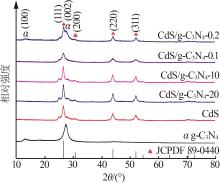
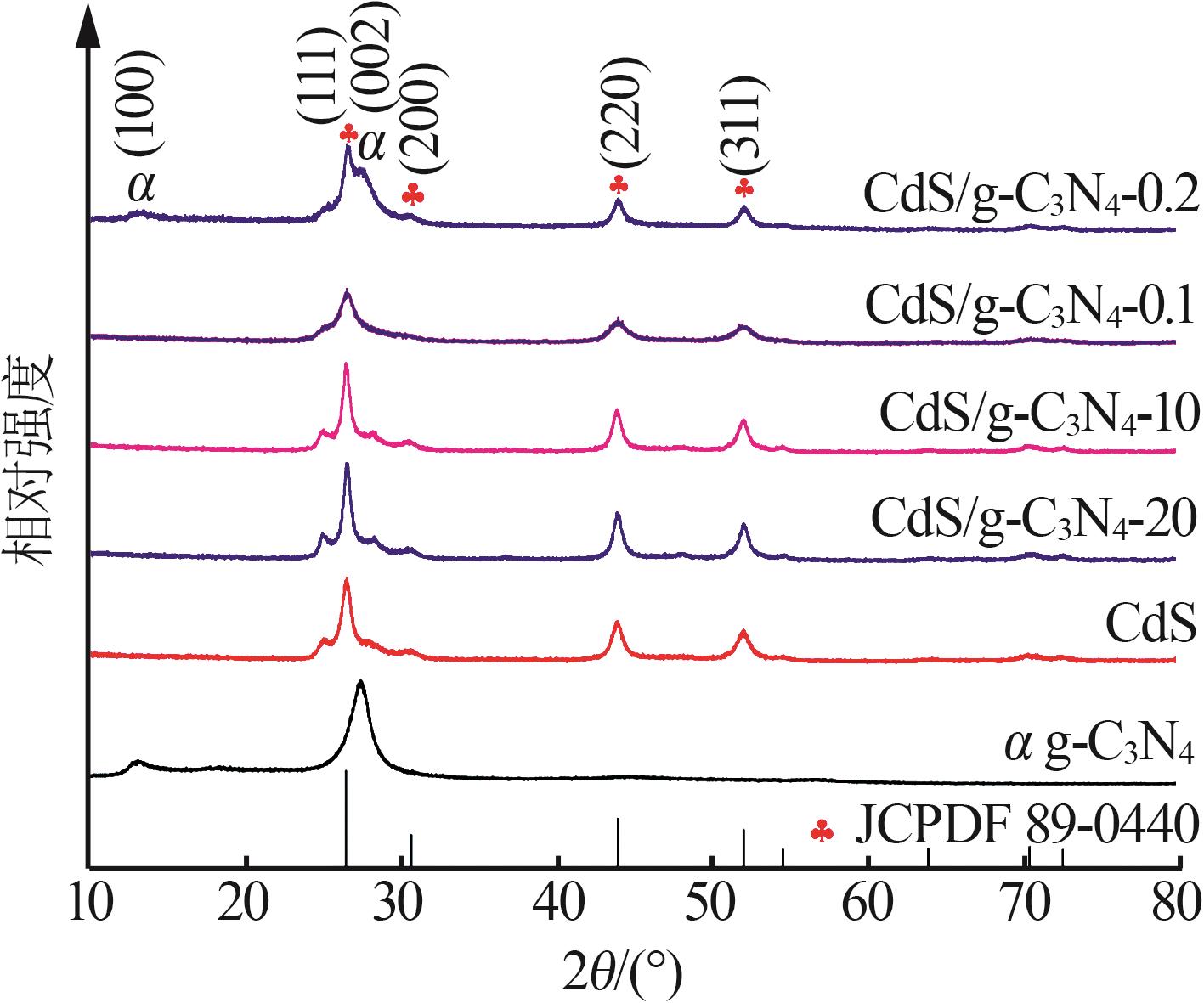
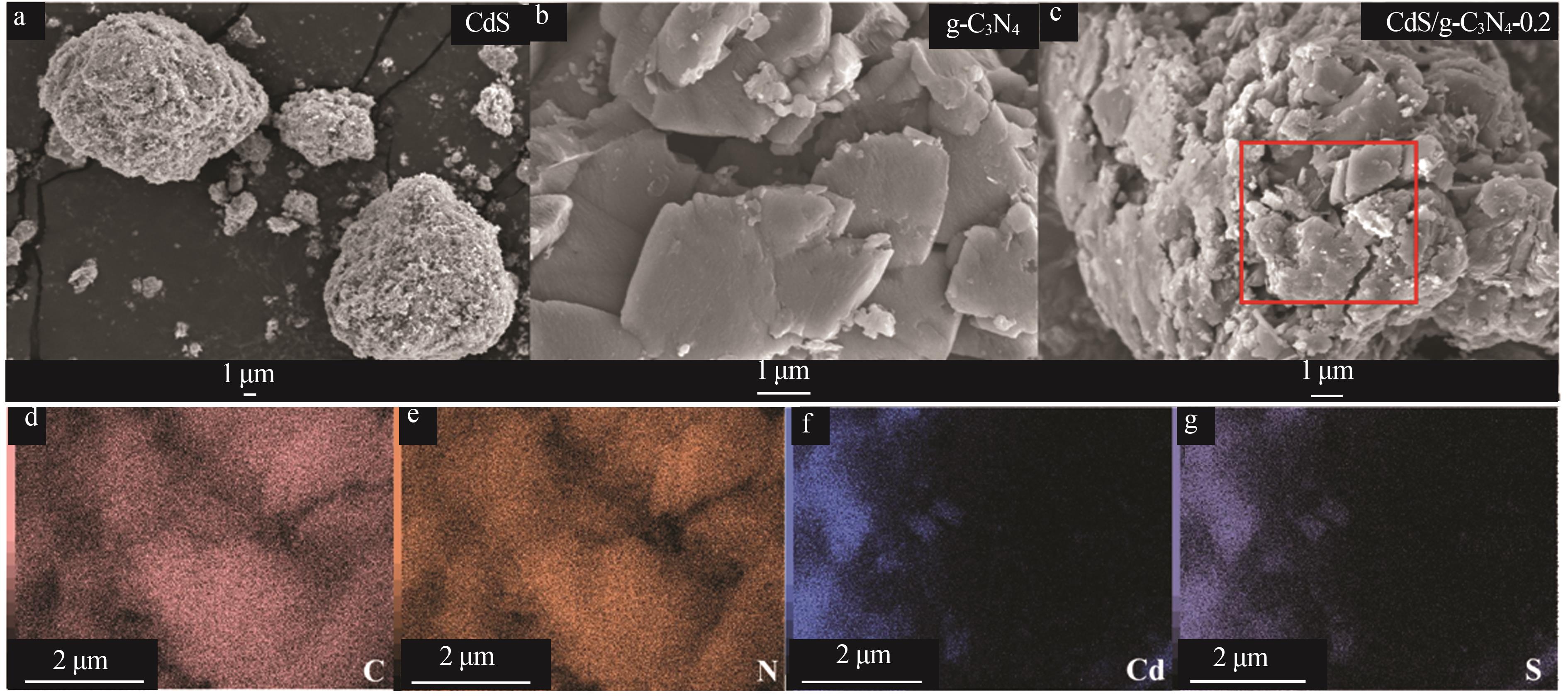
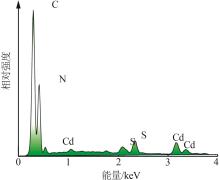
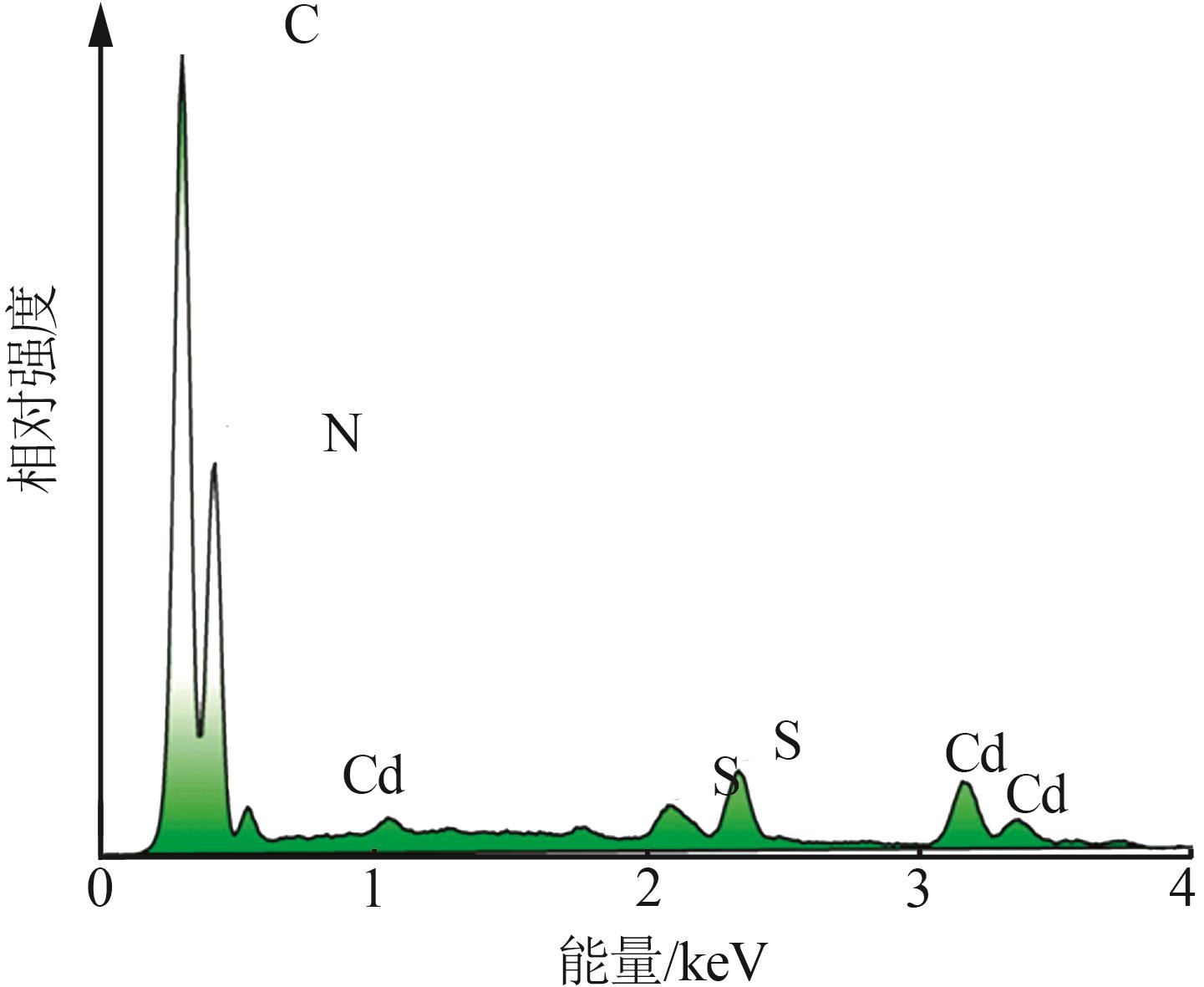
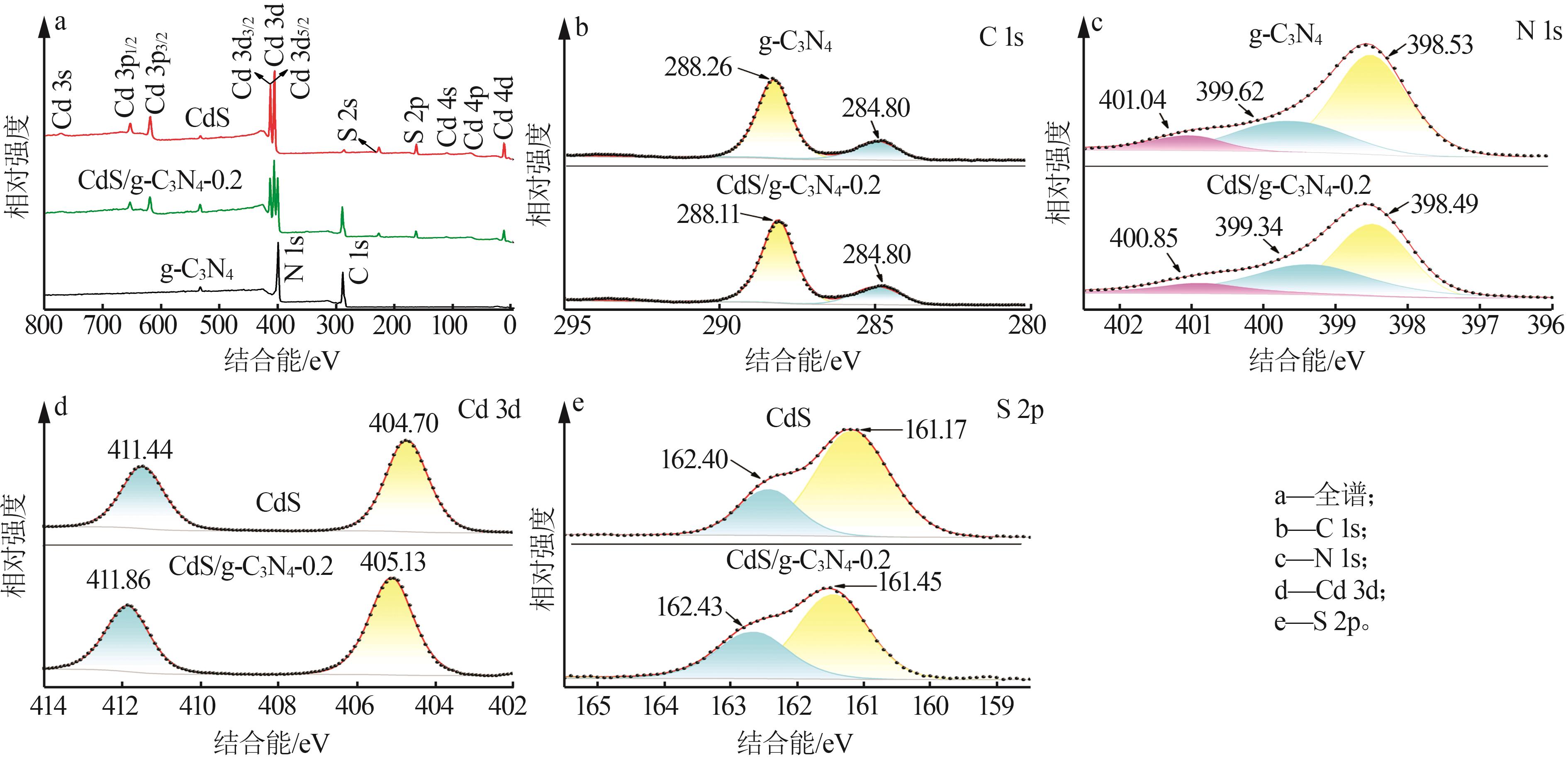
