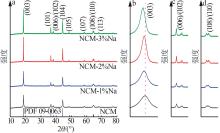
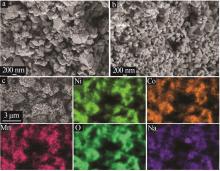
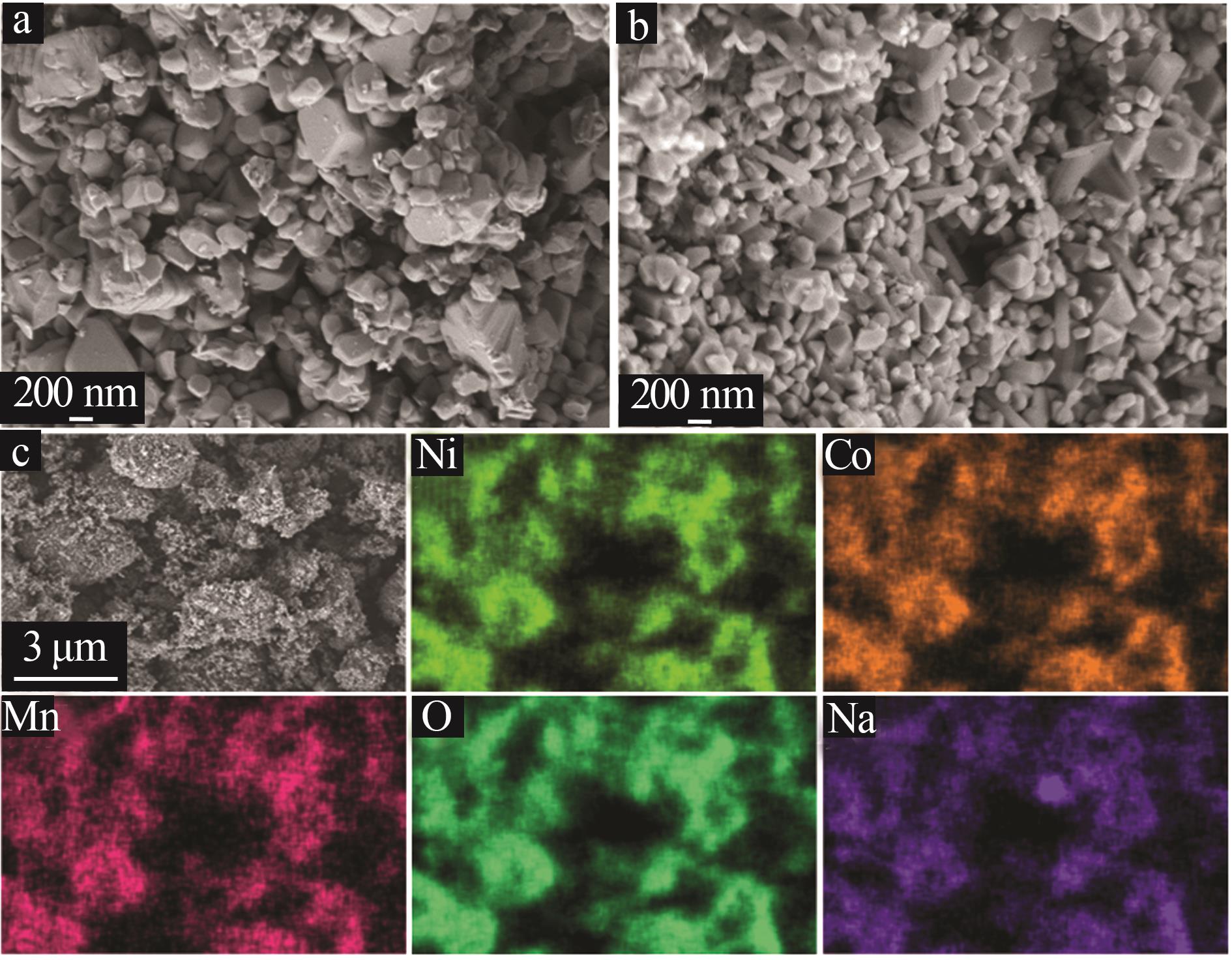
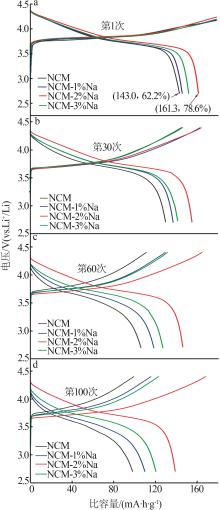
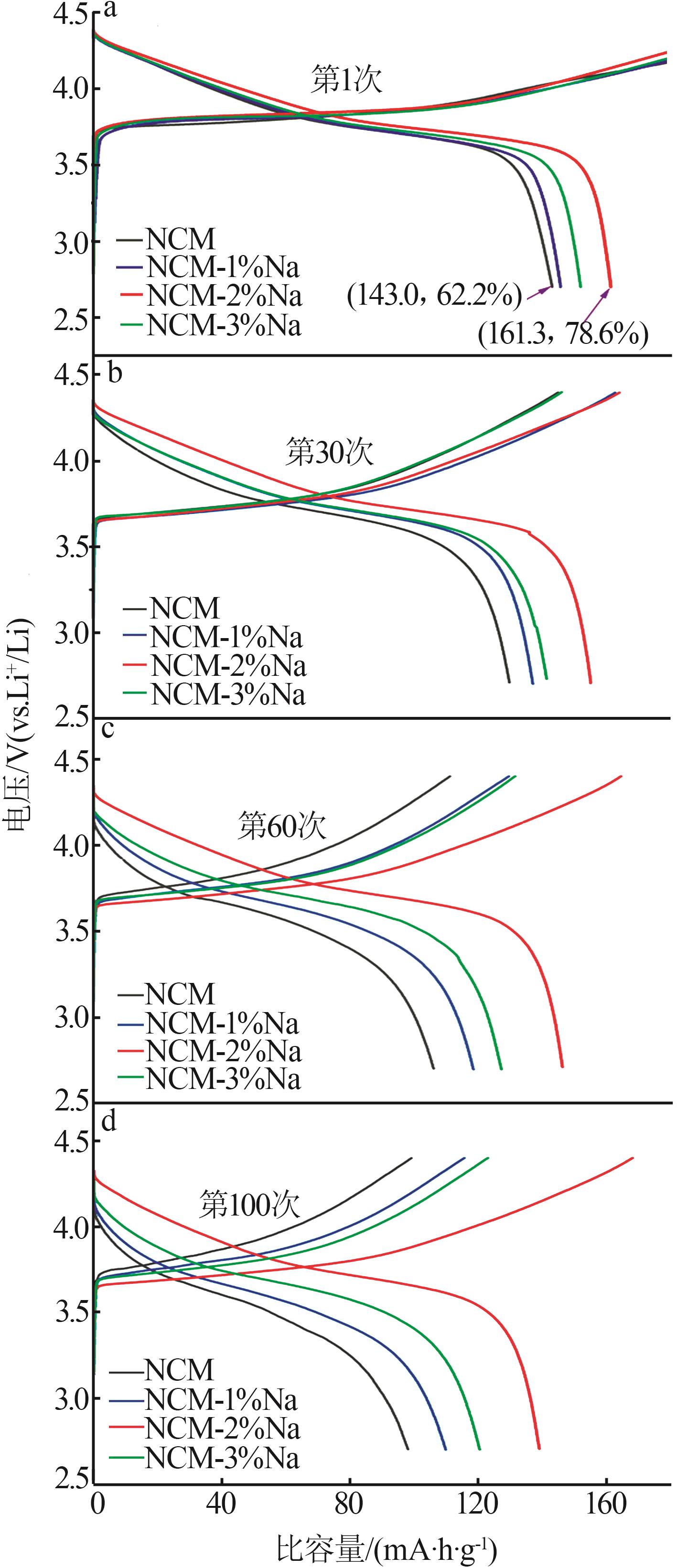
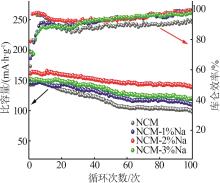
