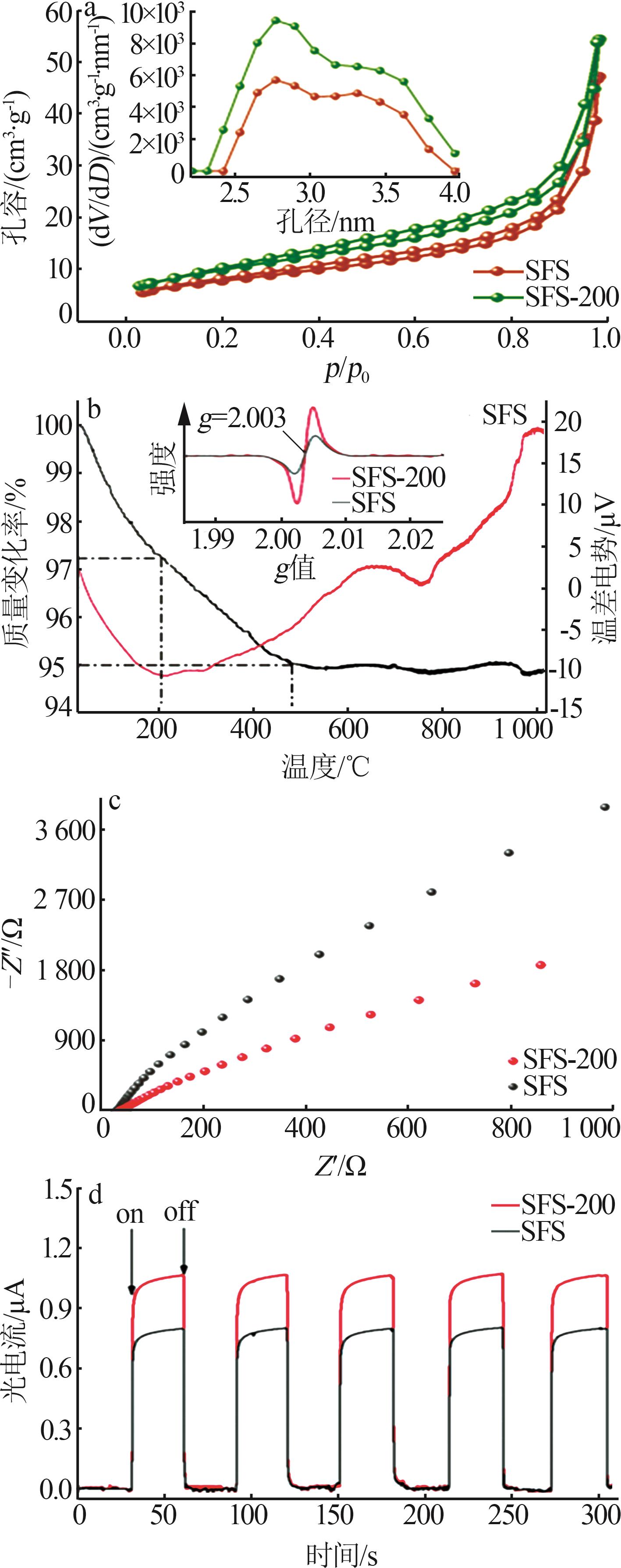
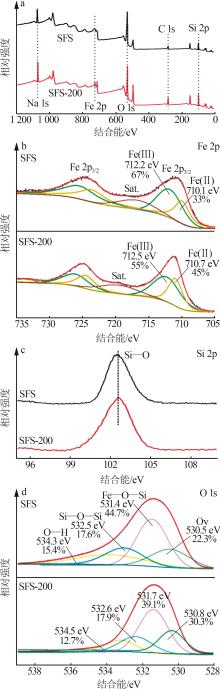
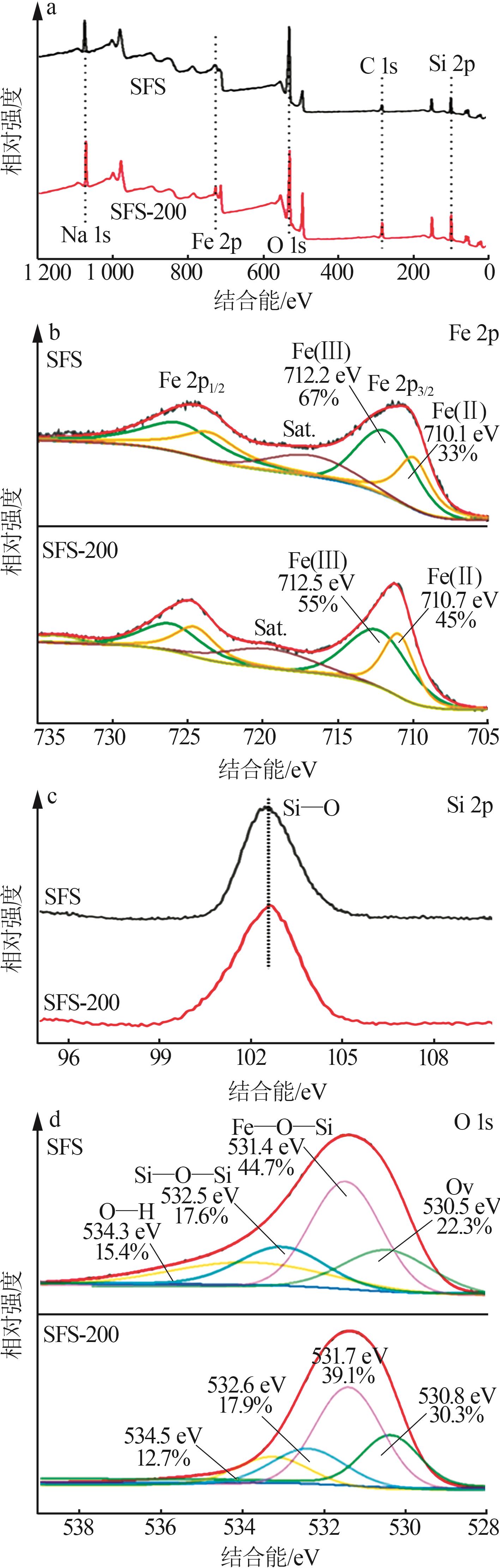
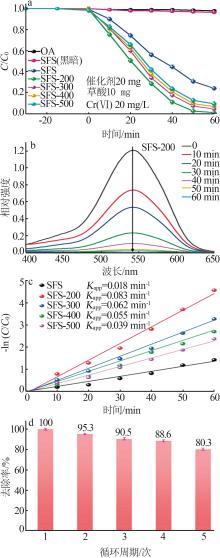
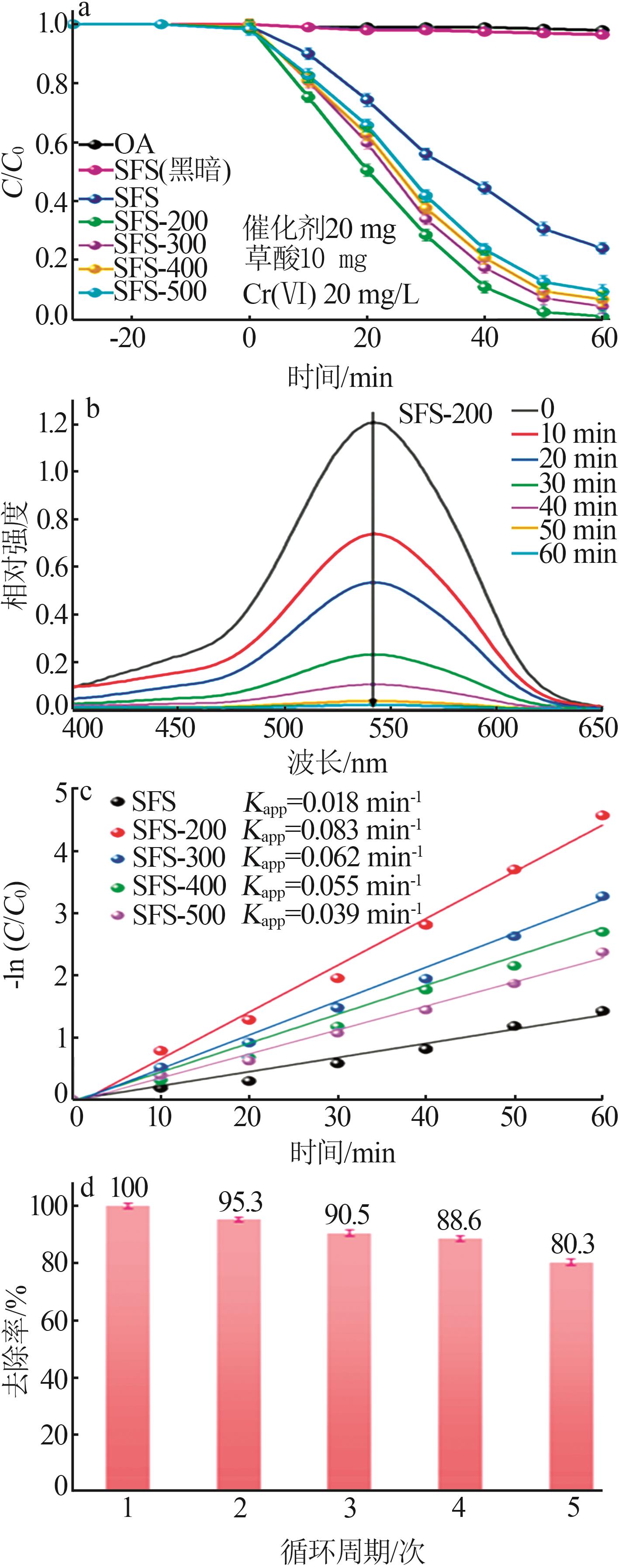
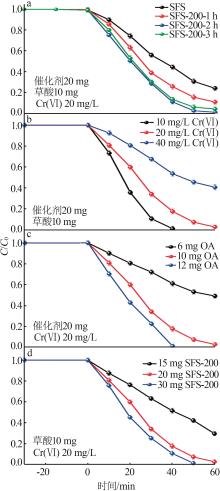
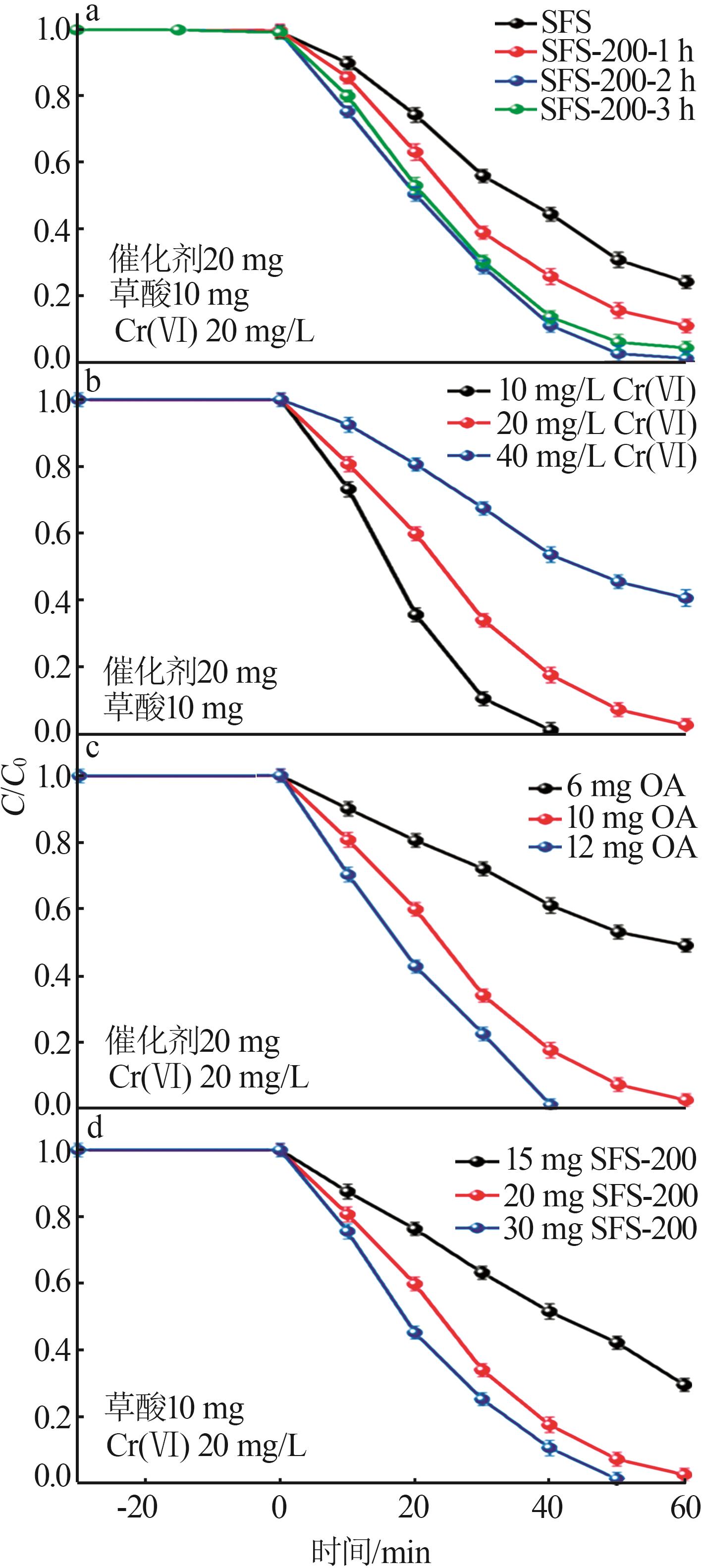
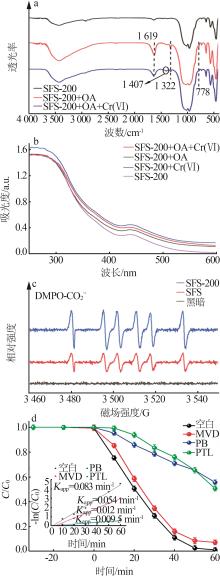
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (10): 141-150.doi: 10.19964/j.issn.1006-4990.2024-0104
• Catalytic Materials • Previous Articles Next Articles
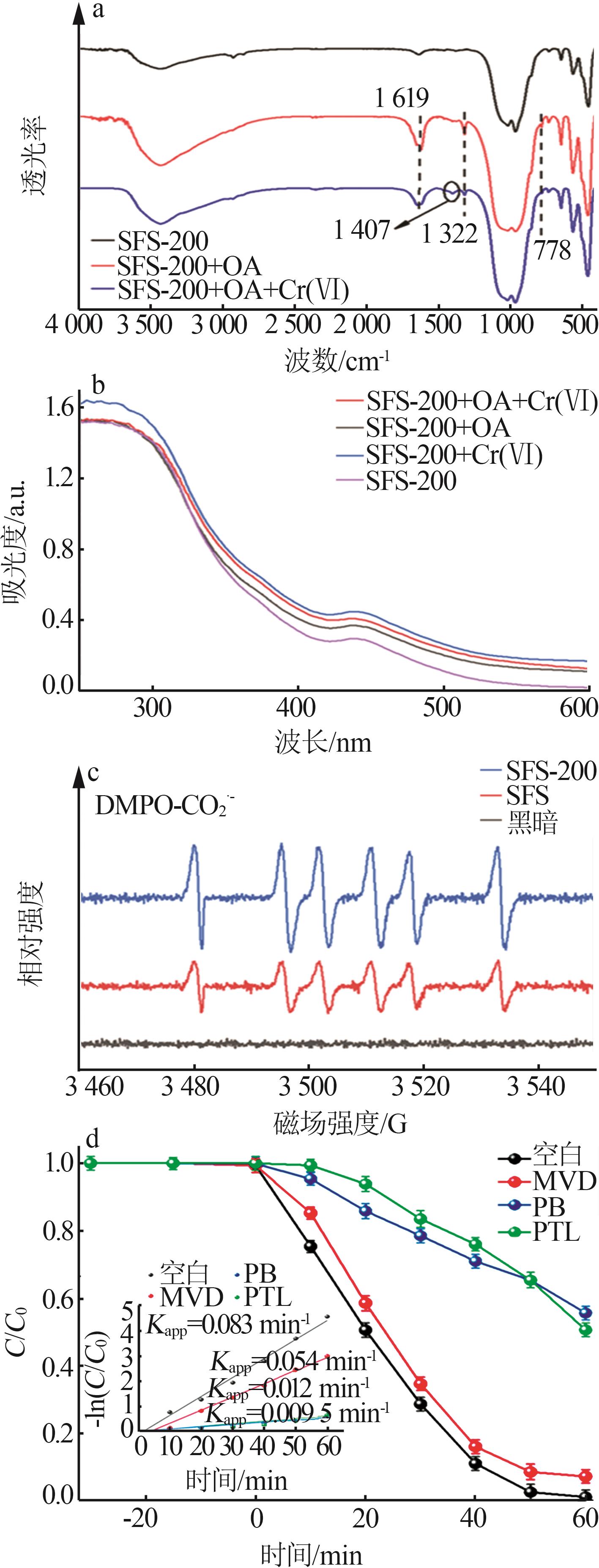
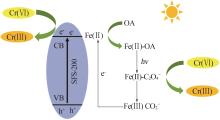
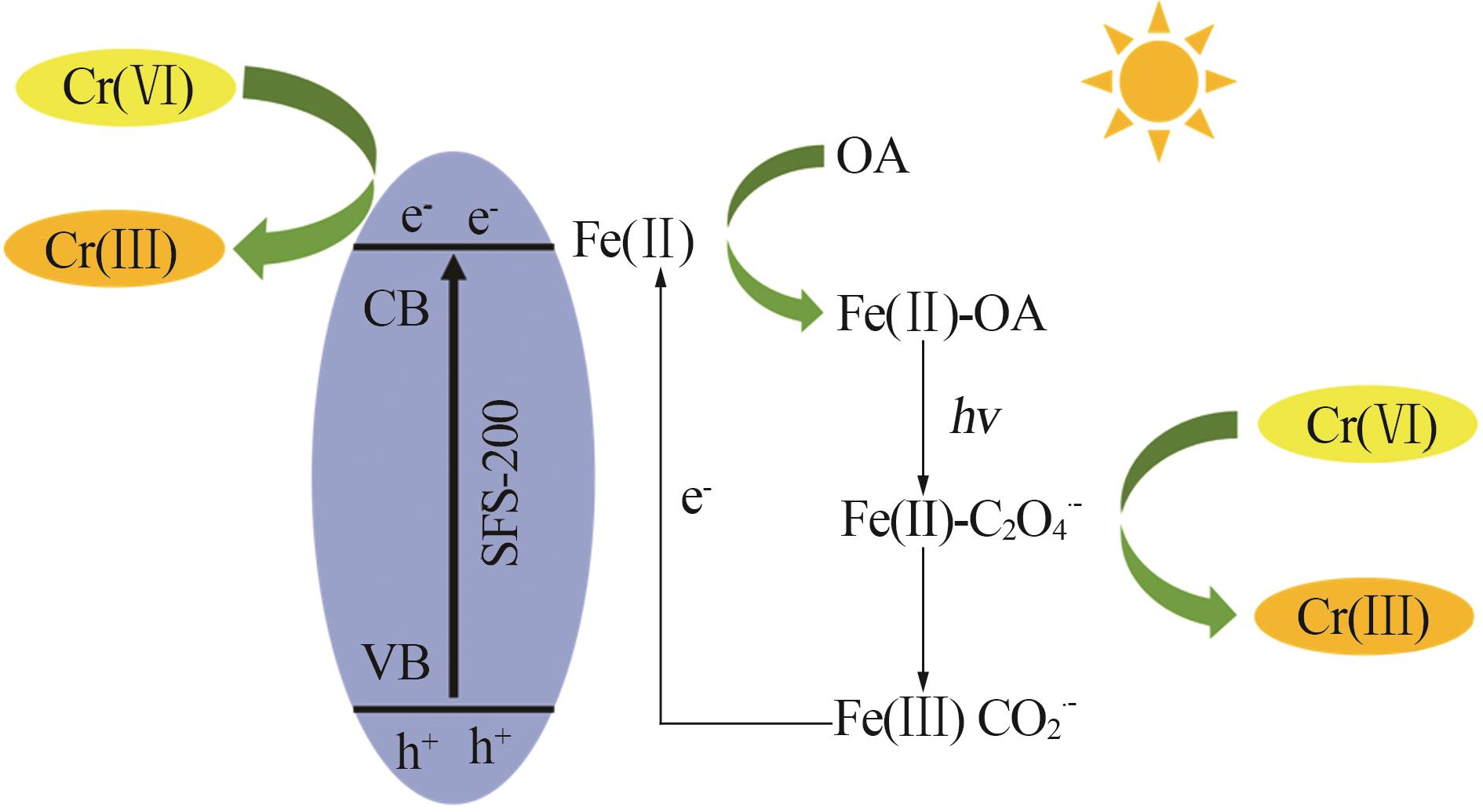
Study on surface regulation of sodium ferric silicate photocatalyst and its enhanced Cr(Ⅵ) photoreduction properties
YAN Yu( ), ZHOU Wenyuan, YANG Yunfei, WU Junshu(
), ZHOU Wenyuan, YANG Yunfei, WU Junshu( ), WANG Jinshu, SUN Lingmin
), WANG Jinshu, SUN Lingmin
- Beijing University of Technology,Beijing 100124,China
-
Received:2024-02-28Online:2024-10-10Published:2024-03-27 -
Contact:WU Junshu E-mail:xpyy98@163.com;junshuwu@bjut.edu.cn
CLC Number:
Cite this article
YAN Yu, ZHOU Wenyuan, YANG Yunfei, WU Junshu, WANG Jinshu, SUN Lingmin. Study on surface regulation of sodium ferric silicate photocatalyst and its enhanced Cr(Ⅵ) photoreduction properties[J]. Inorganic Chemicals Industry, 2024, 56(10): 141-150.
