Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (11): 43-51.doi: 10.19964/j.issn.1006-4990.2024-0684
• Research & Development • Previous Articles Next Articles
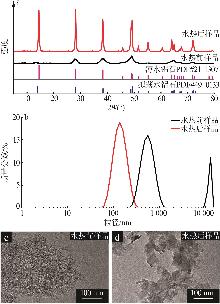
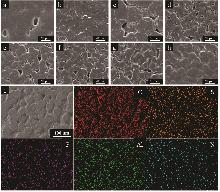
Study on enhancement of ionic conductivity in poly(ethylene oxide)-based solid-state electrolytes by boehmite
TIAN Peng( ), YANG Guanglong, MU Chenxi, CHEN Junyi, NING Guiling
), YANG Guanglong, MU Chenxi, CHEN Junyi, NING Guiling
- School of Chemical Engineering,Dalian University of Technology,Dalian 116023,China
-
Received:2024-12-17Online:2025-11-10Published:2025-07-25
CLC Number:
Cite this article
TIAN Peng, YANG Guanglong, MU Chenxi, CHEN Junyi, NING Guiling. Study on enhancement of ionic conductivity in poly(ethylene oxide)-based solid-state electrolytes by boehmite[J]. Inorganic Chemicals Industry, 2025, 57(11): 43-51.
share this article
Table 1
Ionic conductivity of different solid-state electrolytes at 30 ℃"
| 序号 | 样品名 | n(SN)∶ n(EO) | w(BM)/ % | 离子电导率 σ/(S cm-1) | 脱膜难易程度 |
|---|---|---|---|---|---|
| 1 | PEO-NaTFSI | 0 | 0 | 2.27×10-6 | 容易 |
| 2 | PNS24 | 1∶24 | 0 | 1.53×10-5 | 容易 |
| 3 | PNS16 | 1∶16 | 0 | 1.63×10-5 | 容易 |
| 4 | PNS12 | 1∶12 | 0 | 2.30 0×10-5 | 容易 |
| 5 | PNS8 | 1∶8 | 0 | 4.51×10-5 | 容易 |
| 6 | PNS7 | 1∶7 | 0 | 困难 | |
| 7 | PNS4 | 1∶4 | 0 | 无法 | |
| 8 | PNB10 | 0 | 10 | 3.11×10-6 | 容易 |
| 9 | PNS8B5 | 1∶8 | 5 | 6.36×10-5 | 容易 |
| 10 | PNS8B10 | 1∶8 | 10 | 6.99×10-5 | 容易 |
| 11 | PNS8B15 | 1∶8 | 15 | 6.57×10-5 | 容易 |
| 12 | PNS8B20 | 1∶8 | 20 | 6.15×10-5 | 容易 |
| 13 | PNS8B25 | 1∶8 | 25 | 6.02×10-5 | 容易 |
| 14 | PNS8B30 | 1∶8 | 30 | 5.19×10-5 | 容易 |
| 15 | PNS7B10 | 1∶7 | 10 | 7.77×10-5 | 容易 |
| 16 | PNS6B10 | 1∶6 | 10 | 8.99×10-5 | 容易 |
| 17 | PNS5B10 | 1∶5 | 10 | 1.11×10-4 | 容易 |
| 18 | PNS4B10 | 1∶4 | 10 | 1.43×10-4 | 容易 |
| 19 | PNS3B10 | 1∶3 | 10 | 困难 |
| [1] | SHI Kuangyi, GUAN Bin, ZHUANG Zhongqi,et al.Recent progress and prospects on sodium-ion battery and all-solid-state sodium battery:A promising choice of future batteries for energy storage[J].Energy & Fuels,2024,38(11):9280-9319. |
| [2] | YIN Hang, HAN Chengjun, LIU Qirong,et al.Recent advances and perspectives on the polymer electrolytes for sodium/potassium-ion batteries[J].Small,2021,17(31):2006627. |
| [3] | 康乐,景茂祥,李东红,等.铝酸锂纳米棒改性固态电解质的制备及电化学性能研究[J].无机盐工业,2023,55(8):65-70. |
| KANG Le, JING Maoxiang, LI Donghong,et al.Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte[J].Inorganic Chemicals Industry,2023,55(8):65-70. | |
| [4] | WANG Sen, JIANG Yingjun, HU Xianluo.Ionogel-based membranes for safe lithium/sodium batteries[J].Advanced Materials,2022,34(52):2200945. |
| [5] | YANG Huiling, ZHANG Binwei, KONSTANTINOV K,et al.Progress and challenges for all-solid-state sodium batteries[J].Advanced Energy and Sustainability Research,2021,2(2):2000057. |
| [6] | 晁广召.硅烷偶联剂修饰纳米Al2O3对全固态电池性能提高的研究[D].哈尔滨:哈尔滨工业大学,2021. |
| CHAO Guangzhao.Study on improving the performance of all-solid-state battery by modified nano-Al2O3 with silane coupling agent[D].Harbin:Harbin Institute of Technology,2021. | |
| [7] | YANG Ruilu, ZHANG Zheng, ZHANG Qi,et al.Flexible asymmetric organic-inorganic composite solid-state electrolyte based on PI membrane for ambient temperature solid-state lithium metal batteries[J].Frontiers in Chemistry,2022,10:855800. |