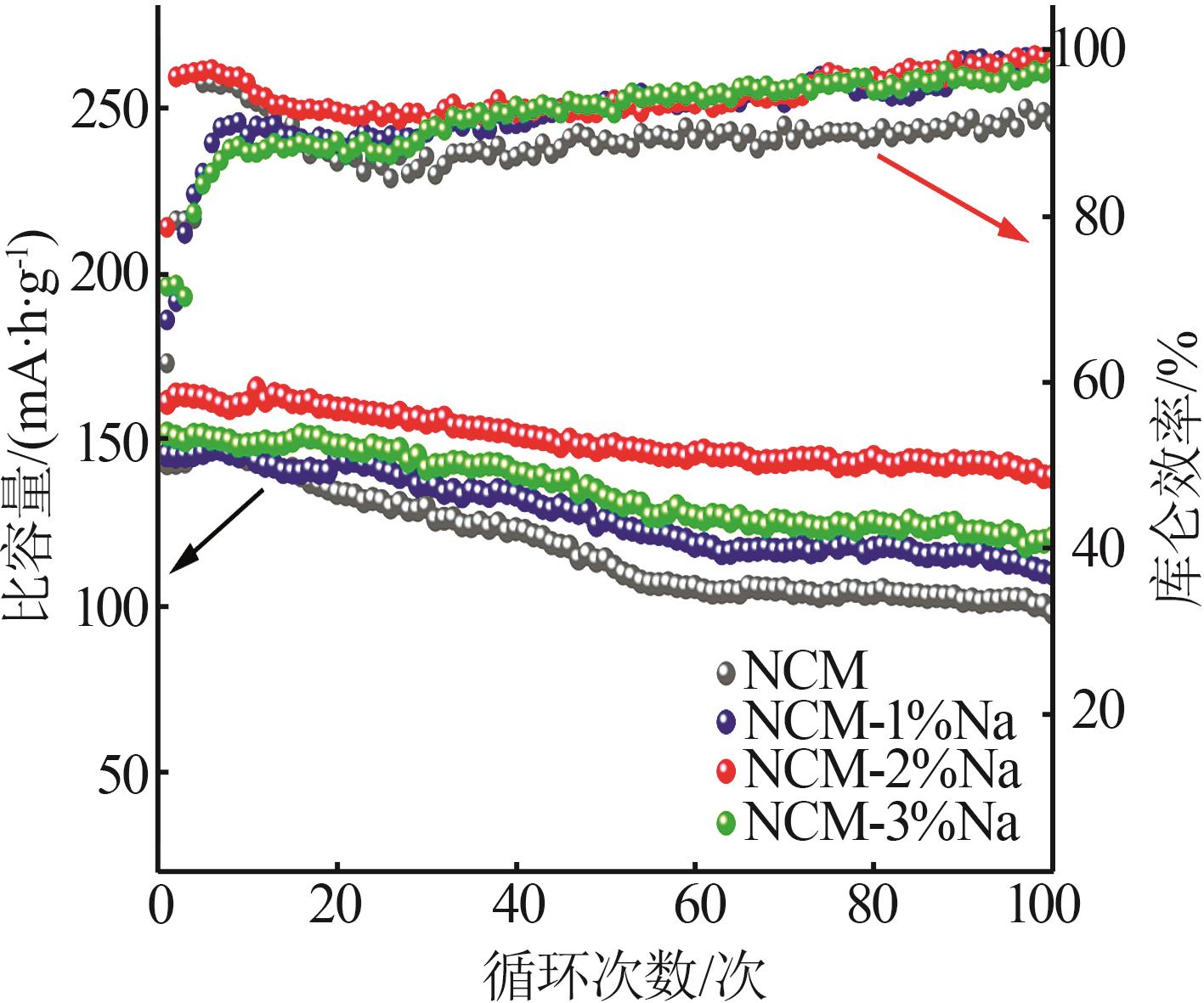
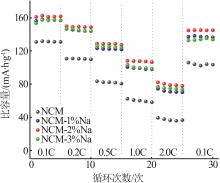
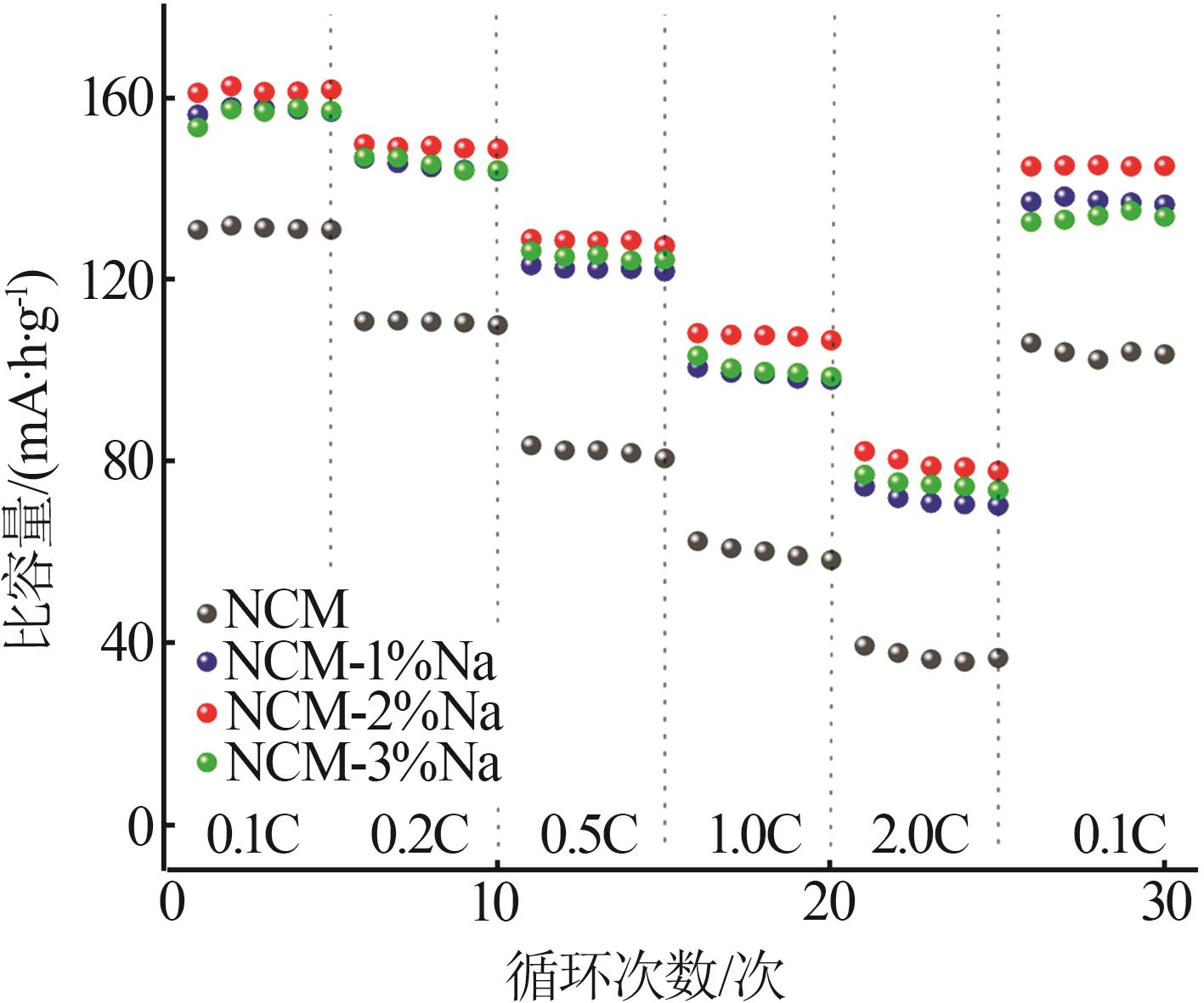
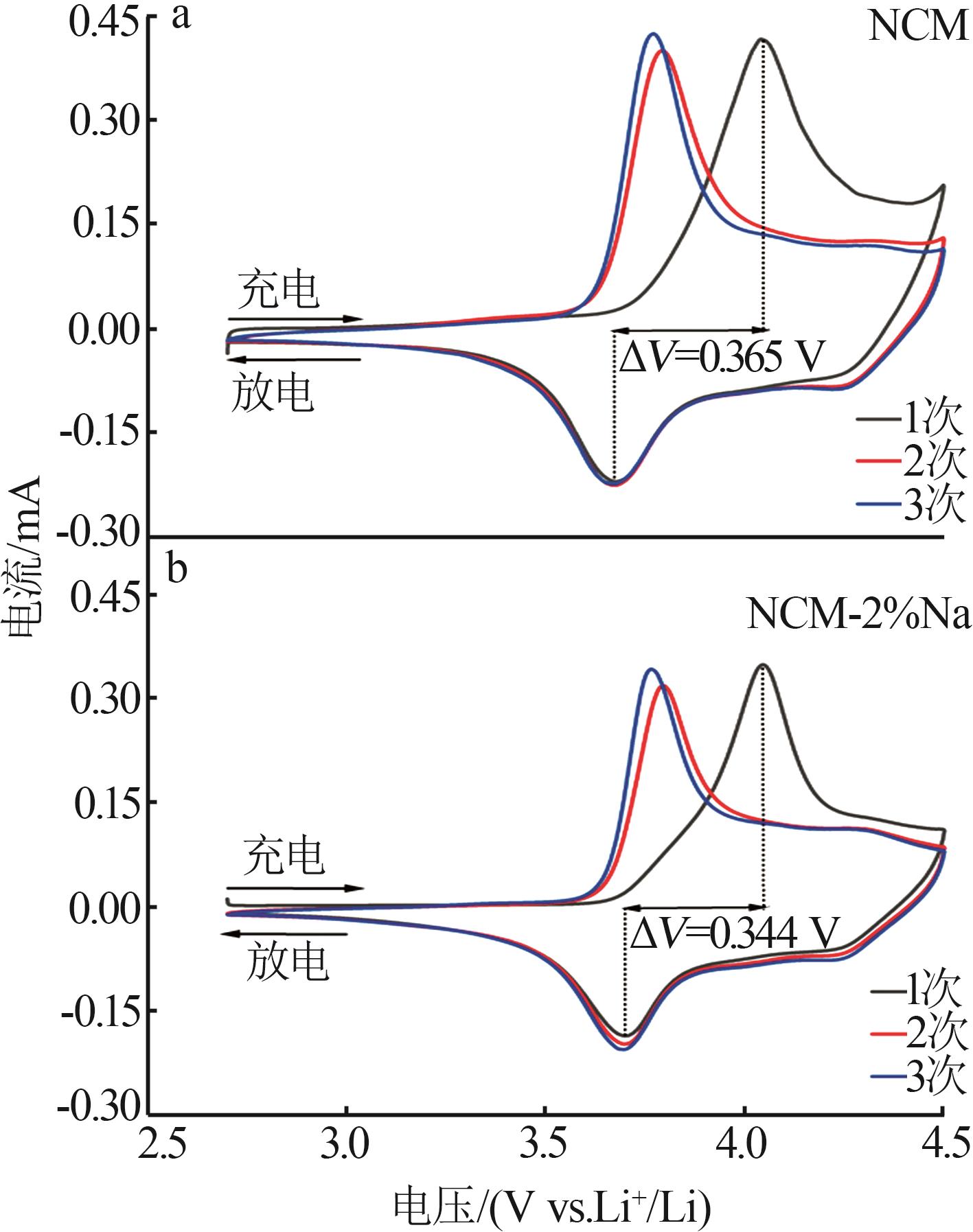
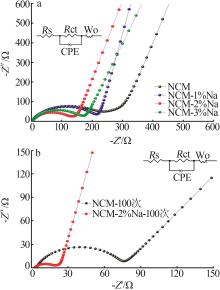