share this article
| 1 | LI Jiahao, XIA Chenggong, CHENG Rong,et al.Passivation of multiple heavy metals in lead⁃zinc tailings facilitated by straw biochar⁃loaded N-doped carbon aerogel nanoparticles:Mechanisms and microbial community evolution[J].The Science of the Total Environment,2022,803:149866. |
| 2 | WU Bingdang, ZHANG Li, WEI Shijie,et al.Reduction of chromate with UV/diacetyl for the final effluent to be below the discharge limit[J].Journal of Hazardous Materials,2020,389:121841. |
| 3 | ZHU Pengfei, WU Xiaolong, XIAO Xingyang,et al.Construction of Bi12O17Cl2/ZnO-CN Z-scheme photocatalyst with ZIF-8 as precursor to remove antibiotics and Cr(Ⅵ) under visible light:Optimization,mechanism and degradation path[J].Journal of Alloys and Compounds,2023,966:171513. |
| 4 | CHEN Dongjie, CHENG Yanling, ZHOU Nan,et al.Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts:A review[J].Journal of Cleaner Production,2020,268:121725. |
| 5 | WANG Chen, LIU Haiyan, WANG Guifang,et al.Photocatalytic removal of metronidazole and Cr(Ⅵ) by a novel Zn3In2S6/Bi2O3 S-scheme heterojunction:Performance,mechanism insight and toxicity assessment[J].Chemical Engineering Journal,2022,450:138167. |
