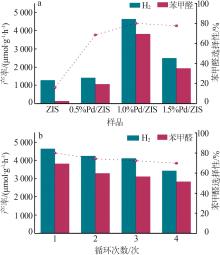
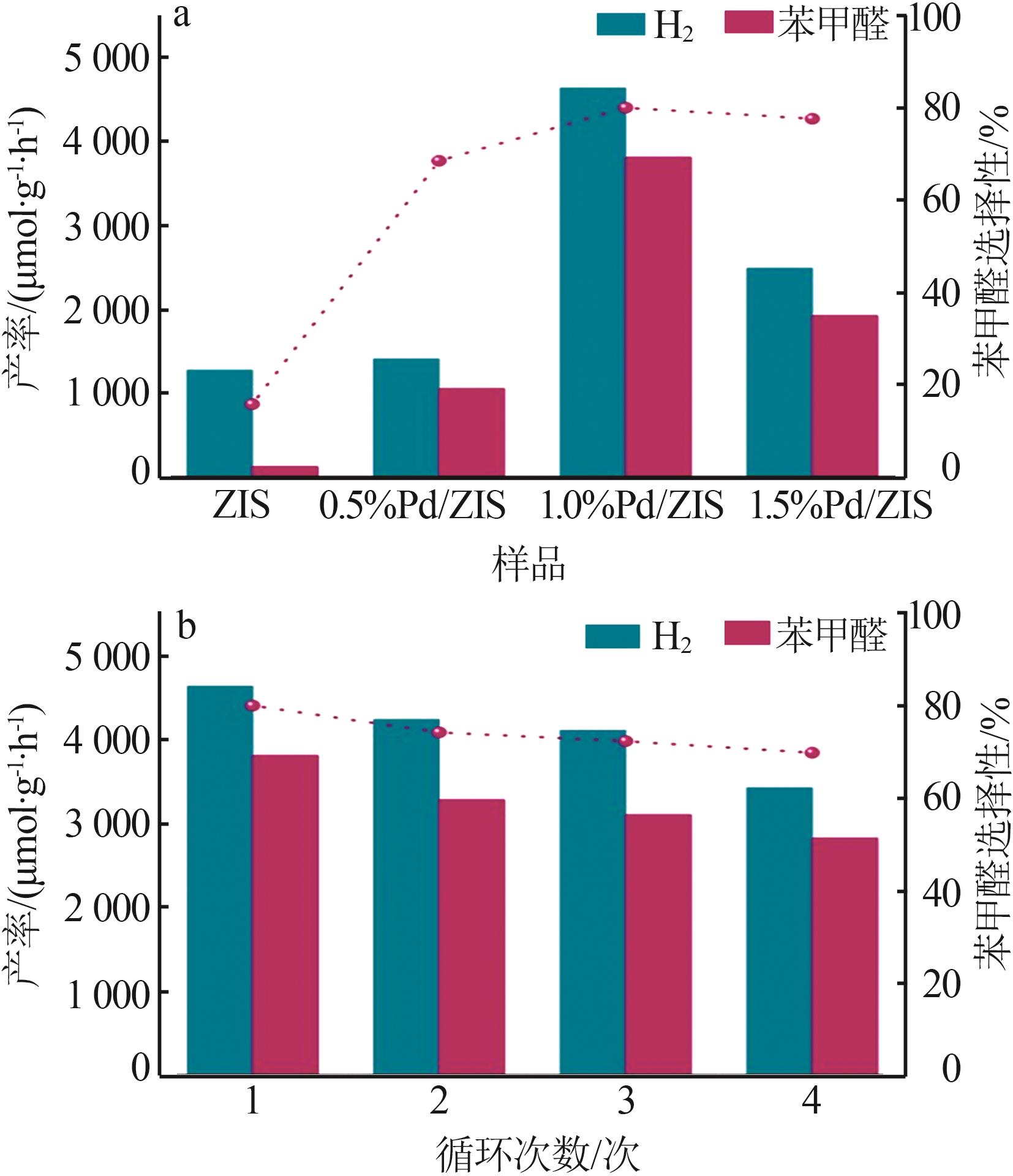
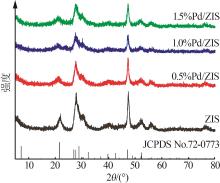
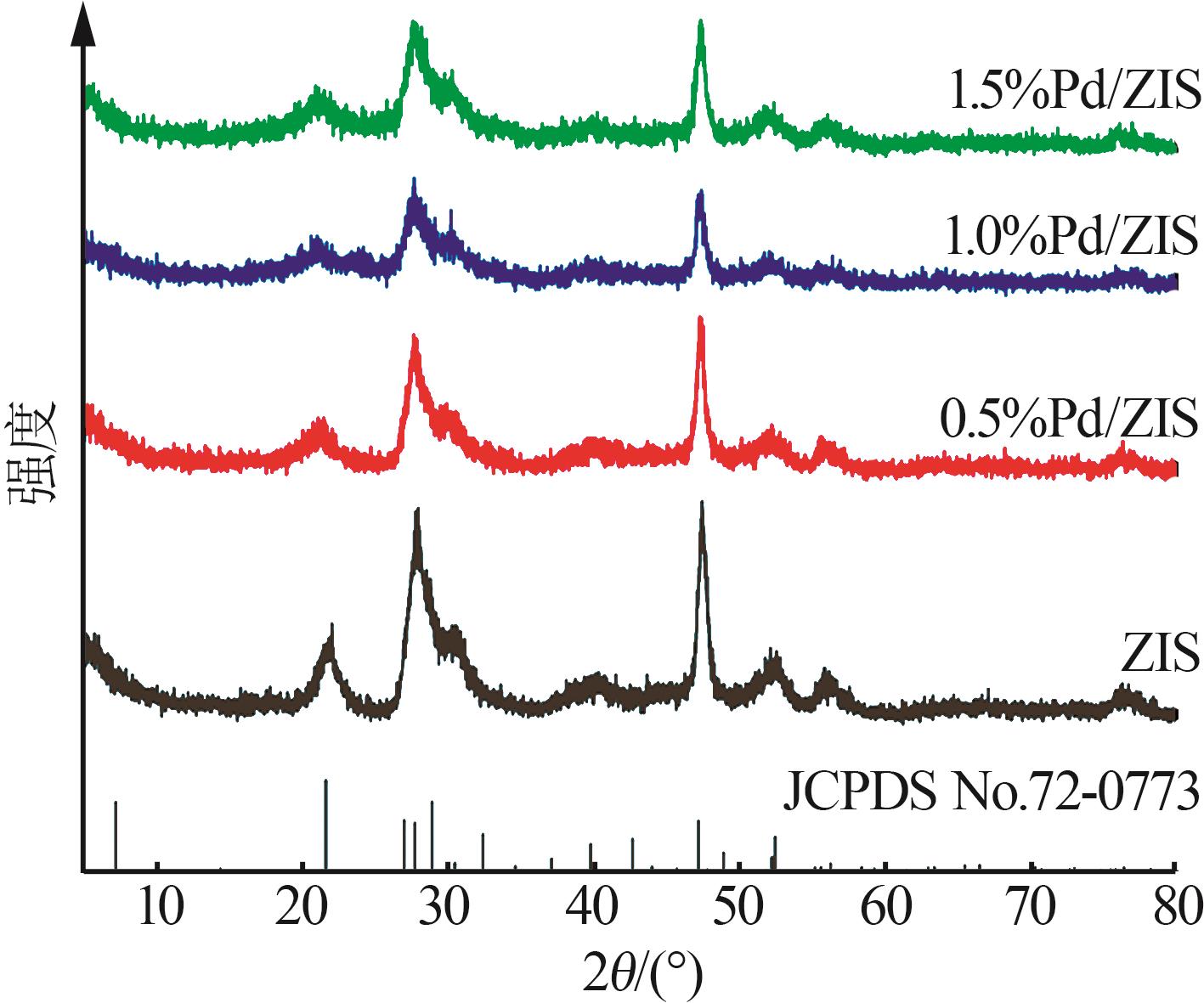
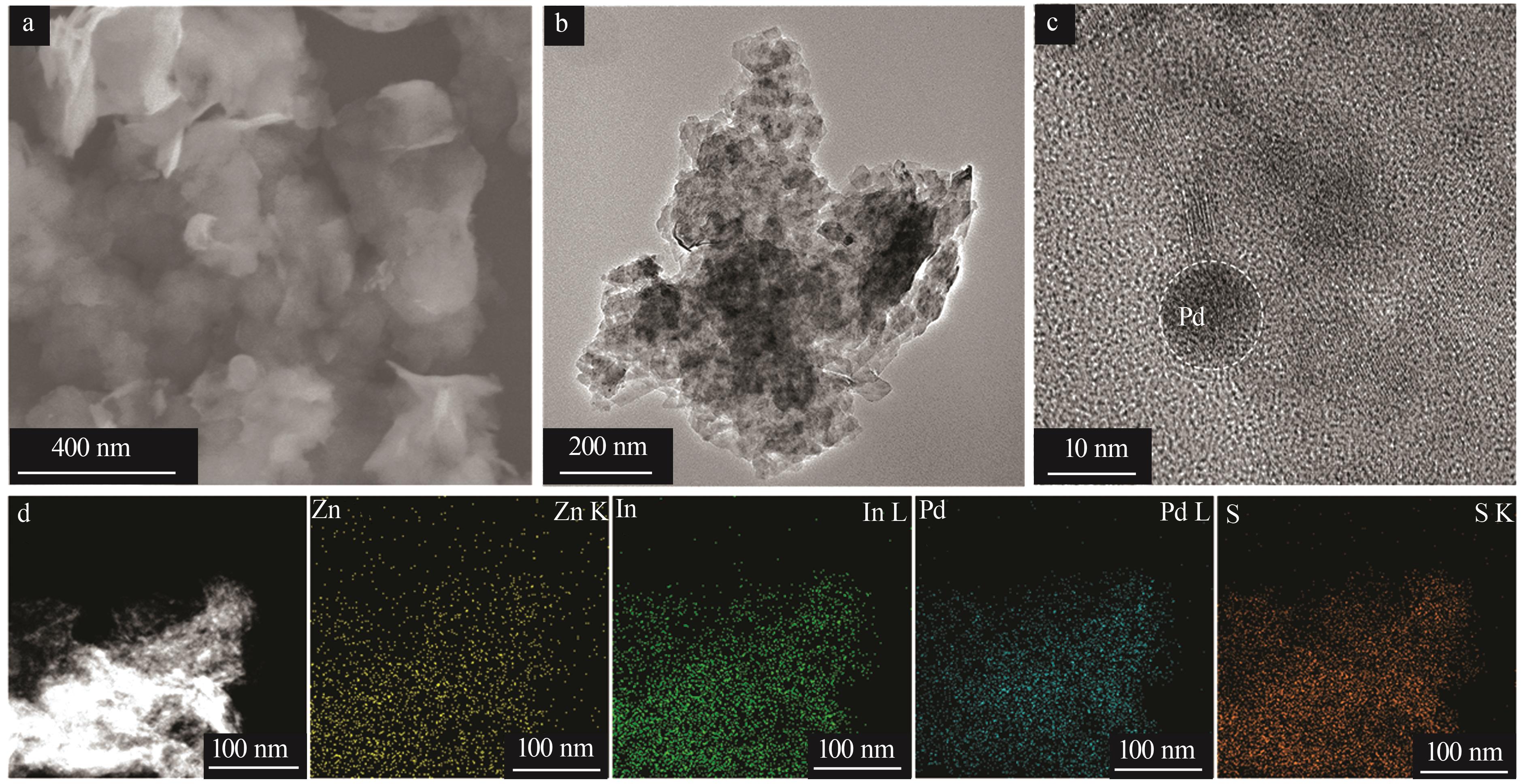
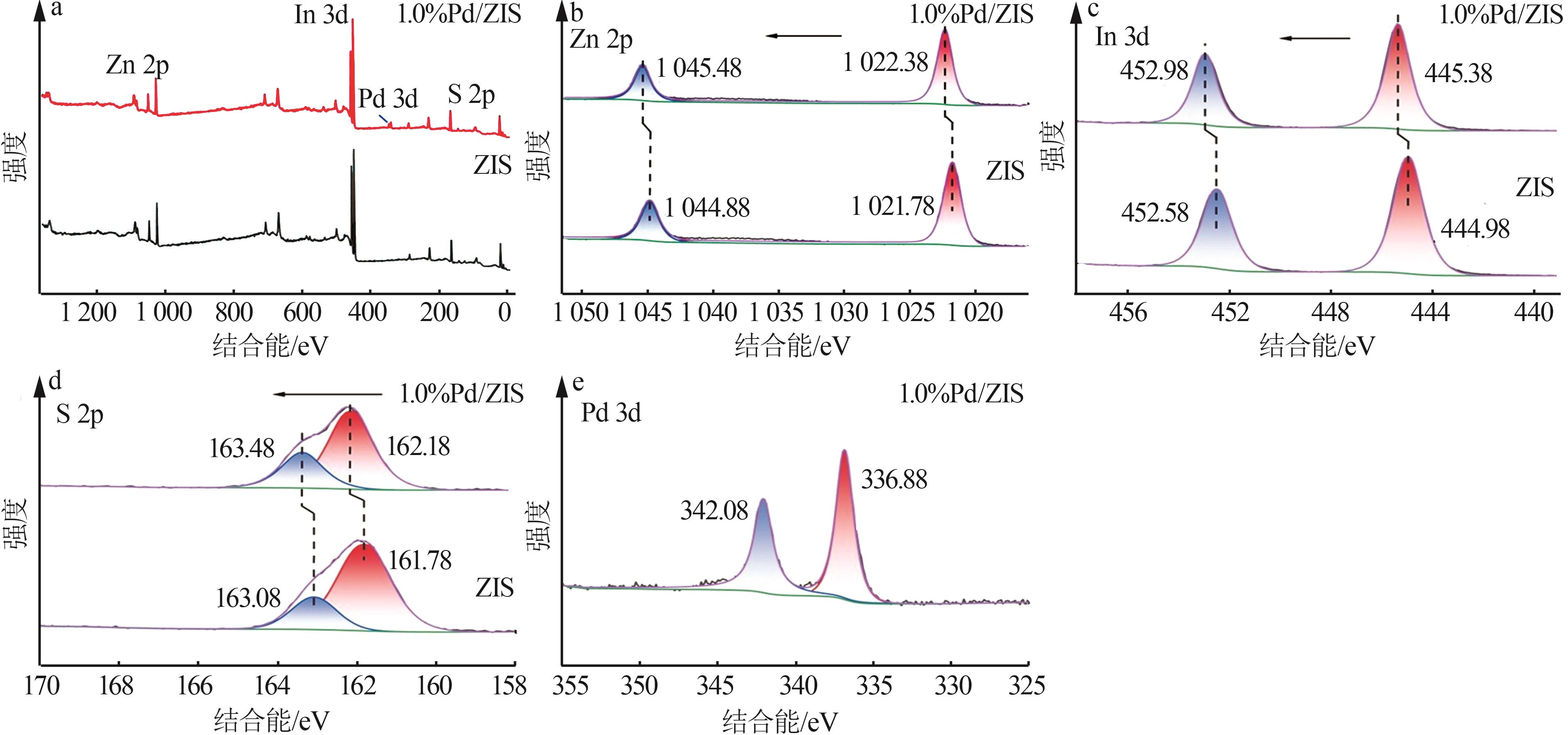
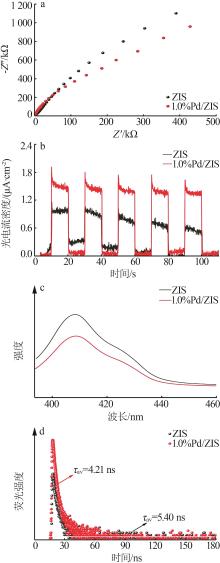
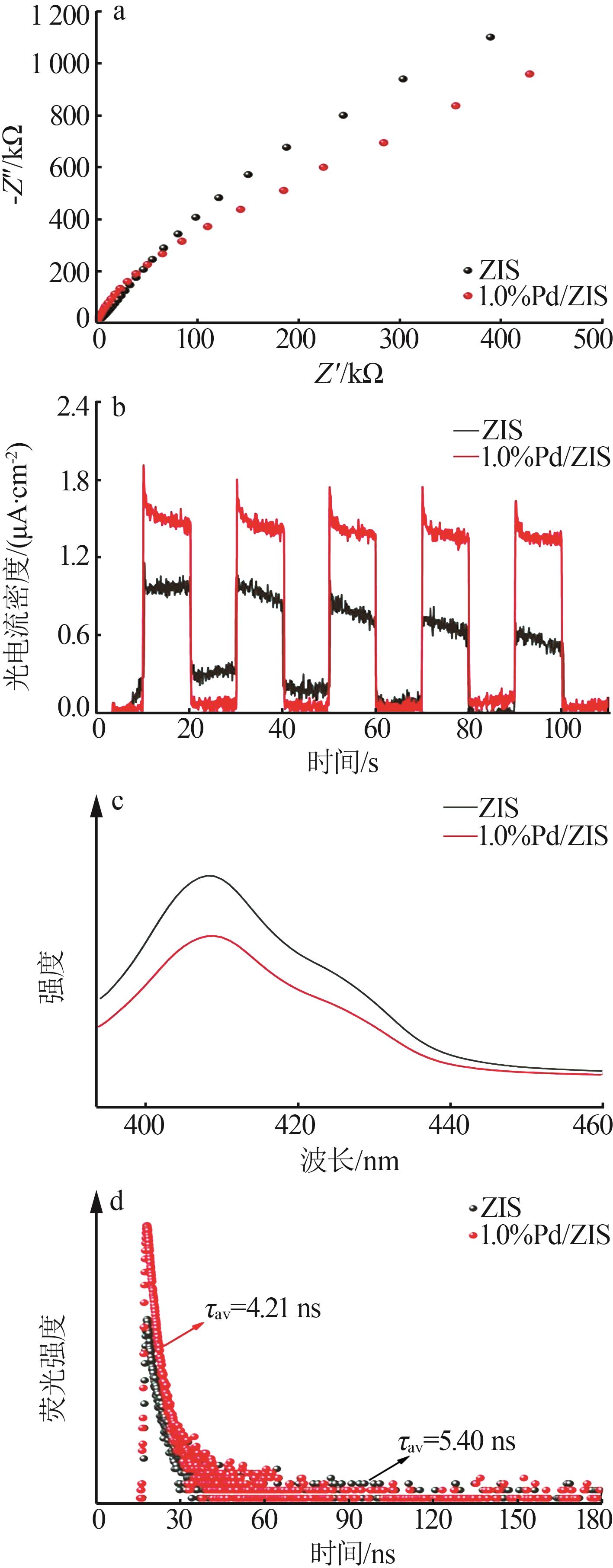