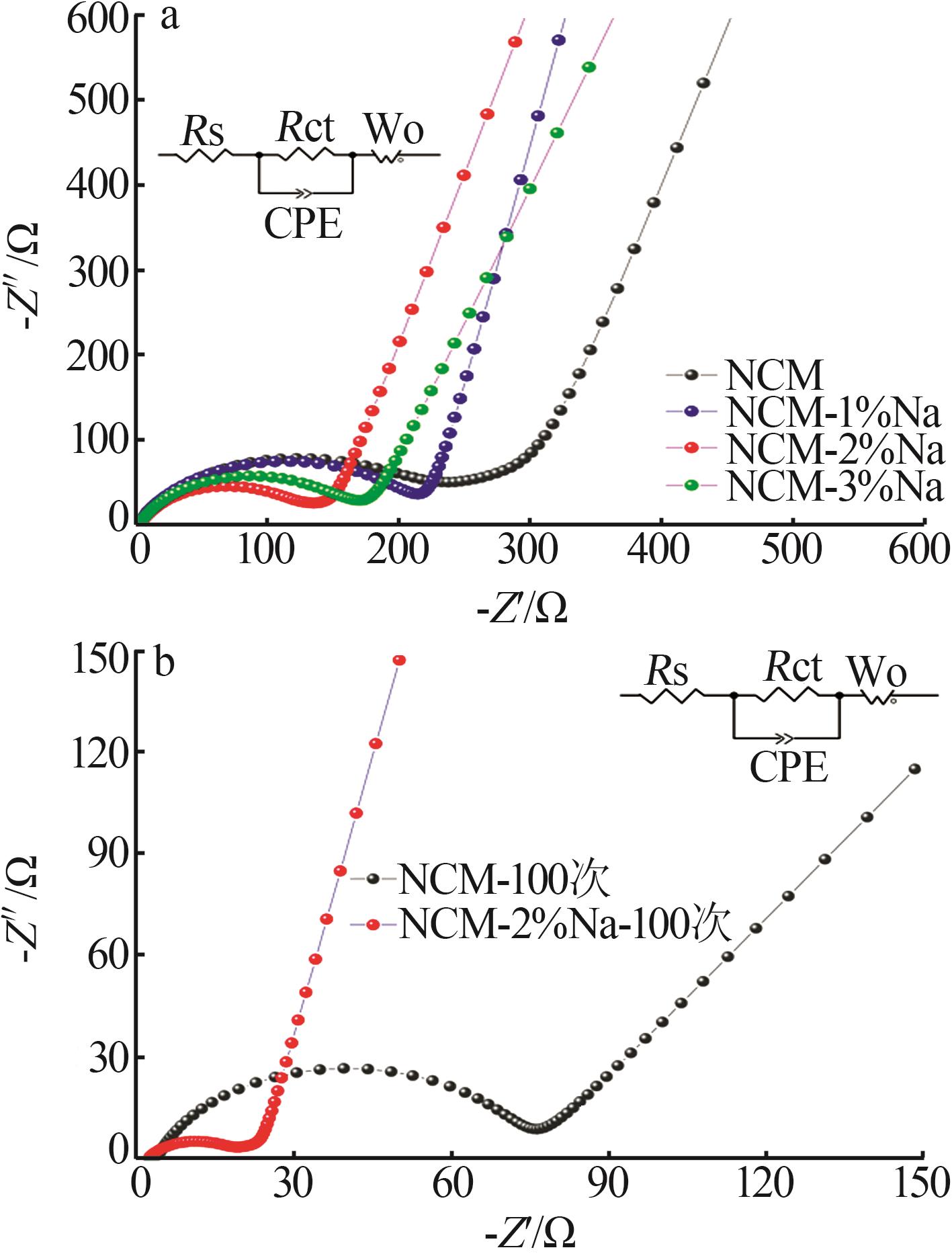
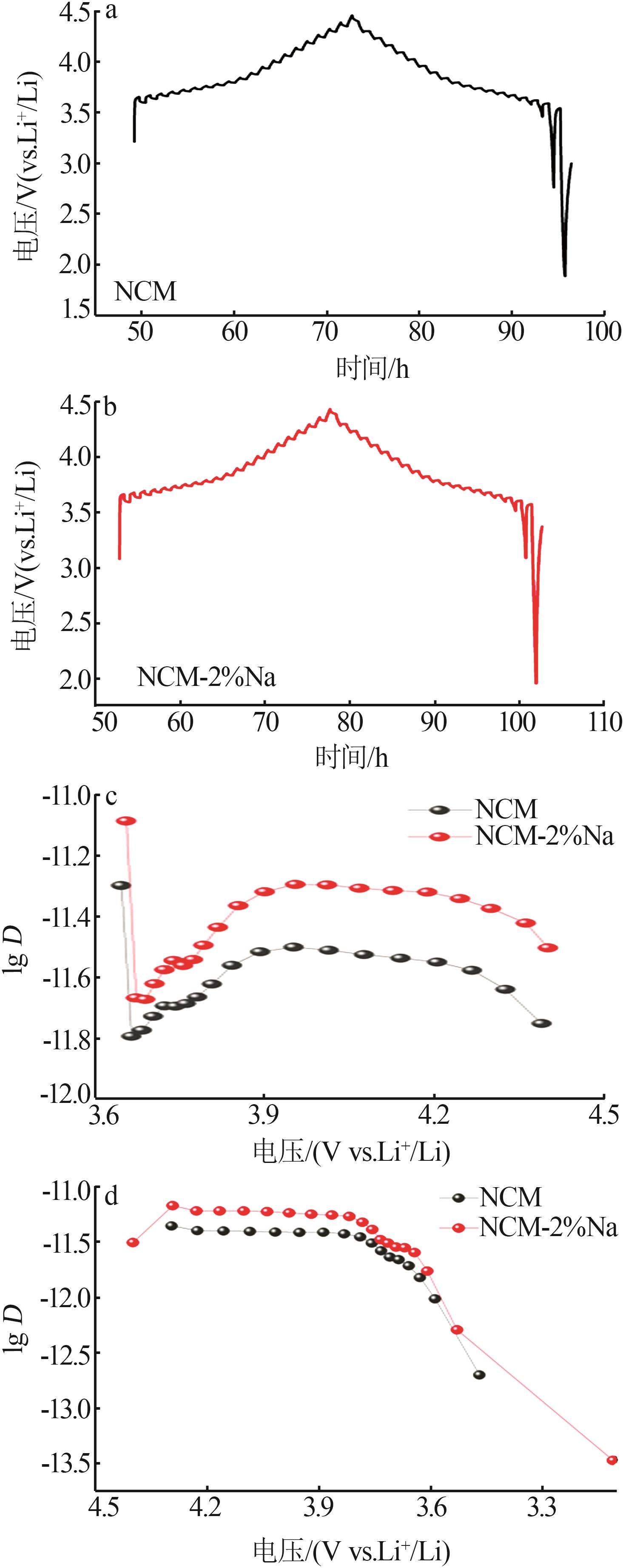
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (3): 43-49.doi: 10.19964/j.issn.1006-4990.2024-0229
• Research & Development • Previous Articles Next Articles
Study on preparation and Na+ doping modification of ternary material LiNi0.65Co0.15Mn0.2O2
- School of Chemistry and Chemical Engineering,Qinghai Minzu University,Key Laboratory of Resource Chemistry and Eco-environmental Protection in Tibetan Plateau of State Ethnic Affairs Commission,Qinghai Provincial Key Laboratory of Nanomaterials and Nanotechnology,Xining 810007,China
-
Received:2024-04-26Online:2025-03-10Published:2025-03-21 -
Contact:XIE Yulong E-mail:473369590@qq.com;yulongxie2012@126.com
CLC Number:
Cite this article
YANG Fu, XIE Yulong. Study on preparation and Na+ doping modification of ternary material LiNi0.65Co0.15Mn0.2O2[J]. Inorganic Chemicals Industry, 2025, 57(3): 43-49.