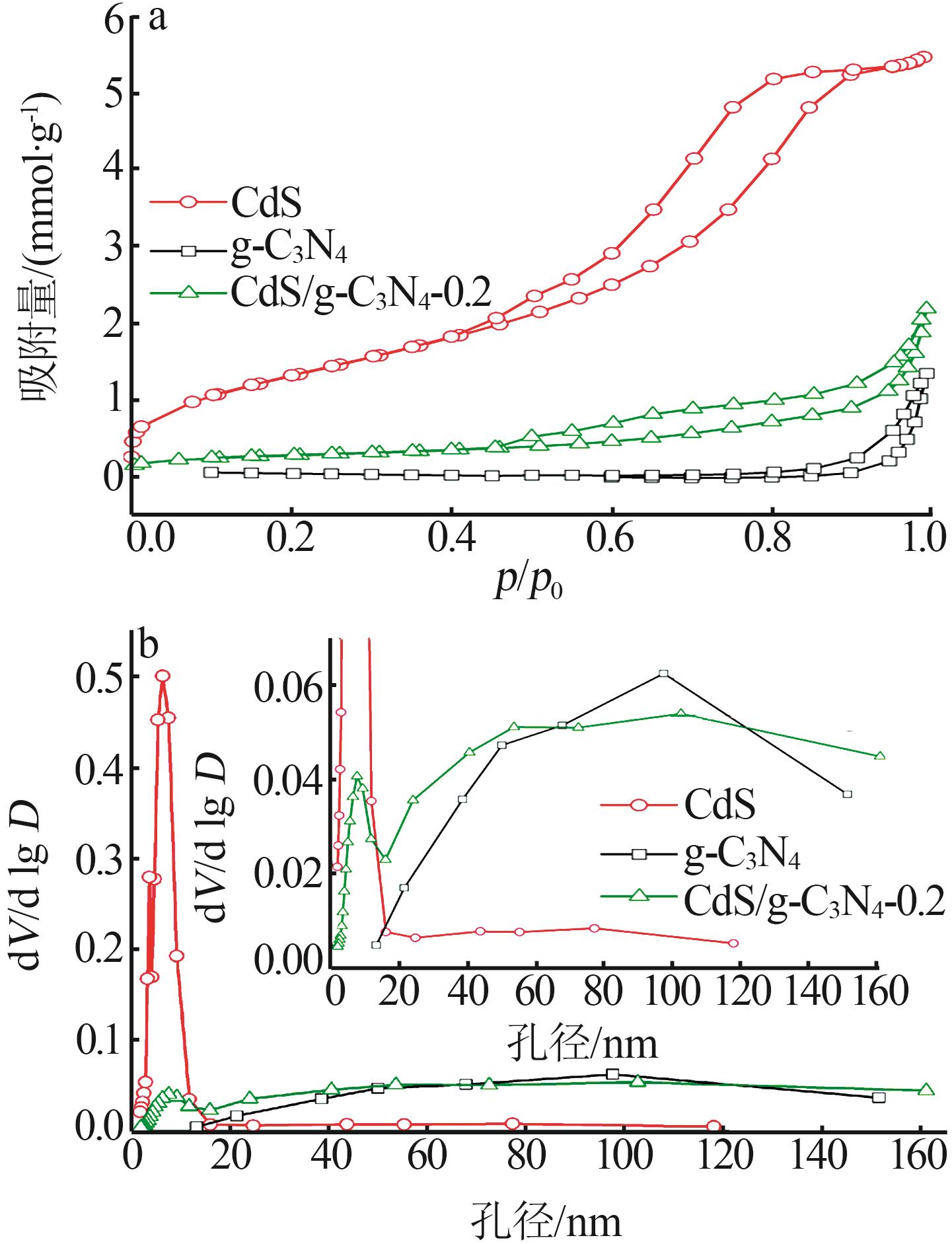
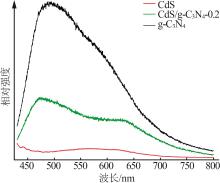
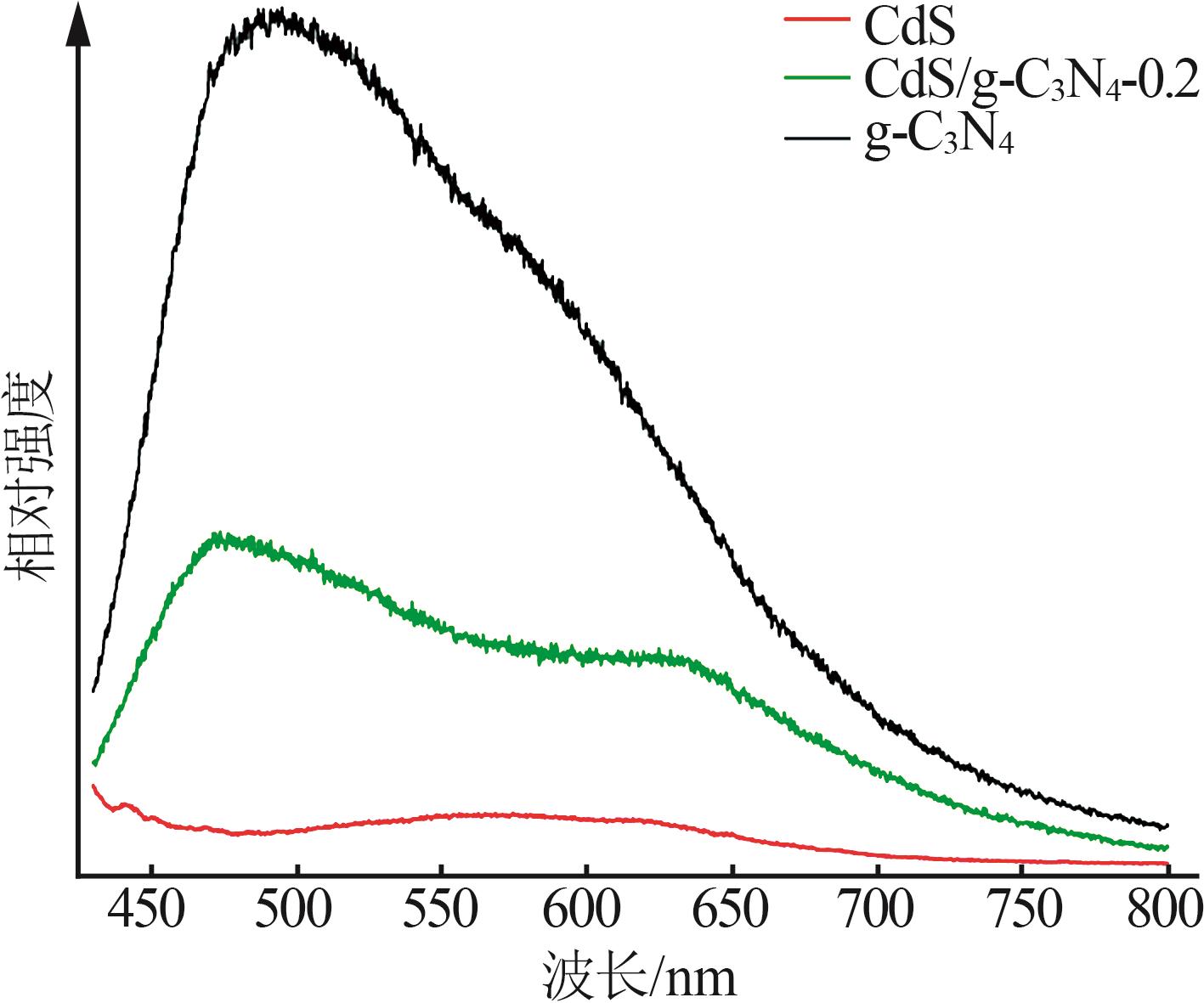
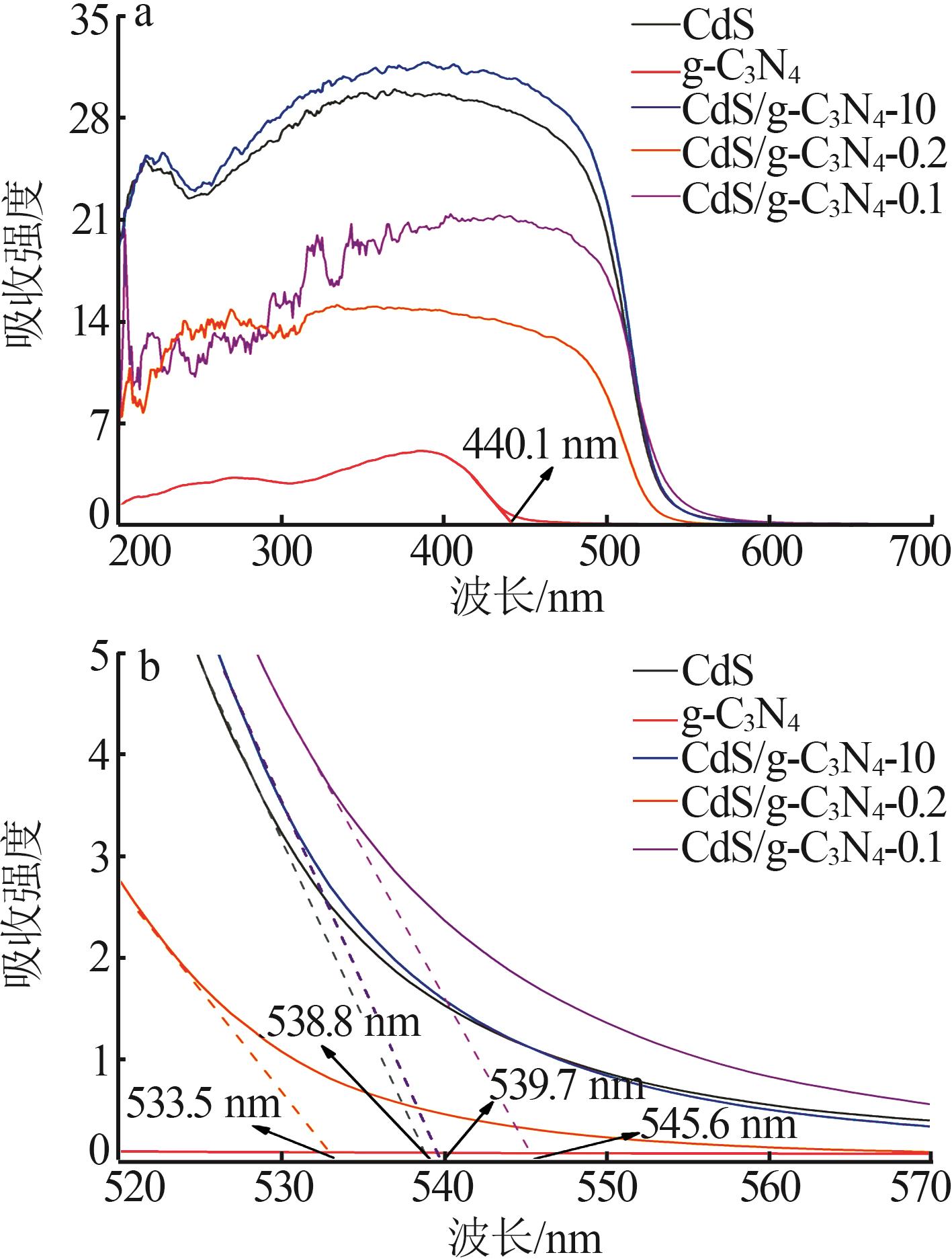
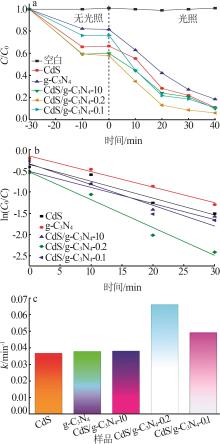
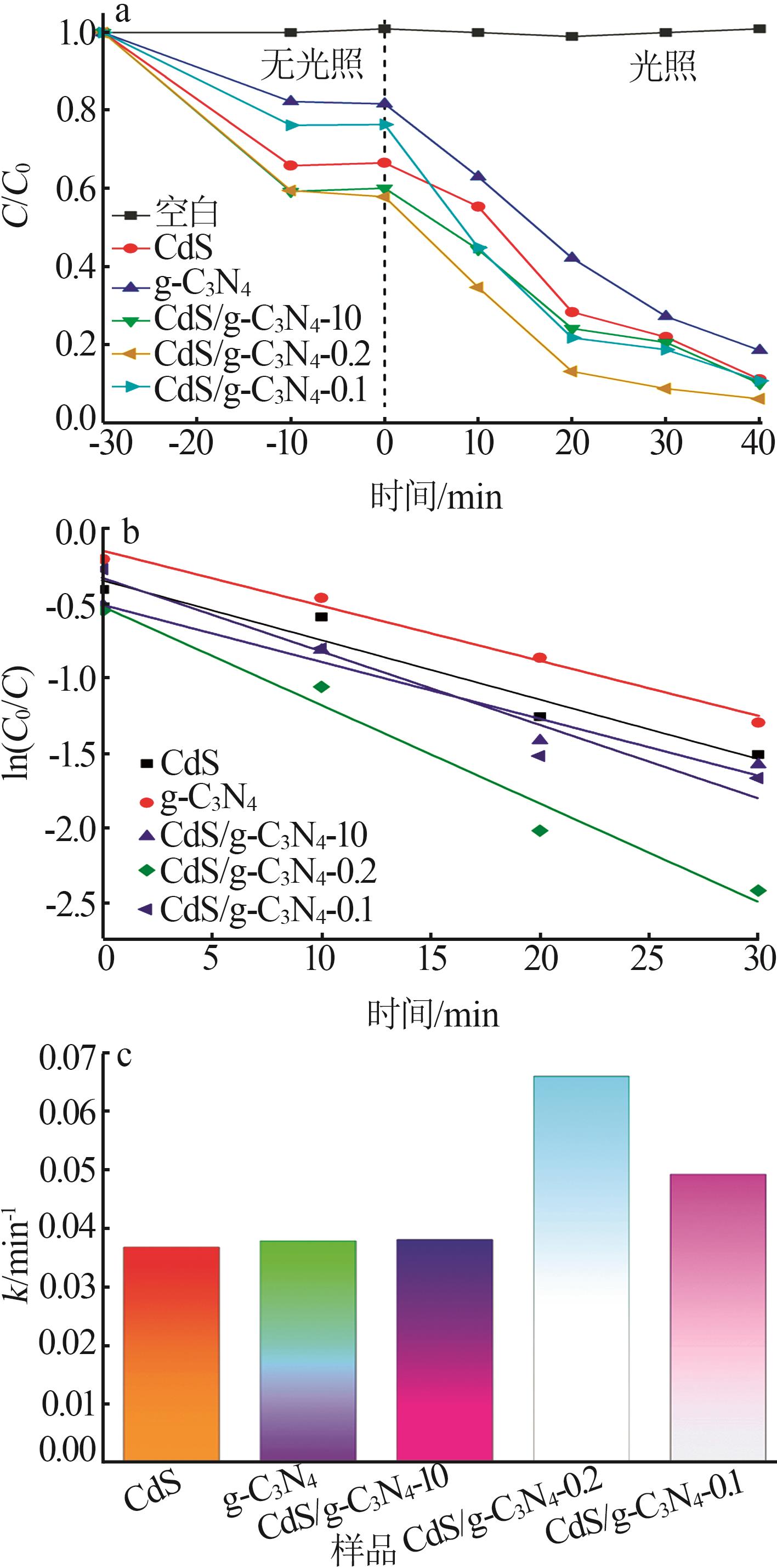