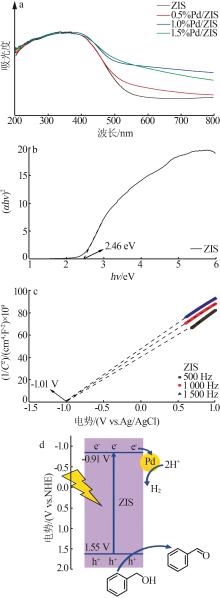
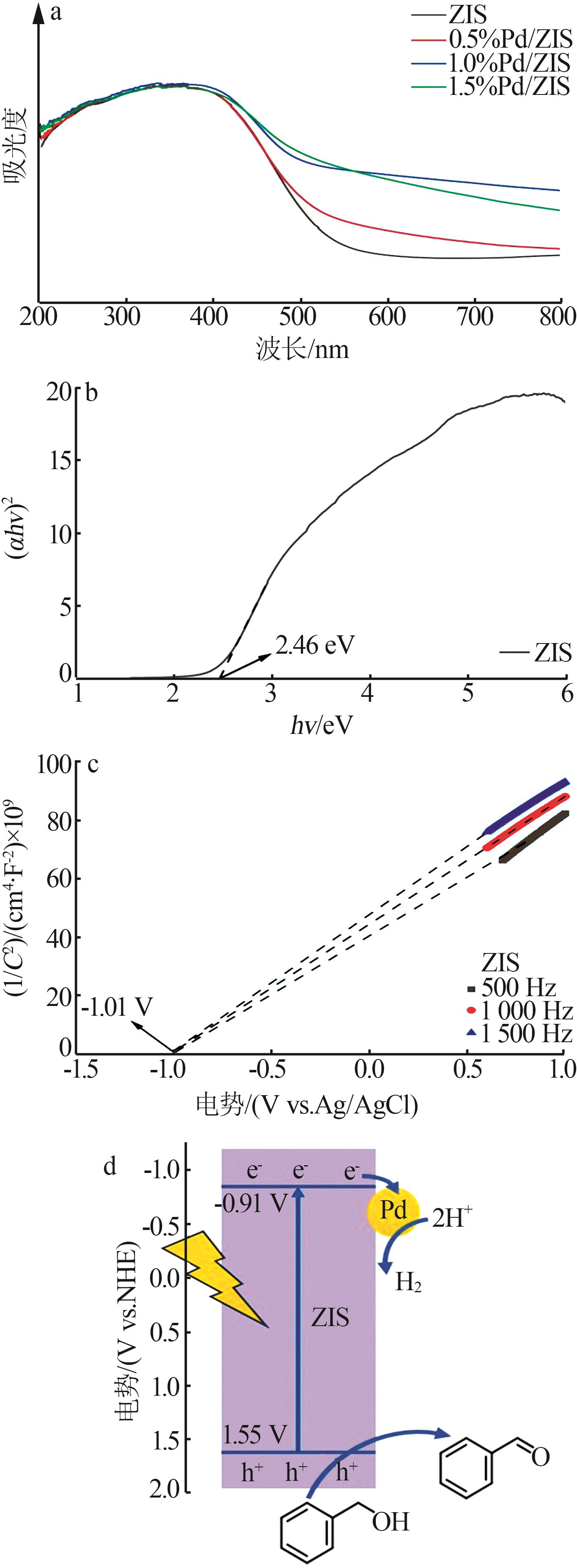
| 1 |
LI Qi, OUYANG Yuxing, LI Hongliang,et al.Photocatalytic conversion of methane:Recent advancements and prospects[J].Angewandte Chemie (International Ed),2022,61(2):e202108069.
|
| 2 |
