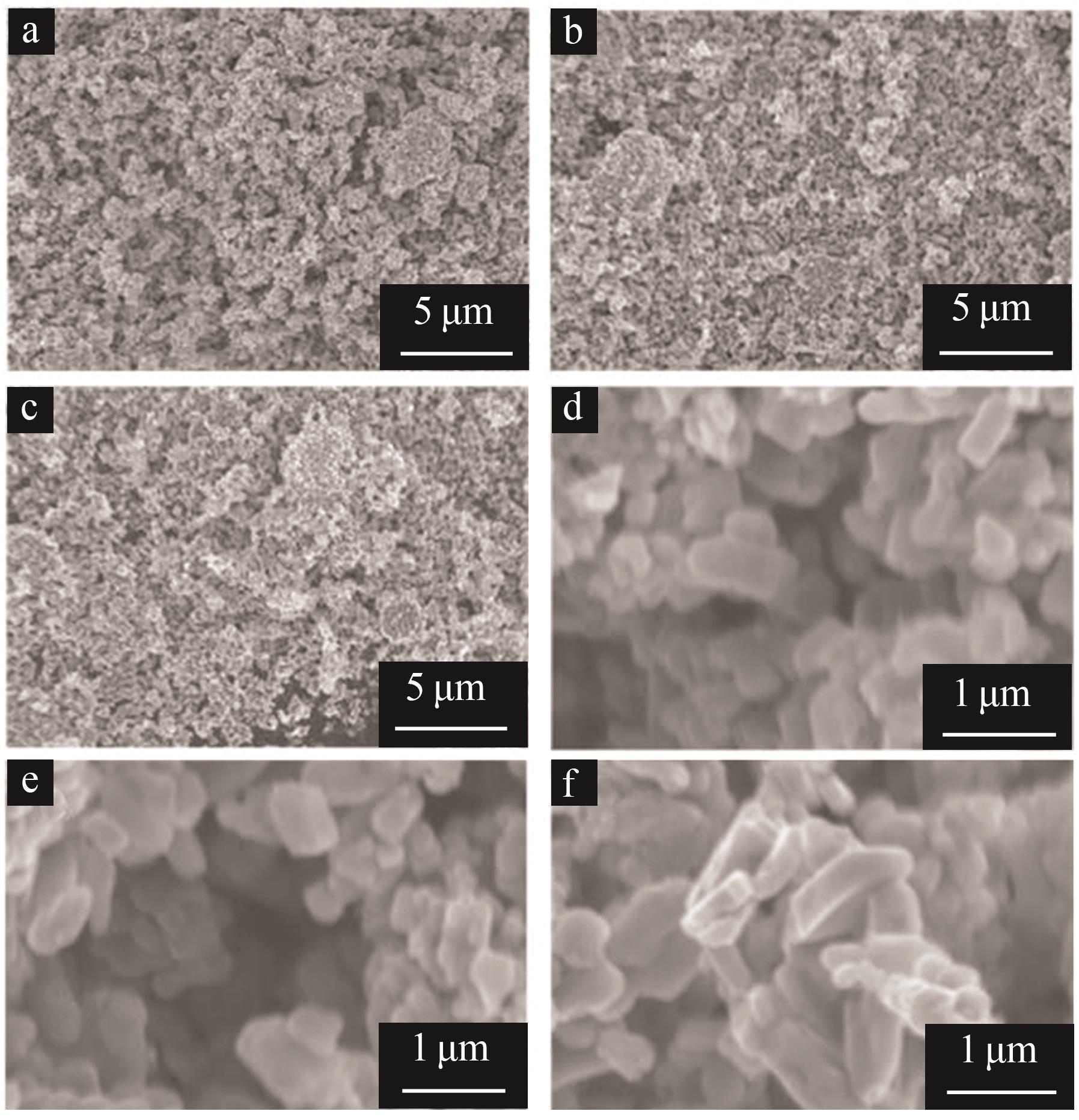
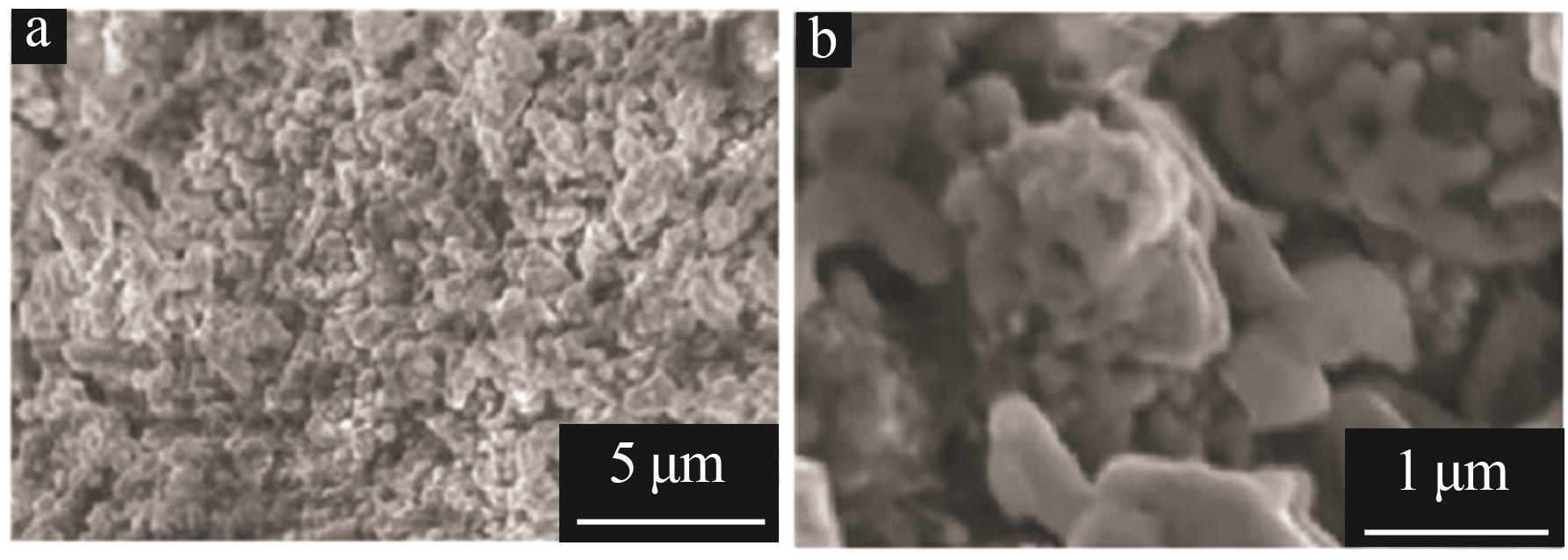
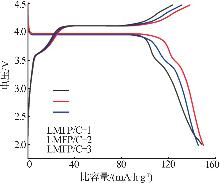
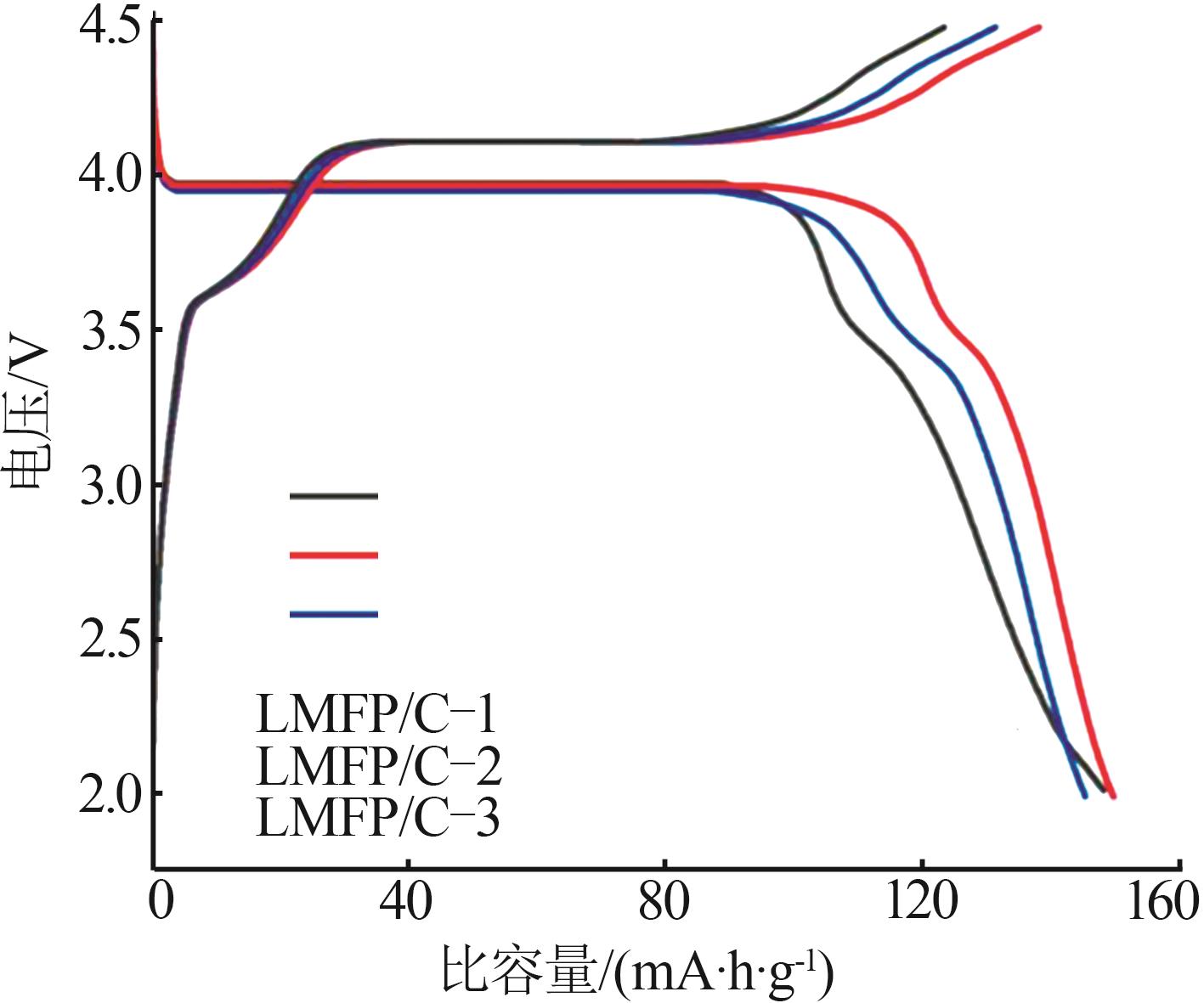
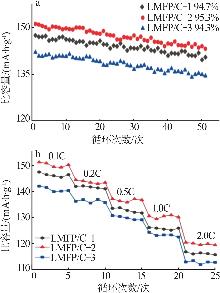
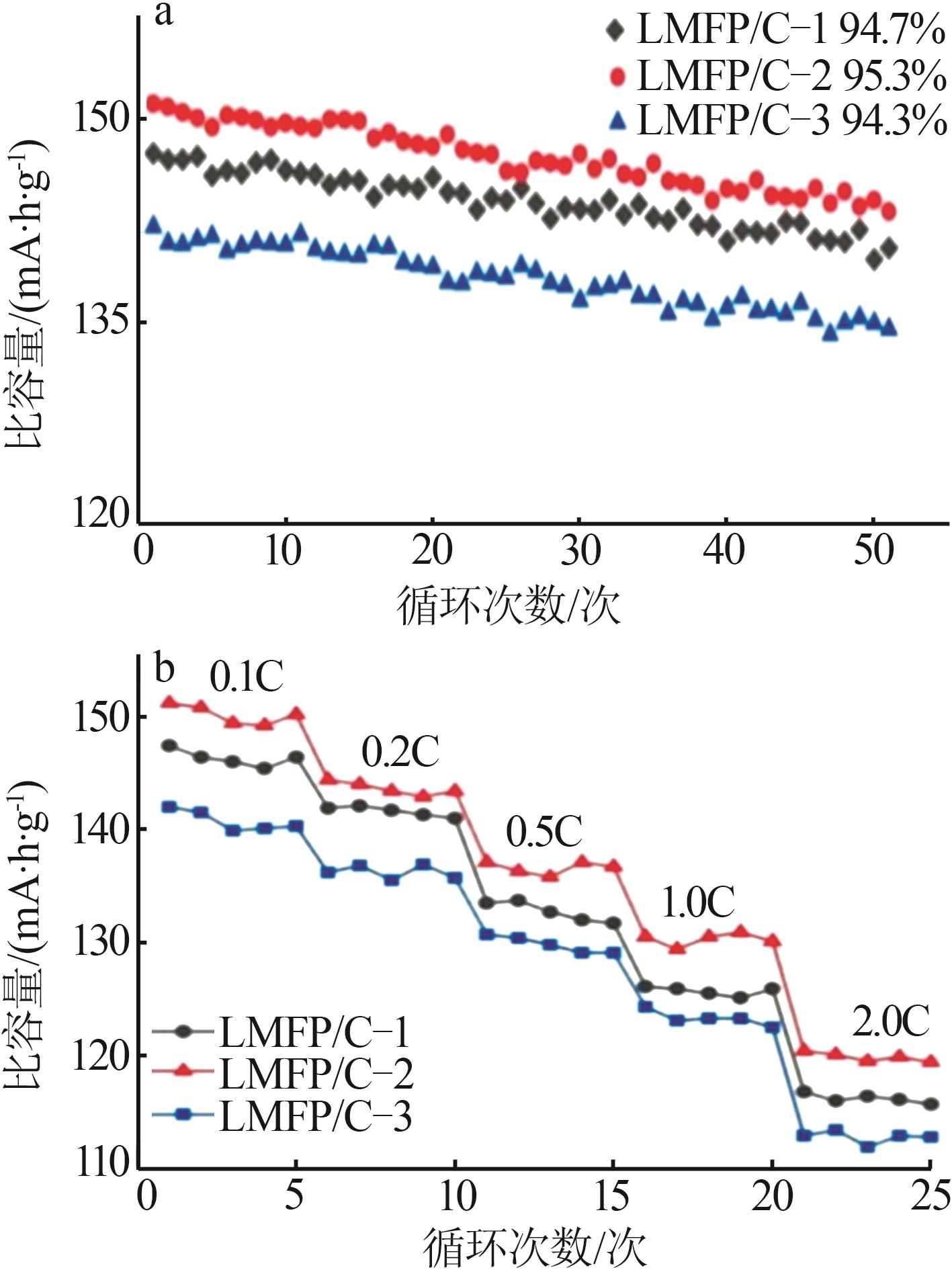