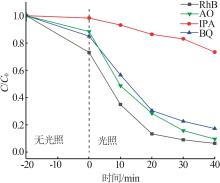
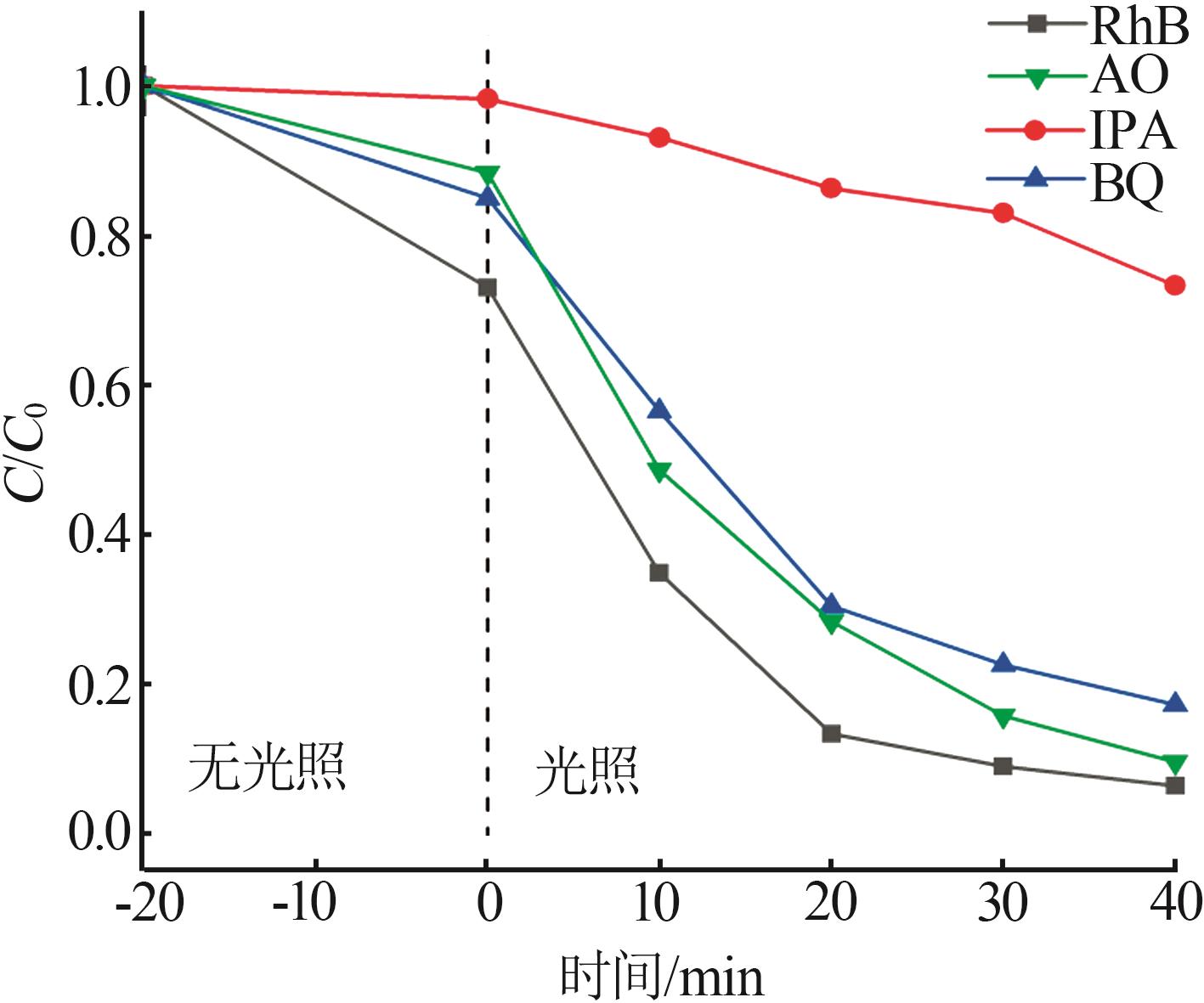
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (3): 124-132.doi: 10.19964/j.issn.1006-4990.2024-0369
• Catalytic Materials • Previous Articles
Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst
LI Zihan1( ), ZHANG Jiaqi1, LI Shizhuo1, LI Xinyu1, LIU Shaozhuo1, WANG Yihao1, HAO Yucui1(
), ZHANG Jiaqi1, LI Shizhuo1, LI Xinyu1, LIU Shaozhuo1, WANG Yihao1, HAO Yucui1( ), LIU Jian1, LI Yanhua2
), LIU Jian1, LI Yanhua2
- 1. School of New Materials and Chemical Engineering,Tangshan University,Tangshan 063099,China
2. Jijianlian(Tangshan) Environmental Testing Technology Co. ,Ltd. ,Tangshan 063010,China
-
Received:2024-07-01Online:2025-03-10Published:2025-03-21 -
Contact:HAO Yucui E-mail:1156646896@qq.com;zljzjh0001@163.com
CLC Number:
Cite this article
LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst[J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132.