| 6 | ZHAO Yitao, LI Le, ZUO Yuanjie,et al.Reduced graphene oxide supported ZnO/CdS heterojunction enhances photocatalytic remo⁃val efficiency of hexavalent chromium from aqueous solution[J].Chemosphere,2022,286(Pt 3):131738. |
| 7 | LI Kexin, HUANG Ziai, ZHU Suiyi,et al.Removal of Cr(Ⅵ) from water by a biochar⁃coupled g-C3N4 nanosheets composite and performance of a recycled photocatalyst in single and combined pollution systems[J].Applied Catalysis B:Environmental,2019,243:386-396. |
| 8 | XU Meng, WU Junshu, WANG Jinshu,et al.The integration of triazine⁃based porous organic polymer with bio⁃waste poplar catkin as water⁃floatable photocatalyst[J].Applied Surface Science,2022,581:152409. |
| 9 | SUN Lingmin, WU Junshu, WANG Jinshu,et al. In-situ constructing nanostructured magnesium ferrite on steel slag for Cr(Ⅵ) photoreduction[J].Journal of Hazardous Materials,2022,422:126951. |
| 10 | SUN Jie, CAO Fengming, ZHANG Ling,et al.The low⁃cost and high⁃efficient photoreduction of Cr(Ⅵ) by oxalic acid synergized with the iron⁃loaded rice straw[J].Journal of Molecular Liquids,2022,351:118552. |
| 11 | HE Heng, LI Yongli, WANG Jinshu,et al.Tunable Fe-deficiency modified sodium ferric silicate for improving photo⁃Fenton⁃like activity[J].Chemical Engineering Journal,2022,450:138141. |
| 12 | WANG Longyang, ZHANG Kejie, QIAN Jianying,et al.S-schemeMOF-on-MOF heterojunctions for enhanced photo⁃Fenton Cr(Ⅵ)reduction and antibacterial effects[J].Chemosphere,2023,344:140277. |