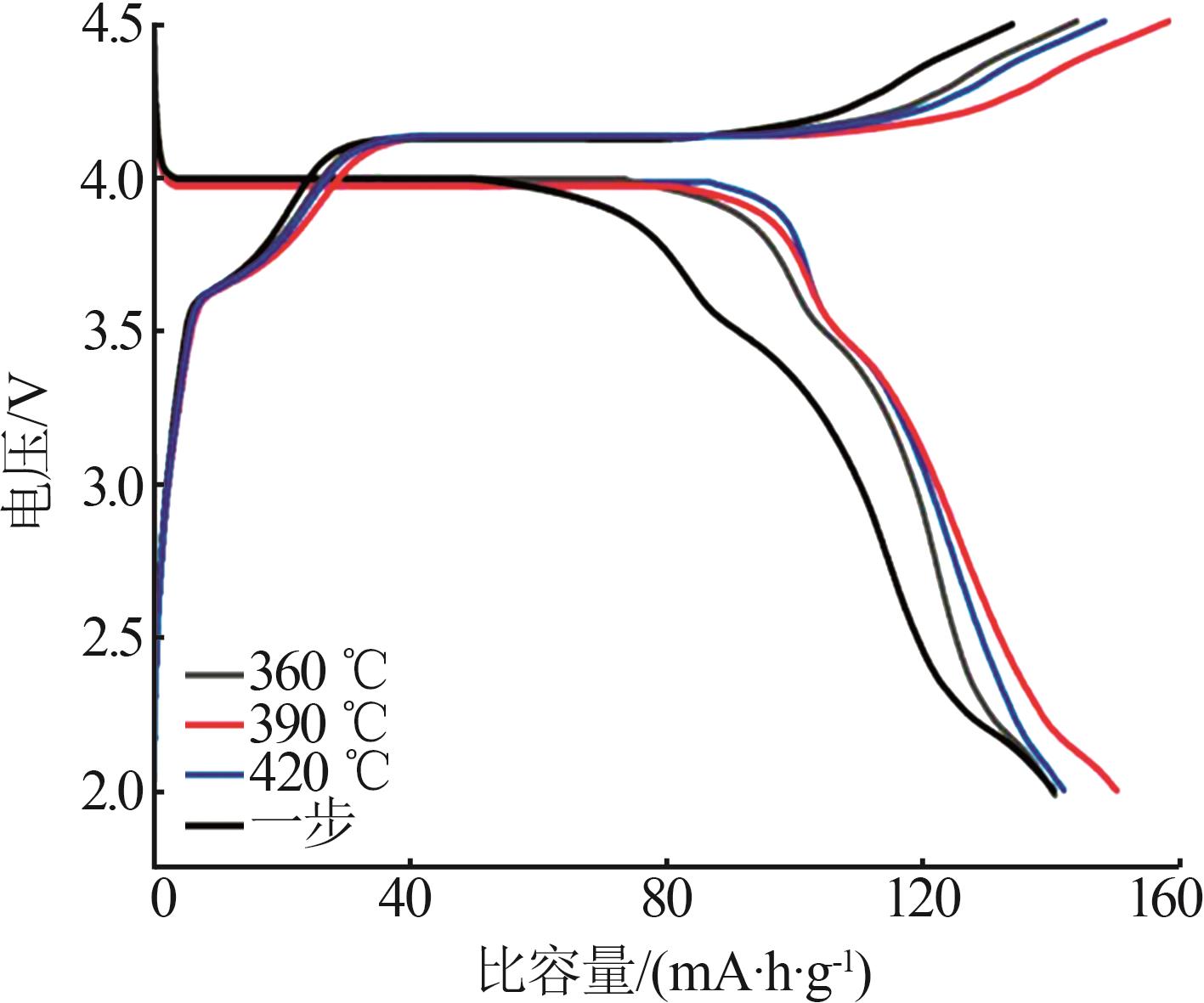
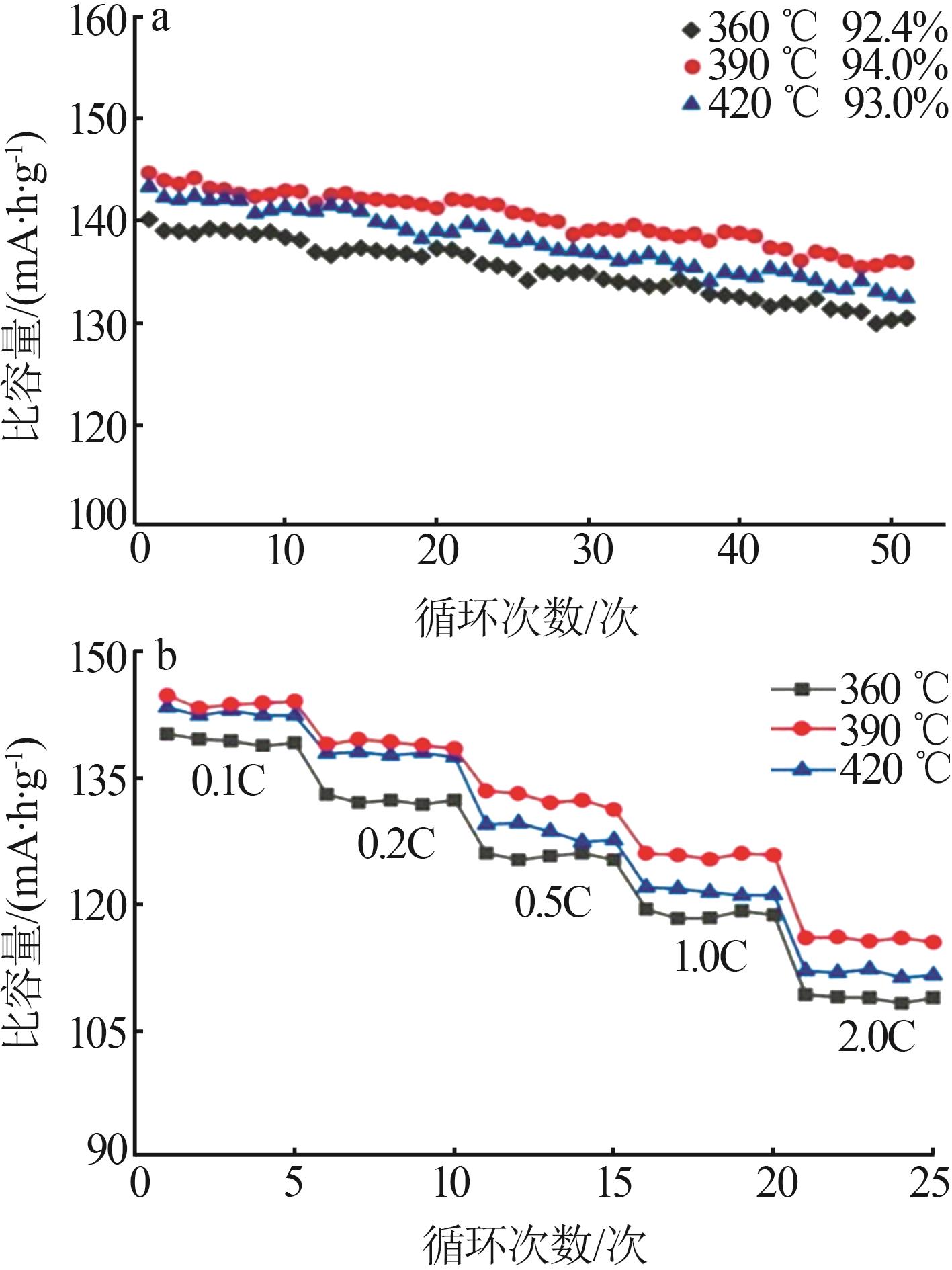
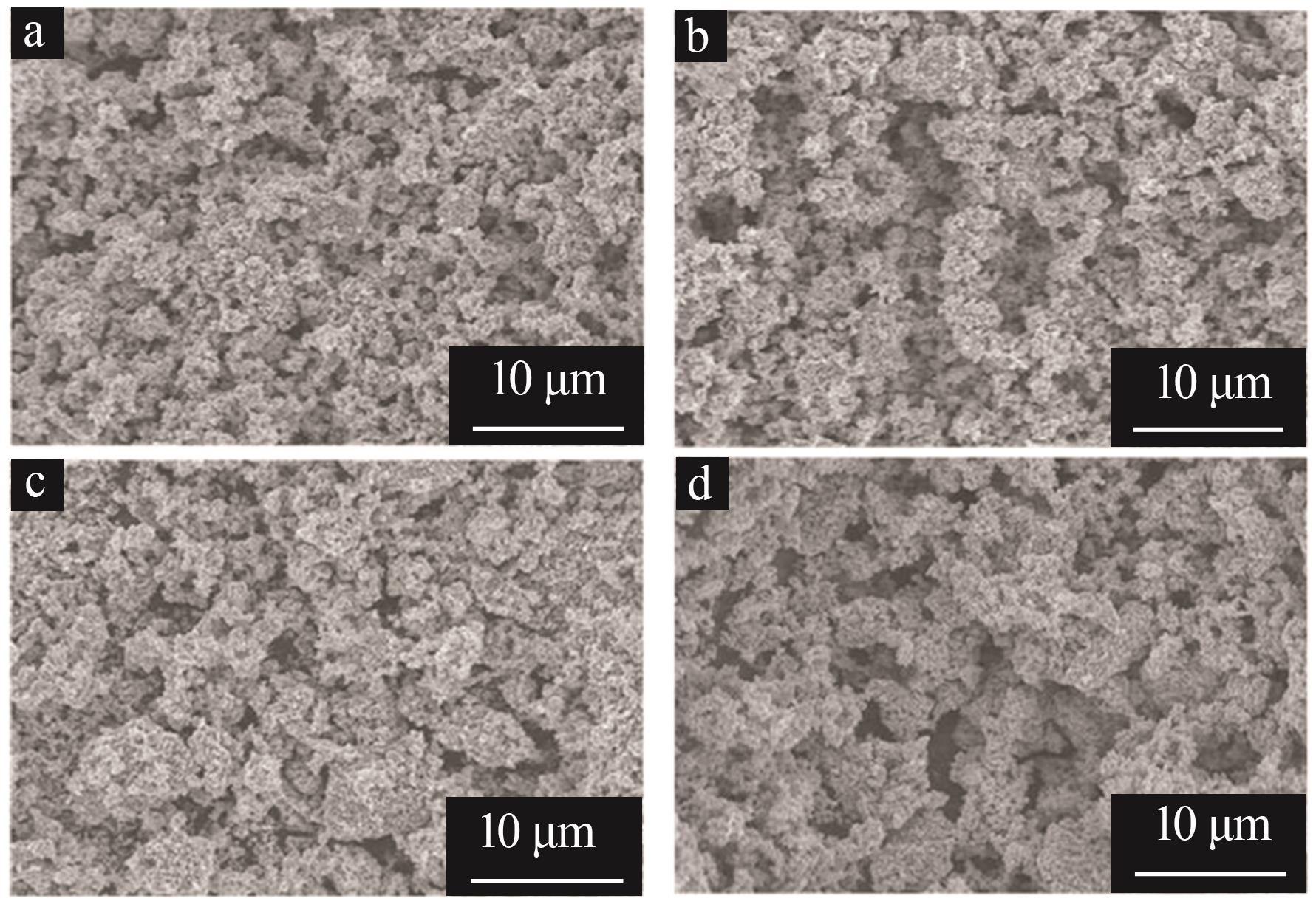
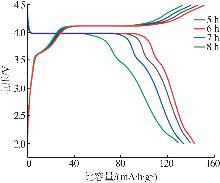
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (11): 36-42.doi: 10.19964/j.issn.1006-4990.2024-0607