| [8] | ZHANG Zhizhen, ZHANG Qiangqiang, REN Cheng,et al.A ceramic/polymer composite solid electrolyte for sodium batteries[J].Journal of Materials Chemistry A,2016,4(41):15823-15828. |
| [9] | 许希军,林见烽,罗雄伟,等.NASICON型Na1+ x Zr2Si x P3- x O12固态电解质及其钠金属电池研究进展[J].无机盐工业,2024,56(11):1-14,38. |
| XU Xijun, LIN Jianfeng, LUO Xiongwei,et al.Recent progress of NASICON-type Na1+ x Zr2Si x P3- x O12 solid electrolyte for sodium metal batteries[J].Inorganic Chemicals Industry,2024,56(11):1-14,38. | |
| [10] | LI Zhiyong, LI Zhuo, FU Jialong,et al.Sodium-ion conducting polymer electrolytes[J].Rare Metals,2023,42(1):1-16. |
| [11] | 刘丽露.钠离子固体电解质及固态钠电池研究[D].北京:中国科学院大学(中国科学院物理研究所),2018. |
| LIU Lilu.Studies on solid state sodium ion electrolytes and solid state sodium batteries[D].Beijing:University of Chinese Academy of Sciences(Institute of Physics,Chinese Academy of Sciences),2018. | |
| [12] | LI Zhuo, FU Jialong, ZHOU Xiaoyan,et al.Ionic conduction in polymer-based solid electrolytes[J].Advanced Science,2023,10(10):2201718. |
| [13] | 马静媛,李妍,周晗洁,等.PEO基有机/无机复合固态电解质的研究进展[J].无机盐工业,2025,57(3):1-8. |
| MA Jingyuan, LI Yan, ZHOU Hanjie,et al.Research progress of PEO based organic/inorganic composite solid electrolyte[J].Inorganic Chemicals Industry,2025,57(3):1-8. | |
| [14] | HE Fei, HU Zhenglin, TANG Wenjing,et al.Vertically heterostructured solid electrolytes for lithium metal batteries[J].Advanced Functional Materials,2022,32(25):2201465. |
| [15] | PAN Kecheng, ZHANG Lan, QIAN Weiwei,et al.A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J].Advanced Materials,2020,32(17):2000399. |
| [16] | ZHANG Jinfang, WANG Yuanyuan, XIA Qingbing,et al.Confining polymer electrolyte in MOF for safe and high-performance all-solid-state sodium metal batteries[J].Angewandte Chemie International Edition,2024,63(16):e202318822. |
| [17] | 赵兰清,侯敏杰,张达,等.固态钠离子电池用PEO基聚合物固体电解质[J].化学进展,2023,35(11):1625-1637. |
| ZHAO Lanqing, HOU Minjie, ZHANG Da,et al.Poly(ethylene oxide)-based solid polymer electrolytes for solid-state sodiumI on batteries[J].Progress in Chemistry,2023,35(11):1625-1637. | |
| [18] | XU Shengjun, SUN Zhenhua, SUN Chengguo,et al.Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature[J].Advanced Functional Materials,2020,30(51):2007172. |
| [19] | QIU Genrui, SHI Yapeng, HUANG Bolong.A highly ionic conductive succinonitrile-based composite solid electrolyte for lithi-um metal batteries[J].Nano Research,2022,15(6):5153-5160. |
| [20] | ZHANG Xueyan, FU Chuankai, CHENG Shichao,et al.Novel PEO-based composite electrolyte for low-temperature all-solid-state lithium metal batteries enabled by interfacial cation-assistance[J].Energy Storage Materials,2023,56:121-131. |
| [21] | ZHOU Dong, HE Yanbing, LIU Ruliang,et al.In situ synthesis of a hierarchical all-solid-state electrolyte based on nitrile materials for high-performance lithium-ion batteries[J].Advanced Energy Materials,2015,5(15):1500353. |
| [22] | CHEN Fei, ZHA Wenping, YANG Dunjie,et al.All-solid-state lithium battery fitted with polymer electrolyte enhanced by solid plasticizer and conductive ceramic filler[J].Journal of the Electrochemical Society,2018,165(14):A3558. |
| [23] | GUO Shiyuan, SU Yuefeng, YAN Kang,et al.Robust and adhesive laminar solid electrolyte with homogenous and fast Li-ion conduction for high-performance all-solid-state lithium metal battery[J].Advanced Science,2024,11(30):2404307. |