share this article
| 1 | YU Wanjing, HE Wenjie, WANG Chaolei,et al.Confinement of TiO2 quantum dots in graphene nanoribbons for high-performance lithium and sodium ion batteries[J].Journal of Alloys and Compounds,2022,898:162856. |
| 2 | YU Wanjing, LIU Fan, ZHANG Lili,et al.Lithiophilic ZnO confined in microscale carbon cubes as a stable host for lithium metal anodes[J].Carbon,2022,196:92-101. |
| 3 | ZHANG Guoliang, LI Gaoyang, WANG Jun,et al.2D SnSe cathode catalyst featuring an efficient facet-dependent selective Li2O2 growth/decomposition for Li-oxygen batteries[J].Advanced Energy Materials,2022,12(21):2103910. |
| 4 | HOU Peiyu, YIN Jiangmei, DING Meng,et al.Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes:Advances and perspectives[J].Small,2017,13(45).Doi:10.1002/smll.201701802. |
| 5 | UDDIN M J, ALABOINA P K, CHO S J.Nanostructured cathode materials synthesis for lithium-ion batteries[J].Materials Today Energy,2017,5:138-157. |
| 6 | LI Wangda, ERICKSON E M, MANTHIRAM A.High-nickel layered oxide cathodes for lithium-based automotive batteries[J].Nature Energy,2020,5:26-34. |
| 7 | ETACHERI V, MAROM R, ELAZARI R,et al.Challenges in the development of advanced Li-ion batteries:A review[J].Energy & Environmental Science,2011,4(9):3243-3262. |
| 8 | 王甲泰,赵段,马莲花,等.锂离子电池正极材料磷酸铁锂的研究进展[J].无机盐工业,2020,52(4):18-22. |
| WANG Jiatai, ZHAO Duan, MA Lianhua,et al.Research progress of LiFePO4 cathode materials for Li-ion battery[J].Inorganic Chemicals Industry,2020,52(4):18-22. | |
| 9 | LI Yilin, FAN Ziqiang, PENG Zhijian,et al.Metal-organic framework-derived LiFePO4/C composites for lithium storage:In situ construction,effective exploitation,and targeted restoration[J].EcoMat,2023,5(12):e12415. |
| 10 | BALOGUN M S, YANG Hao, LUO Yang,et al.Achieving high gravimetric energy density for flexible lithium-ion batteries facilitated by core-double-shell electrodes[J].Energy & Environmental Science,2018,11(7):1859-1869. |
| 11 | WANG Huayu, ZHAO Naiqin, SHI Chunsheng,et al.Interface and doping effect on the electrochemical property of graphene/LiFePO4 [J].The Journal of Physical Chemistry C,2016,120(31):17165-17174. |
| 12 | 周煌,胡晓萍,任稳,等.硫掺杂Na3(VOPO4)2F正极材料的制备及储钠性能[J].无机盐工业,2024,56(2):30-37. |
| ZHOU Huang, HU Xiaoping, REN Wen,et al.Preparation and sodium storage properties of sulfur-doped Na3(VOPO4)2F cathode materials[J].Inorganic Chemicals Industry,2024,56(2):30-37. | |
| 13 | YU Haifeng, WANG Shouliang, HU Yanjie,et al.Lithium-conductive LiNbO3 coated high-voltage LiNi0.5Co0.2Mn0.3O2 cathode with enhanced rate and cyclability[J].Green Energy & Environment,2022,7(2):266-274. |
| 14 | SHIN M R, LEE S J, KIM S J,et al.Preparation and effect of(3-aminopropyl)triethoxysilane-coated LiNi0.5Co0.2Mn0.3O2 cathode material for lithium ion batteries[J].Journal of Nanoscience and Nanotechnology,2020,20(6):3460-3465. |
| 15 | 郭金梅,朱华丽,沈瑞,等.双重改性三元正极材料及其性能研究[J].现代化工,2024,44(1):120-125,132. |
| GUO Jinmei, ZHU Huali, SHEN Rui,et al.Study on properties of dually modified ternary cathode materials[J].Modern Chemical Industry,2024,44(1):120-125,132. | |
| 16 | WEI Hanxin, TANG Linbo, HUANG Yingde,et al.Comprehensive understanding of Li/Ni intermixing in layered transition metal oxides[J].Materials Today,2021,51:365-392. |
| 17 | LIU Qi, WU Zhenqian, SUN Jingying,et al.Facile synthesis of crack-free single-crystalline Al-doped Co-free Ni-rich cathode material for lithium-ion batteries[J].Electrochimica Acta,2023,437:141473. |
| 18 | HE Tao, LU Yun, SU Yuefeng,et al.Sufficient utilization of zirconium ions to improve the structure and surface properties of nickel-rich cathode materials for lithium-ion batteries[J].ChemSusChem,2018,11(10):1639-1648. |
| 19 | YANG Jun, XIA Yongyao.Enhancement on the cycling stability of the layered Ni-rich oxide cathode by in situ fabricating nano-thickness cation-mixing layers[J].Journal of the Electrochemical Society,2016,163(13):A2665-A2672. |