• Research & Development • Previous Articles Next Articles
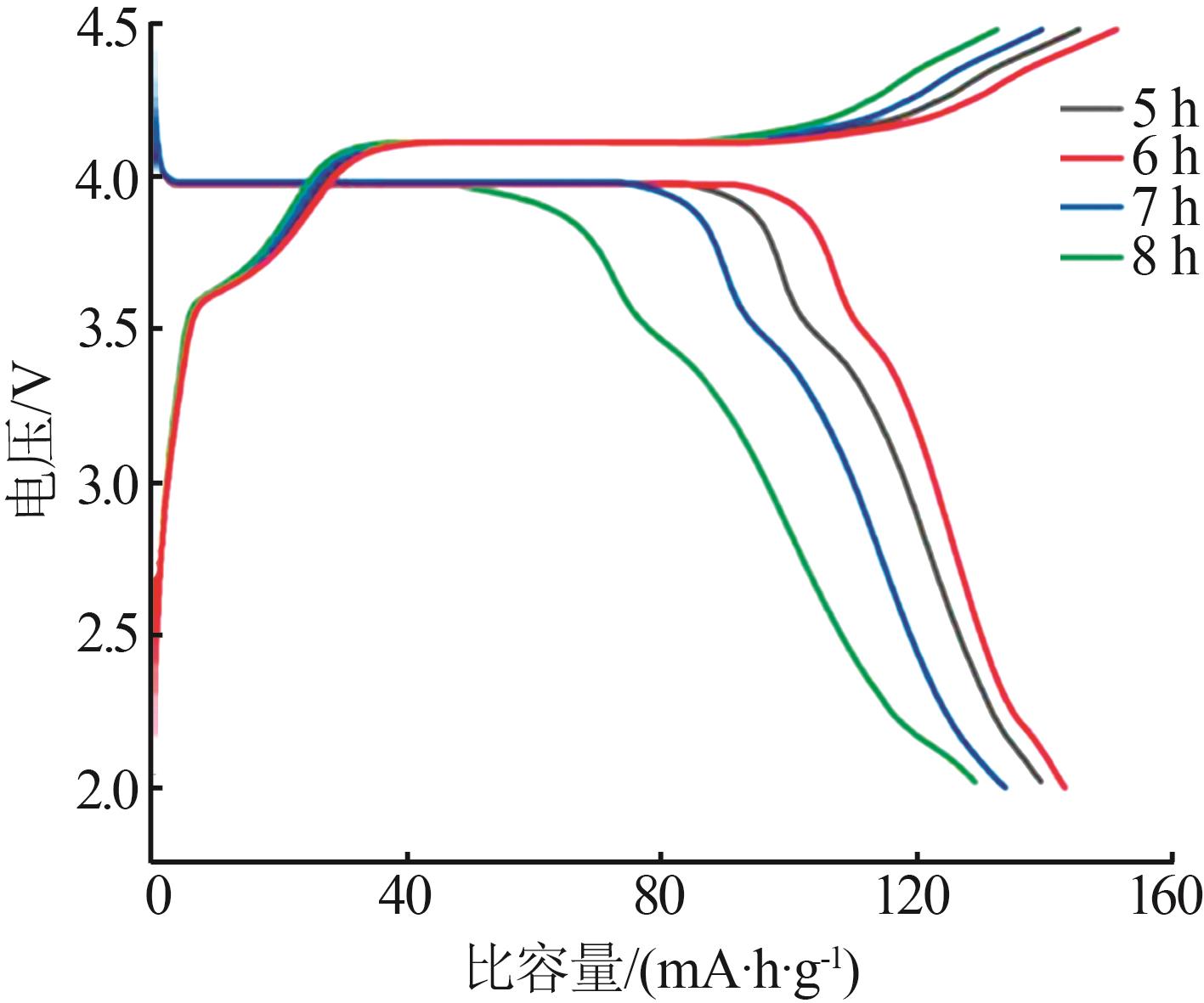
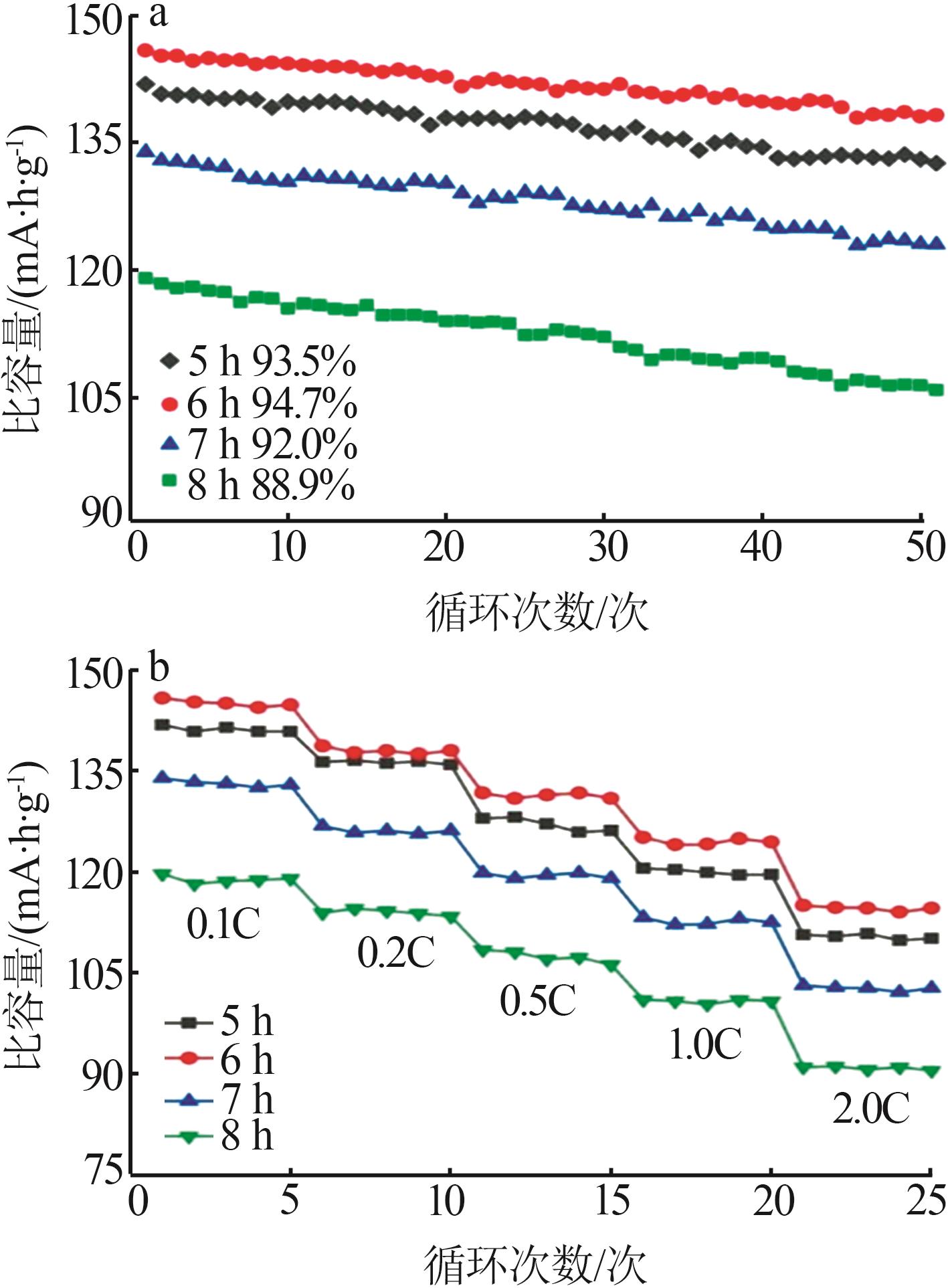
Study on preparation of lithium ferric manganese phosphate cathode materials by two-step carbon coating methods
MIAO Pu1,2( ), LUO Xiaoqiang2(
), LUO Xiaoqiang2( ), HOU Cuihong1, RUAN Haobo2, XUE Lingwei2
), HOU Cuihong1, RUAN Haobo2, XUE Lingwei2
- 1. School of Chemical Engineering,Zhengzhou University,Zhengzhou 450001,China
2. School of Chemical and Environmental Engineering,Pingdingshan University,Pingdingshan 467000,China
-
Received:2024-11-18Online:2025-11-10Published:2025-05-13 -
Contact:LUO Xiaoqiang E-mail:13939952004@163.com;lxq.5566@163.com
CLC Number:
Cite this article
MIAO Pu, LUO Xiaoqiang, HOU Cuihong, RUAN Haobo, XUE Lingwei. Study on preparation of lithium ferric manganese phosphate cathode materials by two-step carbon coating methods[J]. Inorganic Chemicals Industry, 2025, 57(11): 36-42.