share this article
| 1 | 朱佳新,熊裕华,郭锐.二氧化钛光催化剂改性研究进展[J].无机盐工业,2020,52(3):23-27,54. |
| ZHU Jiaxin, XIONG Yuhua, GUO Rui.Research progress in modification of TiO2 photocatalyst[J].Inorganic Chemicals Industry,2020,52(3):23-27,54. | |
| 2 | 司翠青,张璐瑶,曾菲,等.光催化去除水中全氟辛酸及其机理研究[J].环境科学与技术,2023,46(5):76-87. |
| SI Cuiqing, ZHANG Luyao, ZENG Fei,et al.Removal and degradation mechanism study of perfluorooctanoic acid in water by photocatalysis[J].Environmental Science & Technology,2023,46(5):76-87. | |
| 3 | 徐若航,崔丹丹,郝维昌.二维半导体光催化材料研究进展[J].自然杂志,2023,45(5):355-362. |
| XU Ruohang, CUI Dandan, HAO Weichang.Research progress of two-dimensional semiconductor photocatalytic materials[J].Chinese Journal of Nature,2023,45(5):355-362. | |
| 4 | 刘剑,赵文武,郁建元,等.YBO3微米球的水热合成及光催化性能[J].精细化工,2019,36(12):2491-2497. |
| LIU Jian, ZHAO Wenwu, YU Jianyuan,et al.Hydrothermal synthesis and photocatalytic performance of YBO3 microspheres[J].Fine Chemicals,2019,36(12):2491-2497. | |
| 5 | REN Yijie, ZENG Deqian, ONG W J.Interfacial engineering of graphitic carbon nitride(g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion:A review[J].Chinese Journal of Catalysis,2019,40(3):289-319. |
| 6 | 唐贝.ZnO/g-C3N4异质结光催化材料的制备及对吡啶的降解[J].无机盐工业,2024,56(4):133-142. |
| TANG Bei.Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine[J].Inorganic Chemicals Industry,2024,56(4):133-142. | |
| 7 | 陈彰旭,朱丹琛,傅明连.g-C3N4/TiO2复合材料制备及其处理罗丹明B研究[J].无机盐工业,2023,55(7):130-136. |
| CHEN Zhangxu, ZHU Danchen, FU Minglian.Study on preparation of g-C3N4/TiO2 composites and application for rhodamine B removal[J].Inorganic Chemicals Industry,2023,55(7):130-136. | |
| 8 | 任富彦,欧阳二明.g-C3N4改性Bi2O3对盐酸四环素的光催化降解[J].材料研究学报,2023,37(8):633-640. |
| REN Fuyan, OUYANG Erming.Photocatalytic degradation of tetracycline hydrochloride by g-C3N4 modified Bi2O3 [J].Chinese Journal of Materials Research,2023,37(8):633-640. | |
| 9 | 张乾坤,梁海欧,白杰,等.CdS基光催化材料的研究进展[J].化学通报,2023,86(10):1181-1187. |
| ZHANG Qiankun, LIANG Haiou, BAI Jie,et al.Research progress in CdS-based photocatalytic materials[J].Chemistry,2023,86(10):1181-1187. | |
| 10 | 王海涛,施宝旭,赵晓旭,等.高效降解盐酸四环素的CdS/BiOCl复合光催化剂的制备及性能[J].材料导报,2024,38(6):123-130. |
| WANG Haitao, SHI Baoxu, ZHAO Xiaoxu,et al.Preparation and property of CdS/BiOCl materials:A high efficiency photocatalyst for degradation of tetracycline hydrochloride[J].Materials Reports,2024,38(6):123-130. | |
| 11 | 敏世雄,吕功煊.CdS/石墨烯复合材料的制备及其可见光催化分解水产氢性能[J].物理化学学报,2011,27(9):2178-2184. |
| MIN Shixiong, Gongxuan LÜ.Preparation of CdS/graphene composites and photocatalytic hydrogen generation from water under visible light irradiation[J].Acta Physico-Chimica Sinica,2011,27(9):2178-2184. | |
| 12 | 徐元盛,李建伟,马炎,等.硫化镉-二氧化钛/分子筛复合光催化材料制备及性能研究[J].无机盐工业,2019,51(6):83-87. |
| XU Yuansheng, LI Jianwei, MA Yan,et al.Study on preparation and property of CdS-TiO2/zeolite composite catalysts[J].Inorganic Chemicals Industry,2019,51(6):83-87. | |
| 13 | 曹昉,陈甜,冯淑婷,等.多孔化改性对g-C3N4/CdS复合光催化剂制备及光催化活性的影响[J].吉林大学学报(理学版),2019,57(3):684-690. |
| CAO Fang, CHEN Tian, FENG Shuting,et al.Effect of porous modification on preparation and photocatalytic activity of g-C3N4/CdS composite photocatalyst[J].Journal of Jilin University(Science Edition),2019,57(3):684-690. | |
| 14 | 秦建宇,贾密英,张艳峰.g-C3N4基异质结光催化剂研究进展[J].河北师范大学学报(自然科学版),2023,47(3):275-286. |
| QIN Jianyu, JIA Miying, ZHANG Yanfeng.Research progress on g-C3N4 based heterojunction photocatalysts[J].Journal of Hebei Normal University(Natural Science),2023,47(3):275-286. | |
| 15 | 张仰全,李龙飞,周峰,等.Zr掺杂g-C3N4光催化降解有机污染物[J].精细化工,2022,39(10):2112-2121. |
| ZHANG Yangquan, LI Longfei, ZHOU Feng,et al.Zr-doped g-C3N4 photocatalytic degradation of organic pollutants[J].Fine Chemicals,2022,39(10):2112-2121. | |
| 16 | 汪益林,高博,张留科,等.g-C3N4/介孔TiO2的制备及可见光下光催化还原Cr(Ⅵ)试验研究[J].湿法冶金,2023,42(3):282-290. |
| WANG Yilin, GAO Bo, ZHANG Liuke,et al.Preparation of g-C3N4/mesoporous TiO2 and visible-light-driven photocatalytic reduction of Cr(Ⅵ)[J].Hydrometallurgy of China,2023,42(3):282-290. | |
| 17 | MAMARI S AL, SULIMAN F E, KIM Y,et al.Three-dimensional maple leaf CdS/g-C3N4 nanosheet composite for photodegradation of benzene in water[J].Advanced Powder Technology,2023,34(6):104026. |