李佳慧,李克艳,宋春山,等.聚合氮化碳的制备、改性及光催化还原二氧化碳性能研究[J].无机盐工业,2021,53(12):21-28.
|
|
LI Jiahui, LI Keyan, SONG Chunshan,et al.Study on preparation,modification and carbon dioxide photocatalytic reduction performance of polymeric carbon nitride[J].Inorganic Chemicals Industry,2021,53(12):21-28.
|
| 3 |
WANG Qian, DOMEN K.Particulate photocatalysts for light-driven water splitting:Mechanisms,challenges,and design strategies[J].Chemical Reviews,2020,120(2):919-985.
|
| 4 |
LUO Nengchao, MONTINI T, ZHANG Jian,et al.Visible-light-driven coproduction of diesel precursors and hydrogen from lignocellulose-derived methylfurans[J].Nature Energy,2019,4(7):575-584.
|
| 5 |
CHEN Lang, TANG Jie, SONG Luna,et al.Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons[J].Applied Catalysis B:Environmental,2019,242:379-388.
|
| 6 |
ZHANG Meiyu, LI Kongming, HU Chunlian,et al.Co nanoparticles modified phase junction CdS for photoredox synthesis of hydrobenzoin and hydrogen evolution[J].Chinese Journal of Catalysis,2023,47:254-264.
|
| 7 |
JIANG Chunli, WANG Hao, WANG Yongqing,et al.All solid-state Z-scheme CeO2/ZnIn2S4 hybrid for the photocatalytic selective oxidation of aromatic alcohols coupled with hydrogen evoluti-on[J].Applied Catalysis B:Environmental,2020,277:119235.