| [24] | WEI Xiuqin, WANG Qiulin, SONG Zhenglin,et al.Effect of hydroxyls and particle size on the electrochemical performance of boehmite coated PE separators for lithium-ion batteries[J].Solid State Ionics,2021,366-367:115652. |
| [25] | 冯锟,朱勇,张凯强,等.勃姆石纳米片增强锂离子电池隔膜性能研究[J].无机材料学报,2022,37(9):1009-1016. |
| FENG Kun, ZHU Yong, ZHANG Kaiqiang,et al.Boehmite nanosheets-coated separator with enhanced performance for lithi-um-ion batteries[J].Journal of Inorganic Materials,2022,37(9):1009-1016. | |
| [26] | GAO Tingting, TIAN Peng, XU Qianjin,et al.Class of boehmite/polyacrylonitrile membranes with different thermal shutdown temperatures for high-performance lithium-ion batteries[J].ACS Applied Materials & Interfaces,2023,15(1):2112-2123. |
| [27] | ZHAO Weiran, TIAN Peng, GAO Tingting,et al.Different-grain-sized boehmite nanoparticles for stable all-solid-state lithium metal batteries[J].Nanoscale,2024,16(23):11163-11173. |
| [28] | ALSULAMI Q A.Structural,dielectric,and magnetic studies based on MWCNTs/NiFe2O4/ZnO nanoparticles dispersed in poly-mer PVA/PEO for electromagnetic applications[J].Journal of Materials Science:Materials in Electronics,2021,32(3):2906-2924. |
| [29] | BANDYOPADHYAY S, GUPTA A, SRIVASTAVA R,et al.Bio-inspired design of electrospun poly(acrylonitrile) and novel ionene based nanofibrous mats as highly flexible solid state polymer electrolyte for lithium batteries[J].Chemical Engineering Journal,2022,440:135926. |
| [30] | SHENG Ouwei, JIN Chengbin, LUO Jianmin,et al.Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity,excellent mechanical properties,and flame-retardant performance[J].Nano Letters,2018,18(5):3104-3112. |
| [31] | XU Hao, LIU Shuai, LI Zhiang,et al.Ti3C2T x MXene enhanced PEO/SN-based solid electrolyte for high-performance Li metal battery[J].Journal of Materials Science & Technology,2025,219:101-112. |
| [32] | WANG Han, SUN Yongjiang, LIU Qing,et al.An asymmetric bilayer polymer-ceramic solid electrolyte for high-performance sodium metal batteries[J].Journal of Energy Chemistry,2022,74:18-25. |
| [33] | LI Bangxing, YI Xianlin, XIE Zhenjun,et al.A promising composite room temperature solid electrolyte via incorporating LLZTO into cross-linked ETPTA/PEO/SN matrix for all solid state lithi- um batteries[J].Ionics,2024,30(4):2007-2017. |
| [34] | FU Wenjing, WEI Cundi, ZUO Jing,et al.A facile temperature-controlled“green” method to prepare multi-kinds of high-quality alumina hydrates via a Ga-In-Sn-alloyed aluminum-water interface reaction[J].ACS Omega,2022,7(23):19775-19783. |
| [35] | LIU Lehao, MO Jinshan, LI Jingru,et al.Comprehensively-modified polymer electrolyte membranes with multifunctional PMIA for highly-stable all-solid-state lithium-ion batteries[J].Journal of Energy Chemistry,2020,48:334-343. |
| [36] | SONG Jiechen, XU Yuxing, ZHOU Yuncheng,et al.Incorporating 2D γ-Al2O3 nanosheets into the flexible PEO-based solid electrolyte for lithium metal batteries[J].Electrochimica Acta,2023,437:141504. |
| [37] | WANG Meihuang, TIAN Liying, CAO Yu,et al.Surface positive-charged modification of inorganic fillers to optimize lithium ion conductive pathways in composite polymer electrolytes for lithium-metal batteries[J].Journal of Colloid and Interface Science,2023,630:634-644. |
| [38] | SU Yanxia, MU Zheshen, QIU Yuqian,et al.Embedding of laser generated TiO2 in poly(ethylene oxide) with boosted Li+ conduction for solid-state lithium metal batteries[J].ACS Applied Materials & Interfaces,2023,15(48):55713-55722. |