share this article
| [1] | PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B.Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J].Journal of the Electrochemical Society,1997,144(4):1188-1194. |
| [2] | 潘晓晓,庄树新,孙雨晴,等.动力型磷酸铁锂正极材料改性的研究进展[J].无机盐工业,2023,55(6):18-26. |
| PAN Xiaoxiao, ZHUANG Shuxin, SUN Yuqing,et al.Research progress on modification of dynamic Ferrous lithium phosphate cathode materials[J].Inorganic Chemicals Industry,2023,55(6):18-26. | |
| [3] | 李志军,王红英,汤伟杰,等.锂离子电池正极材料LiMnPO4/C的固相法制备及性能研究[J].人工晶体学报,2015,44(3):773-778. |
| LI Zhijun, WANG Hongying, TANG Weijie,et al.Study on synthesis and characterization of LiMnPO4/C cathode materials for lithium ion batteries by solid state method[J].Journal of Synthetic Crystals,2015,44(3):773-778. | |
| [4] | FU L J, LIU H, LI C,et al.Electrode materials for lithium secondary batteries prepared by sol-gel methods[J].Progress in Materials Science,2005,50(7):881-928. |
| [5] | ZHANG Ping, LIU Debo.Effect of organic carbon coating prepared by hydrothermal method on performance of lithium iron phosphate battery[J].Alexandria Engineering Journal,2023,80:1-7. |
| [6] | GUO Ju, WU Fuyong.Preparation of nano-spherical iron phosphate by hydrothermal method and its application as the precursor of lithium iron phosphate[J].Journal of Electrochemical Energy Conversion and Storage,2021,18:011014. |
| [7] | ZHANG Junxi, XU Mingyu, CAO Xiaowei,et al.A synthesis route for lithium iron phosphate prepared by improved coprecipitati-on[J].Functional Materials Letters,2010,3(3):177-180. |
| [8] | 李晶,秦元斌,宁晓辉.改进高温固相法制备磷酸锰铁锂正极材料[J].材料导报,2020,34(16):16001-16005. |
| LI Jing, QIN Yuanbin, NING Xiaohui.Preparation of lithium manganese iron phosphate cathode material by improved high-temperature solid-state method[J].Materials Reports,2020,34(16):16001-16005. | |
| [9] | NATARAJAN S, ARAVINDAN V.Burgeoning prospects of spent lithium-ion batteries in multifarious applications[J].Advanced Energy Materials,2018,8(33):1802303. |
| [10] | 张彬,张萍,向晓刚,等.不同钴含量对三元正极材料性能的影响研究[J].无机盐工业,2021,53(11):91-94. |
| ZHANG Bin, ZHANG Ping, XIANG Xiaogang,et al.Study on effect of different cobalt content on properties of ternary cathode materials[J].Inorganic Chemicals Industry,2021,53(11):91-94. | |
| [11] | 吴兆国,曹骏,杨则恒.基于水热/溶剂热法制备不同形貌LiNi0.8Co0.1Mn0.1O2锂离子电池正极材料[J].无机盐工业,2022,54(2):72-77,105. |
| WU Zhaoguo, CAO Jun, YANG Zeheng.Preparation of LiNi0.8Co0.1Mn0.1O2 cathode materials with different morphologies based on hydrothermal/solvothermal method[J].Inorganic Chemicals Industry,2022,54(2):72-77,105. | |
| [12] | 马镰仁,谢红艳.采用两步固相法配合表面活性剂制备LiMn0.7Fe0.3PO4/C正极材料的研究[J].无机盐工业,2024,56(11):39-44. |
| MA Lianren, XIE Hongyan.Study on preparation of LiMn0.7Fe0.3PO4/C cathode materials by two-step solid-phase method with surfactant[J].Inorganic Chemicals Industry,2024,56(11):39-44. | |
| [13] | 杜柯,伍斌,胡国荣,等.两步固相法制备正极材料LiNi0.8Co0.2O2 [J].电池,2011,41(5):264-267. |
| DU Ke, WU Bin, HU Guorong,et al.Synthesis of cathode material LiNi0.8Co0.2O2 by two-step solid state method[J].Battery Bimonthly,2011,41(5):264-267. | |
| [14] | 佟健,石迪辉,曹雁冰,等.两步碳包覆工艺制备锂离子电池正极复合材料LiFePO4/C[J].湖南有色金属,2015,31(1):44-50. |
| TONG Jian, SHI Dihui, CAO Yanbing,et al.Synthesis of double carbon-coated LiFePO4/C composite cathode material by two-step synthesis route[J].Hunan Nonferrous Metals,2015,31(1):44-50. | |
| [15] | WI S, PARK J, LEE S,et al.Synchrotron-based X-ray absorption spectroscopy for the electronic structure of Lix Mn0.8Fe0.2PO4 mesocrystal in Li+ batteries[J].Nano Energy,2017,31:495-503. |
| [16] | AN Liwei, LI Zhenfei, REN Xin,et al.Low-cost synthesis of LiMn0.7Fe0.3PO4/C cathode materials with Fe2O3 and Mn3O4 via two-step solid-state reaction for lithium-ion battery[J].Ionics,2019,25(7):2997-3007. |
| [17] | 赵炯,李专,邹凌峰,等.溶胶-凝胶法结合二步烧结制备陶瓷刚玉磨料的微结构控制及晶粒细化机理[J].粉末冶金材料科学与工程,2022,27(3):319-326. |