|
| 8 |
白红亮,王俊生,王艳.Au-Pd/ZrO2可见光下催化苯甲醇氧化反应条件探索[J].无机盐工业,2020,52(4):100-103.
|
|
BAI Hongliang, WANG Junsheng, WANG Yan.Study on oxidation reacting conditions of benzyl alcohol catalyzed by Au-Pd/ZrO2 under visible light[J].Inorganic Chemicals Industry,2020,52(4):100-103.
|
| 9 |
CHEN Mengjie, LU Simin, PENG Yueyi,et al.Tracking the electrocatalytic activity of a single palladium nanoparticle for the hydrogen evolution reaction[J].Chemistry-A European Journal,2021,27(46):11799-11803.
|
| 10 |
DU Chun, ZHANG Qian, LIN Zhaoyong,et al.Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution[J].Applied Catalysis B:Environmental,2019,248:193-201.
|
| 11 |
ZHAO Yanchun, YANG Xiulin, TIAN Jianniao,et al.Methanol electro-oxidation on Ni@Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media[J].International Journal of Hydrogen Energy,2010,35(8):3249-3257.
|
| 12 |
DU Jun, SHI Hainan, WU Jiaming,et al.Interface and defect engineering of a hollow TiO2@ZnIn2S4 heterojunction for highly enhanced CO2 photoreduction activity[J].ACS Sustainable Chemistry & Engineering,2023,11(6):2531-2540.
|
| 13 |
YANG Guang, CHEN Daimei, DING Hao,et al.Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency[J].Applied Catalysis B:Environmental,2017,219:611-618.
|
| 14 |
肖勇,贾雅梅,关登,等.锡掺杂溴铅铯钙钛矿纳米晶的合成、结构及发光性能[J].无机盐工业,2023,55(6):63-69.
|
|
XIAO Yong, JIA Yamei, GUAN Deng,et al.Synthesis,structure and luminescent performance of Sn2+-doped CsPbBr3 perovskite nanocrystals[J].Inorganic Chemicals Industry,2023,55(6):63-69.
|
| 15 |
ZHAO Shuangchao, LI Keyan, DU Jun,et al.Facile construction of a hollow In2S3/polymeric carbon nitride heterojunction for efficient visible-light-driven CO2 reduction[J].ACS Sustainable Chemistry & Engineering,2021,9(17):5942-5951.
|
| 16 |
XIE Ziyu, CHEN Jing, CHEN Yanxin,et al.A Z-scheme Pd modified ZnIn2S4/P25 heterojunction for enhanced photocatalytic hydrogen evolution[J].Applied Surface Science,2022,579:152003.
|
| 17 |
CHEN Zhihao, SHAHID M Z, JIANG Xinyan,et al.Regulating the active sites of Cs2AgBiCl6 by doping for efficient coupling of photocatalytic CO2 reduction and benzyl alcohol oxidation[J].Small,2024,20(1):2304756.
|
| 18 |
MENG Sugang, YE Xiangju, ZHANG Jinghu,et al.Effective use of photogenerated electrons and holes in a system:Photocataly-tic selective oxidation of aromatic alcohols to aldehydes and hydrogen production[J].Journal of Catalysis,2018,367:159- 170.
|
| 19 |
XING Fangshu, ZENG Renyou, CHENG Chuchu,et al.POM-incorporated ZnIn2S4 Z-scheme dual-functional photocatalysts for cooperative benzyl alcohol oxidation and H2 evolution in aqueous solution[J].Applied Catalysis B:Environmental,2022,306:121087.
|
| 20 |
WANG Chunyang, TANG Yuan, GENG Zikang,et al.Modulating charge accumulation via electron interaction for photocatalytic hydrogen evolution:A case of fabricating palladium sites on ZnIn2S4 nanosheets[J].ACS Catalysis,2023,13(17):11687-11696.
|
 ), LI Keyan(
), LI Keyan( ), GUO Xinwen(
), GUO Xinwen( )
)