| [39] | WANG Xiaoen, ZHU Haijin, GIRARD G A,et al.Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids[J].Journal of Materials Chemistry A,2017,5(45):23844-23852. |
| [40] | SHEN Lin, DENG Shungui, JIANG Rongrong,et al.Flexible composite solid electrolyte with 80% Na3.4Zr1.9Zn0.1Si2.2P0.8O12 for solid-state sodium batteries[J].Energy Storage Materials,2022,46:175-181. |
| [1] | TIAN Peng, JIAO Dandan, LI Fan, NING Guiling. Study on synthesis of high purity pseudoboehmite by hydrolyzing isooctanol aluminum [J]. Inorganic Chemicals Industry, 2025, 57(10): 49-54. |
| [2] | XU Xijun, LIN Jianfeng, LUO Xiongwei, ZHAO Jingwei, HUO Yanping. Recent progress of NASICON-type Na1+x Zr2Si x P3-x O12 solid electrolyte for sodium metal batteries [J]. Inorganic Chemicals Industry, 2024, 56(11): 1-14. |
| [3] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| [4] | TIAN Peng, ZHOU Ruohui, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Synthesis and dehydration dynamics of boehmite microcrystalline with different particle sizes [J]. Inorganic Chemicals Industry, 2023, 55(11): 27-36. |
| [5] | YANG Yongyu,TIAN Peng,ZHOU Ruohui,XU Qianjin,LIU Kunji,NING Guiling. Effect of gibbsite activation on preparation of boehmite by hydrothermal method [J]. Inorganic Chemicals Industry, 2022, 54(9): 55-62. |
| [6] | LI Fan,YANG Yuzhe,TIAN Peng,LI Wei,NING Guiling. Preparation of high purity pseudo-boehmite by hydrolysis of aluminium sec-butoxide [J]. Inorganic Chemicals Industry, 2022, 54(7): 78-84. |
| [7] | TIAN Peng,LI Wei,YANG Yuzhe,LIU Kunji,XU Qianjin,NING Guiling. Preparation of high purity pseudo-boehmite by hydrolysis of aluminium isopropanol [J]. Inorganic Chemicals Industry, 2022, 54(2): 54-59. |
| [8] | SHI Kai,ZHAO Xu,ZHANG Hongzhi,REN Yixuan,LI Xiaofeng. Study on synthesis of SAPO-34 molecular sieve by silicon-modified pseudo-boehmite [J]. Inorganic Chemicals Industry, 2022, 54(10): 149-154. |
| [9] | Yang Yongyu,Gao Tingting,Tian Peng,Xu Qianjin,Liu Kunji,Ning Guiling. Research progress of lithium-ion battery separator modified with inorganic ultrafine powder [J]. Inorganic Chemicals Industry, 2021, 53(6): 49-58. |
| [10] | Xiong Xiaoyun,Hu Qingxun,Zhao Hongjuan,Gao Xionghou. Impact of alumina matrix on cracking performance of heavy oil molecule of FCC catalyst [J]. Inorganic Chemicals Industry, 2019, 51(8): 83-87. |
| [11] | Nan Jun,Yu Qun,Yu Haibin,Zhang Jingcheng,Xiao Han,Zhu Jinjian,Li Xiaoyun. Study on a new production process of pseudo-boehmite and its application in hydrofining catalysts [J]. Inorganic Chemicals Industry, 2019, 51(6): 80-82. |
| [12] | LI Xiao-Yun, YU Hai-Bin, SUN Yan-Min, LI Shi-Peng, ZENG Xian-Jun, SUI Yun-Le, ZHOU Peng. Inorganic aluminum salt assisted hydrothermal synthesis of pseudoboehmite by activated alumina [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 35-. |
| [13] | ZHANG Chun-Li, YE Xue-Hai, REN Chun-Yan. Study on succinonitrile as additive of electrolyte solution [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 65-. |
| [14] | WANG Yu-Ling. Production of large pore volume active alumina [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(4): 25-. |
| [15] | SHANG Lian-Di, WANG Hui-Hui. Production and modification of activated alumina [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(1): 1-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||