| 18 | WANG Jing, PAN Runhui, HAO Qi,et al.Constructing defect-mediated CdS/g-C3N4 by an in situ interlocking strategy for cocatalyst-free photocatalytic H2 production[J].Applied Surface Science,2022,599:153875. |
| 19 | 张文康,梁燕艺,刘瑶,等.CdS/g-C3N4复合材料的制备及其光催化性能研究[J].广西科技大学学报,2018,29(4):66-73. |
| ZHANG Wenkang, LIANG Yanyi, LIU Yao,et al.Study on the preparation and photocatalytic performance of CdS/g-C3N4 composite catalyst[J].Journal of Guangxi University of Science and Technology,2018,29(4):66-73. | |
| 20 | 张伟,尤奇正,舒金锴,等.改性牡蛎壳粉/Ce-N-TiO2吸附-光催化降解草甘膦[J].环境工程学报,2023,17(5):1398-1408. |
| ZHANG Wei, YOU Qizheng, SHU Jinkai,et al.Adsorption-photocatalysis degradation of glyphosate by the modified oyster shell powder/Ce-N-TiO2 [J].Chinese Journal of Environmental Engineering,2023,17(5):1398-1408. | |
| 21 | LIU Jian, ZHOU Haijing, WANG Xiaoyan,et al.Synthesis and photocatalytic activity of EuBO3 photocatalyst with different morphological characteristics[J].Journal of Nanoparticle Research,2023,25(4):63. |
| 22 | 赵聪越,李春灵,陈明慧,等.具有高效光催化析氢和污染物降解性能的三维多孔V2O5/g-C3N4制备[J].材料研究与应用,2022,16(4):518-527. |
| ZHAO Congyue, LI Chunling, CHEN Minghui,et al.Preparation of 3D porous V2O5/g-C3N4 with efficient photocatalytic hydrogen evolution and pollutant degradation performance[J].Materials Research and Application,2022,16(4):518-527. | |
| 23 | POURSHIRBAND N, NEZAMZADEH-EJHIEH A.An efficient Z-scheme CdS/g-C3N4 nano catalyst in methyl orange photodegradation:Focus on the scavenging agent and mechanism[J].Journal of Molecular Liquids,2021,335:116543. |
| 24 | ZHANG Botao, ZHANG Yang, TENG Yanguo,et al.Sulfate radical and its application in decontamination technologies[J].Critical Reviews in Environmental Science and Technology,2015,45(16):1756-1800. |
| 25 | KARIMI-SHAMSABADI M, NEZAMZADEH-EJHIEH A.Comparative study on the increased photoactivity of coupled and supported manganese-silver oxides onto a natural zeolite nano-particles[J].Journal of Molecular Catalysis A:Chemical,2016,418:103-114. |
| [1] | SHI Wangfang, ZHANG Yongsheng. Study on NO x degradation performance of concrete-based non-metallic boron doped nitrogen-rich carbon nitride [J]. Inorganic Chemicals Industry, 2025, 57(3): 116-123. |
| [2] | MA Jun, JIN Yang, LI Jun, CHEN Ming, WANG Yubin. Study on photochemical synthesis of H2O2 in coiled flow inverter microreactor [J]. Inorganic Chemicals Industry, 2025, 57(2): 50-56. |
| [3] | LIU Huangfei, ZHANG Li, LIU Tao. Research progress of fast synthesis technologies of zeolites [J]. Inorganic Chemicals Industry, 2025, 57(2): 36-43. |
| [4] | SUN Qinghao, LI Keyan, GUO Xinwen. Study on photocatalytic benzyl alcohol oxidation coupled with hydrogen production over Pd/ZnIn2S4 nanosheets [J]. Inorganic Chemicals Industry, 2025, 57(1): 113-119. |
| [5] | LIU Guangming. Study on photocatalytic and mechanical properties of C3N5/NH2-MIL-125(Ti) modified concrete mortar [J]. Inorganic Chemicals Industry, 2025, 57(1): 120-128. |
| [6] | ZHANG Guoqiang, RONG Xilin, XIAO Zhenfang, XUE Ziran, CHENG Hao, FENG Jun, LIU Quan, LU Yao, HUANG Wenyi. Study on preparation and photocatalytic properties of bagasse carbon aerogels loaded with zinc oxide nanoparticles [J]. Inorganic Chemicals Industry, 2024, 56(8): 131-138. |
| [7] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [8] | LIU Min, HUANG Xiu, ZHANG Liyuan. Research progress of S-type heterojunction photocatalysts [J]. Inorganic Chemicals Industry, 2024, 56(7): 18-27. |
| [9] | SU Baocai, ZHANG Qin, XIE Yuanjian, CAI Pingxiong, PAN Yuanfeng. Advances in synthesis methods and structural modification of LiMnFePO4 materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 28-36. |
| [10] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [11] | LI Yuxing, ZHANG Jincai, CHENG Fangqin. Research progress of preparation and growth mechanism of various crystalline nano-calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(5): 1-10. |
| [12] | LI Jiangpeng, ZHANG Huibin. Synergistic degradation of methylene blue by photo-Fenton and photocatalytic with 3D porous LaFeO3/CeO2/SrTiO3 [J]. Inorganic Chemicals Industry, 2024, 56(5): 141-148. |
| [13] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [14] | TANG Bei. Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine [J]. Inorganic Chemicals Industry, 2024, 56(4): 133-142. |
| [15] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||