| 13 | WEN Jiaxin, FU Wenyang, DING Shihu,et al.Pyrogallic acid modified nanoscale zero⁃valent iron efficiently removed Cr(Ⅵ) by improving adsorption and electron selectivity[J].Chemical Engineering Journal,2022,443:136510. |
| 14 | CHEN Yongjun, MA Rui, PU Xunchi,et al.The characterization of a novel magnetic biochar derived from sulfate⁃reducing sludge and its application for aqueous Cr(Ⅵ) removal through synergistic effects of adsorption and chemical reduction[J].Chemosphere,2022,308(Pt 1):136258. |
| 15 | YAN Yu, WU Junshu, WANG Jinshu,et al.Rationally engineering magadiite heavy metal adsorbent for p-nitrophenol hydrogenation reduction[J].Applied Clay Science,2023,245:107143. |
| 16 | LI Shijie, WANG Chunchun, LIU Yanping,et al.S-scheme MIL-101(Fe) octahedrons modified Bi2WO6 microspheres for photocatalytic decontamination of Cr(Ⅵ) and tetracycline hydrochloride:Synergistic insights,reaction pathways,and toxicity analy⁃sis[J].Chemical Engineering Journal,2023,455:140943. |
| 17 | ZHOU Shiliang, ZEIER W G, KEMEI M C,et al.Hydrothermal preparation and magnetic properties of NaFeSi2O6:Nanowires vs bulk samples[J].Inorganic Chemistry,2014,53(23):12396-12401. |