| 20 | ZHANG Chunfang, WAN Jiajia, LI Yixiao,et al.Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping[J].Journal of Materials Chemistry A,2020,8(14):6893-6901. |
| 21 | WU Liping, TANG Xincun, CHEN Xi,et al.Improvement of electrochemical reversibility of the Ni-rich cathode material by gallium doping[J].Journal of Power Sources,2020,445:227337. |
| 22 | LU Yang, JIN Hongfei, MO Yan,et al.Synthesis and characterization of Cu-doped LiNi0.6Co0.2Mn0.2O2 materials for Li-ion batteries[J].Journal of Alloys and Compounds,2020,844:156180. |
| 23 | LV Yeting, CHENG Xu, QIANG Wenjiang,et al.Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping[J].Journal of Power Sources,2020,450:227718. |
| 24 | JEONG M, KIM H, LEE W,et al.Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries[J].Journal of Power Sources,2020,474:228592. |
| [1] | SONG Jiaxi, JI Renfei, CHEN Jun, LIN Sen, YU Jianguo. Research on characteristics analysis and pretreatment on deeply deactivated power battery ternary cathode materials [J]. Inorganic Chemicals Industry, 2025, 57(2): 44-49. |
| [2] | TIAN Peng, ZHANG Haoran, XU Jingang, MOU Chenxi, XU Qianjin, NING Guiling. Study on aluminum sol modified anode and cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2024, 56(9): 44-53. |
| [3] | CHEN Xue, OUYANG Quansheng, SHAO Jiaojing. Recent research progress of lithium-sulfur batteries based on solid-solid reaction mechanism [J]. Inorganic Chemicals Industry, 2024, 56(9): 12-23. |
| [4] | SU Baocai, ZHANG Qin, XIE Yuanjian, CAI Pingxiong, PAN Yuanfeng. Advances in synthesis methods and structural modification of LiMnFePO4 materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 28-36. |
| [5] | WANG Junting, MA Hang, ZHA Zuotong, WAN Banglong, ZHANG Zhenhuan. Research progress of iron phosphate industrial wastewater treatment process [J]. Inorganic Chemicals Industry, 2024, 56(6): 26-33. |
| [6] | LIU Dexin, MA Tengyue, AN Jinling, LIU Jinrong, HE Weiyan. Study on cathode material design and electrochemical properties of manganese-based sodium ion battery [J]. Inorganic Chemicals Industry, 2024, 56(3): 51-55. |
| [7] | ZHOU Huang, HU Xiaoping, REN Wen, CAO Xinxin. Preparation and sodium storage properties of sulfur-doped Na3(VOPO4)2F cathode materials [J]. Inorganic Chemicals Industry, 2024, 56(2): 30-37. |
| [8] | GE Jianhua, XIE Minyan, OUYANG Quansheng, SHAO Jiaojing. Advances in regeneration processes of cathode materials for spent power batteries [J]. Inorganic Chemicals Industry, 2024, 56(12): 79-87. |
| [9] | ZHAO Runze, QIAN A′niu. Research progress of lithium recovery for spent lithium-ion batteries and preparation in battery-grade lithium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(12): 70-78. |
| [10] | LIU Jiasheng, LUO Xiaoqiang, HOU Cuihong, XUE Lingwei. Effects of fluorine doping on electrochemical behavior of LiMn0.8Fe0.2PO4/C cathode materials [J]. Inorganic Chemicals Industry, 2024, 56(11): 45-50. |
| [11] | LIU Juan, JIANG Qinglai, ZHANG Yueyi. Study on Al-Zn co-doping of 4.6 V high voltage lithium cobalt oxide cathode materials [J]. Inorganic Chemicals Industry, 2024, 56(11): 59-64. |
| [12] | MA Lianren, XIE Hongyan. Study on preparation of LiMn0.7Fe0.3PO4/C cathode materials by two-step solid-phase method with surfactant [J]. Inorganic Chemicals Industry, 2024, 56(11): 39-44. |
| [13] | LU Junhao. Study on full element recycling process of retired ternary power lithium battery [J]. Inorganic Chemicals Industry, 2023, 55(6): 92-103. |
| [14] | ZHU Zhihong, ZHU Yongfang. Study on preparation and properties of silicon doped lithium manganate by self-propagating combustion [J]. Inorganic Chemicals Industry, 2023, 55(5): 66-70. |
| [15] | TIAN Peng, XU Jingang, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Preparation of nano-alumina slurry and its application in modifying lithium-ion battery cathode material [J]. Inorganic Chemicals Industry, 2023, 55(12): 43-49. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||