| ZHAO Jiong, LI Zhuan, ZOU Lingfeng,et al.Microstructure control and grain refinement mechanism of ceramic corundum abrasives prepared by sol-gel combined with two step sintering process[J].Materials Science and Engineering of Powder Metallurgy,2022,27(3):319-326. | |
| [18] | 冷科,李统业,喻冲,等.两步烧结制备细晶核燃料的研究[J].核动力工程,2019,40(S2):171-176. |
| LENG Ke, LI Tongye, YU Chong,et al.Study on preparation of fine-grained nuclear fuel by two-step sintering[J].Nuclear Power Engineering,2019,40,40(S2):171-176. |
| [1] | WANG Zehui, LI Enze, QIU Xunzhao, SHANG Wenxin, LI Shasha, FENG Guobin, SHI Caixia. Research progress on anode and cathode electrocatalysts for hydrogen production by directly seawater electrolysis [J]. Inorganic Chemicals Industry, 2025, 57(8): 1-11. |
| [2] | FU Tongtong, HE Ting, ZHOU Shiyu, GU Shuai. Study on self-driven leaching of spent lithium-ion batteries cathodes and anodes with galvanic cell structure [J]. Inorganic Chemicals Industry, 2025, 57(7): 35-43. |
| [3] | SHEN Qirongxin, AI Dengdeng, JIN Shengping, XI Ruheng, HOU Xiaoyi. Study on effect of lithium concent on electrochemical performance of LiNi0.90Mn0.07Al0.03O2 cathode materials [J]. Inorganic Chemicals Industry, 2025, 57(7): 50-56. |
| [4] | DANG Jianmeng, ZHANG Zhichao, CAO Zhongkai, LI Zixuan, SHEN Jixue. Research on improving electrochemical performance of Ni-rich cathode materials under fast-charging operation [J]. Inorganic Chemicals Industry, 2025, 57(7): 14-23. |
| [5] | ZHENG Tianxin, YANG Yu, HUO Dongxing. Research progress on recycling technology of cathode metal materials for used lithium-ion batteries [J]. Inorganic Chemicals Industry, 2025, 57(6): 1-8. |
| [6] | YANG Fu, XIE Yulong. Study on preparation and Na+ doping modification of ternary material LiNi0.65Co0.15Mn0.2O2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 43-49. |
| [7] | SONG Jiaxi, JI Renfei, CHEN Jun, LIN Sen, YU Jianguo. Research on characteristics analysis and pretreatment on deeply deactivated power battery ternary cathode materials [J]. Inorganic Chemicals Industry, 2025, 57(2): 44-49. |
| [8] | ZHANG Shanshan, ZENG Yule, ZHANG Ting, LIN Sen, LIU Chenglin. Research progress of cathode pre-lithiation technology for lithium-ion batteries [J]. Inorganic Chemicals Industry, 2025, 57(1): 1-13. |
| [9] | TIAN Peng, ZHANG Haoran, XU Jingang, MOU Chenxi, XU Qianjin, NING Guiling. Study on aluminum sol modified anode and cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2024, 56(9): 44-53. |
| [10] | CHEN Xue, OUYANG Quansheng, SHAO Jiaojing. Recent research progress of lithium-sulfur batteries based on solid-solid reaction mechanism [J]. Inorganic Chemicals Industry, 2024, 56(9): 12-23. |
| [11] | SU Baocai, ZHANG Qin, XIE Yuanjian, CAI Pingxiong, PAN Yuanfeng. Advances in synthesis methods and structural modification of LiMnFePO4 materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 28-36. |
| [12] | WANG Junting, MA Hang, ZHA Zuotong, WAN Banglong, ZHANG Zhenhuan. Research progress of iron phosphate industrial wastewater treatment process [J]. Inorganic Chemicals Industry, 2024, 56(6): 26-33. |
| [13] | ZHOU Haitao, WEN Chengqin, ZHENG Ling, SUN Jie. Research on boron nitride modified film for cathode interface of metallic lithium battery [J]. Inorganic Chemicals Industry, 2024, 56(4): 85-89. |
| [14] | LIU Jie, SHI Xueru, WEI Shubing, CAO Xinxin. Artificial interface layer construction and sodium storage performance of P2-type layered oxide cathode [J]. Inorganic Chemicals Industry, 2024, 56(3): 39-44. |
| [15] | LIU Dexin, MA Tengyue, AN Jinling, LIU Jinrong, HE Weiyan. Study on cathode material design and electrochemical properties of manganese-based sodium ion battery [J]. Inorganic Chemicals Industry, 2024, 56(3): 51-55. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||