| 18 | LIU Huanhuan, TIAN Kunfei, NING Jiqiang,et al.One⁃step solvothermal formation of Pt nanoparticles decorated Pt2+-dopedα-Fe2O3 nanoplates with enhanced photocatalytic O2 evolution[J].ACS Catalysis,2019,9(2):1211-1219. |
| 19 | IBRAHIM I, KALTZOGLOU A, ATHANASEKOU C,et al.Magnetically separable TiO2/CoFe2O4/Ag nanocomposites for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light[J].Chemical Engineering Journal,2020,381:122730. |
| 20 | KATABATHINI N, MAKSOD I H A EL, MOKHTAR M.Cu,Fe and Mn oxides intercalated SiO2 pillared magadiite and ilerite catalysts for NO decomposition[J].Applied Catalysis A:General,2021,616:118100. |
| 21 | Zengqiang CI, YUE Yanxue, XIAO Jingting,et al.Spectroscopic and modeling investigation of U(Ⅵ) removal mechanism on nanoscale zero⁃valent iron/clay composites[J].Journal of Colloid and Interface Science,2023,630:395-403. |
| 22 | BAO Chaosheng, WANG Hu, WANG Caiyun,et al.Cooperation of oxygen vacancy and FeⅢ/FeⅡ sites in H2-reduced Fe-MIL-101 for enhanced Fenton⁃like degradation of organic pollutan⁃ts[J].Journal of Hazardous Materials,2023,441:129922. |
| 23 | LIU Zishuai, HUANG Jing, ZHANG Yimin,et al.Separation and recovery of iron impurities from a complex oxalic acid solution containing vanadium by K3Fe(C2O4)3·3H2O crystallization[J].Separation and Purification Technology,2020,232:115970. |
| 24 | WU Junshu, WANG Jinshu, DU Yucheng,et al.Chemically controlled growth of porous CeO2 nanotubes for Cr(Ⅵ) photoreduction[J].Applied Catalysis B:Environmental,2015,174-175:435-444. |
| 25 | LEI Dashi, XUE Juanqin, PENG Xiangyu,et al.Oxalate enhanced synergistic removal of chromium(Ⅵ) and arsenic(Ⅲ) over ZnFe2O4/g-C3N4:Z-scheme charge transfer pathway and photo⁃Fenton like reaction[J].Applied Catalysis B:Environmental,2021,282:119578. |
| 26 | JIANG Bo, GONG Yifan, GAO Jianan,et al.The reduction of Cr(Ⅵ) to Cr(Ⅲ) mediated by environmentally relevant carboxylic acids:State⁃of⁃the⁃art and perspectives[J].Journal of Hazardous Materials,2019,365:205-226. |
| 27 | NIU Weiya, SUN Jie, ZHANG Ling,et al.The enhanced removal of highly toxic Cr(Ⅵ) by the synergy of uniform fiber ball loaded with Fe(OH)3 and oxalate acid[J].Chemosphere,2021,262:127806. |
| 28 | KRETSCHMER I, SENN A M, MEICHTRY J M,et al.Photocatalytic reduction of Cr(Ⅵ) on hematite nanoparticles in the presence of oxalate and citrate[J].Applied Catalysis B:Environmental,2019,242:218-226. |
| 29 | ZHANG Ling, SUN Jie, NIU Weiya,et al.The synergetic role of rice straw in enhancing the process of Cr(Ⅵ) photoreduction by oxalic acid[J].Environmental Pollution,2020,265(Pt A):115013. |
| [1] | SHI Wangfang, ZHANG Yongsheng. Study on NO x degradation performance of concrete-based non-metallic boron doped nitrogen-rich carbon nitride [J]. Inorganic Chemicals Industry, 2025, 57(3): 116-123. |
| [2] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [3] | SHEN Xiaoqian, ZHOU Fei, LIU Wanchen, XU Lu, WU Junshu. Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance [J]. Inorganic Chemicals Industry, 2025, 57(2): 57-67. |
| [4] | SUN Qinghao, LI Keyan, GUO Xinwen. Study on photocatalytic benzyl alcohol oxidation coupled with hydrogen production over Pd/ZnIn2S4 nanosheets [J]. Inorganic Chemicals Industry, 2025, 57(1): 113-119. |
| [5] | LIU Guangming. Study on photocatalytic and mechanical properties of C3N5/NH2-MIL-125(Ti) modified concrete mortar [J]. Inorganic Chemicals Industry, 2025, 57(1): 120-128. |
| [6] | WEN Huizi, XI Luyao, HE Shuyu, TAN Shanyi, ZHANG Liwen, CHEN Shaohua, DU Yaguang. Research on Na2CO3 enhanced food additive wastewater hydrothermal wet reduction and detoxification of chromite ore processing residue [J]. Inorganic Chemicals Industry, 2025, 57(1): 83-89. |
| [7] | ZHANG Guoqiang, RONG Xilin, XIAO Zhenfang, XUE Ziran, CHENG Hao, FENG Jun, LIU Quan, LU Yao, HUANG Wenyi. Study on preparation and photocatalytic properties of bagasse carbon aerogels loaded with zinc oxide nanoparticles [J]. Inorganic Chemicals Industry, 2024, 56(8): 131-138. |
| [8] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [9] | LIU Min, HUANG Xiu, ZHANG Liyuan. Research progress of S-type heterojunction photocatalysts [J]. Inorganic Chemicals Industry, 2024, 56(7): 18-27. |
| [10] | LI Jiangpeng, ZHANG Huibin. Synergistic degradation of methylene blue by photo-Fenton and photocatalytic with 3D porous LaFeO3/CeO2/SrTiO3 [J]. Inorganic Chemicals Industry, 2024, 56(5): 141-148. |
| [11] | TANG Bei. Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine [J]. Inorganic Chemicals Industry, 2024, 56(4): 133-142. |
| [12] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [13] | ZUO Guangling, WANG Minghui, PENG Yunying, DU Jia, YE Hongyong. Study on hnoneycomb-like LaVO4/Bi2O3 heterojunction for photocatalytic degradation of tetracycline hydrochloride [J]. Inorganic Chemicals Industry, 2024, 56(11): 158-164. |
| [14] | CUI Xiangdong, LIU Sile. Study on photoelectric performance analysis of g-C3N5 nanorods and removal of Cr(Ⅵ) and methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(10): 159-168. |
| [15] | MA Yihong, CHEN Xingtao, TANG Lei. Treatment of printing wastewater by chemical coagulation-TiO2/g-C3N5 photocatalytic degradation [J]. Inorganic Chemicals Industry, 2024, 56(10): 151-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||