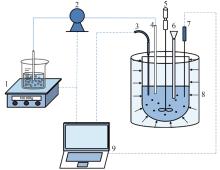
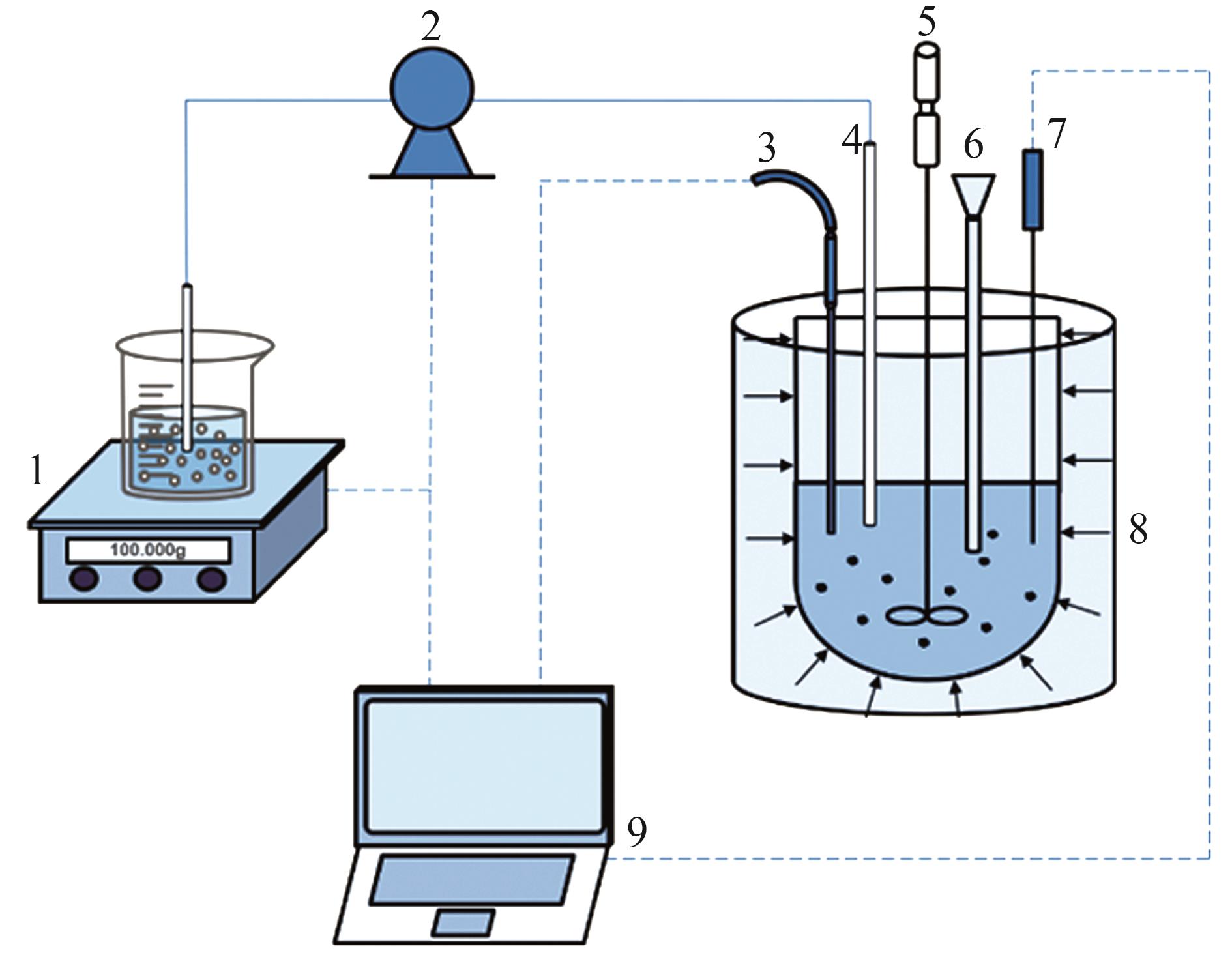
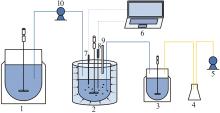
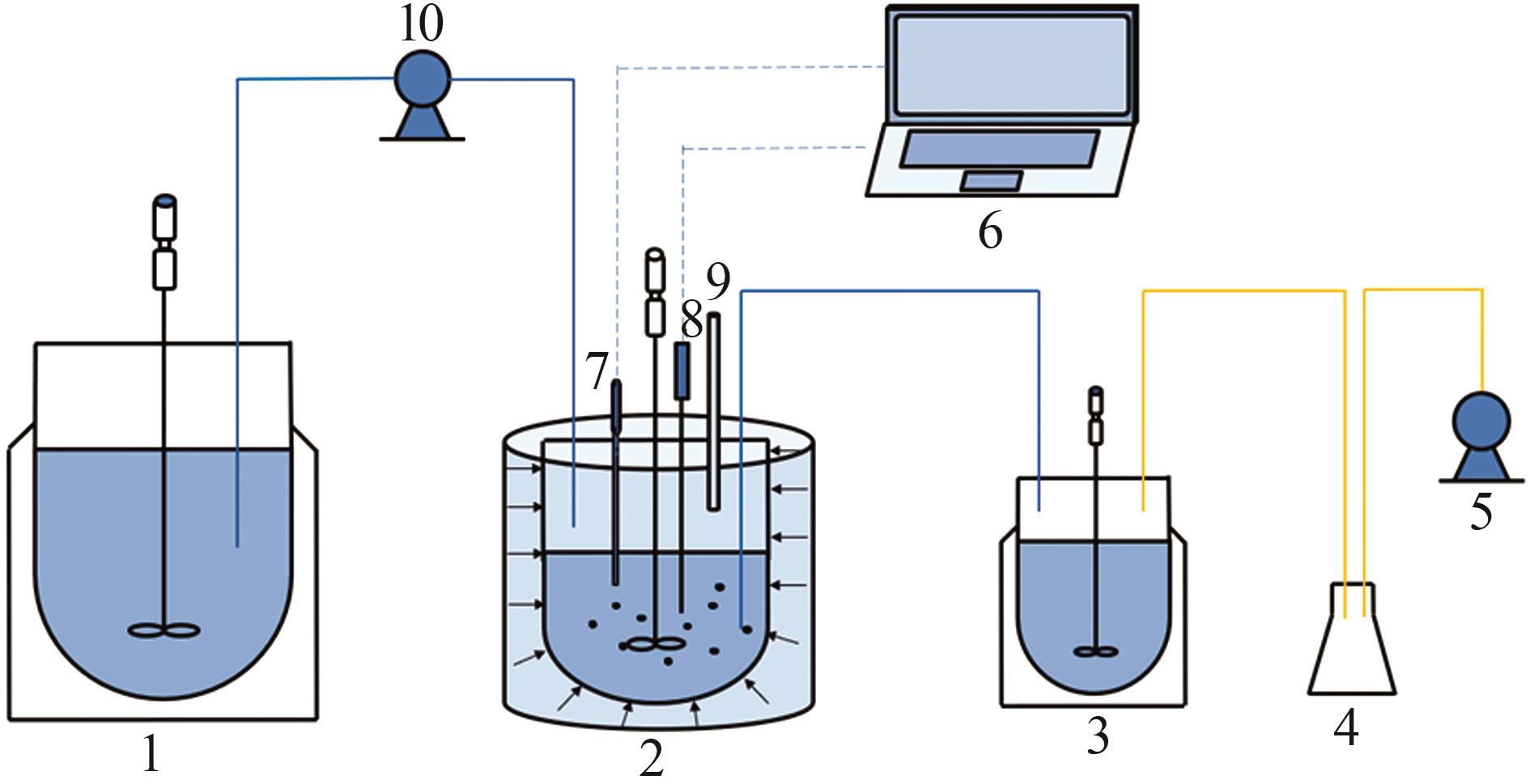
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (8): 40-46.doi: 10.19964/j.issn.1006-4990.2023-0531
• Research & Development • Previous Articles Next Articles
Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system
SU Hang( ), SONG Jitian(
), SONG Jitian( ), HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong
), HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong
- Tianjin Key Laboratory of Integrated Design and On?line Monitoring for Light Industry & Food Machinery and Equipment,College of Mechanical Enginnering,Tianjin University of Science and Technology,Tianjin 300222,China
-
Received:2023-11-09Online:2024-08-10Published:2024-09-26 -
Contact:SONG Jitian E-mail:wieei6375@163.com;songjt@tust.edu.cn
CLC Number:
Cite this article
SU Hang, SONG Jitian, HUANG Zhiqiang, DONG Qing, ZHANG Yaxiong. Study on crystallization kinetics of manganese sulfate monohydrate in H2SO4-H2O binary system[J]. Inorganic Chemicals Industry, 2024, 56(8): 40-46.
share this article
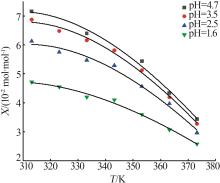
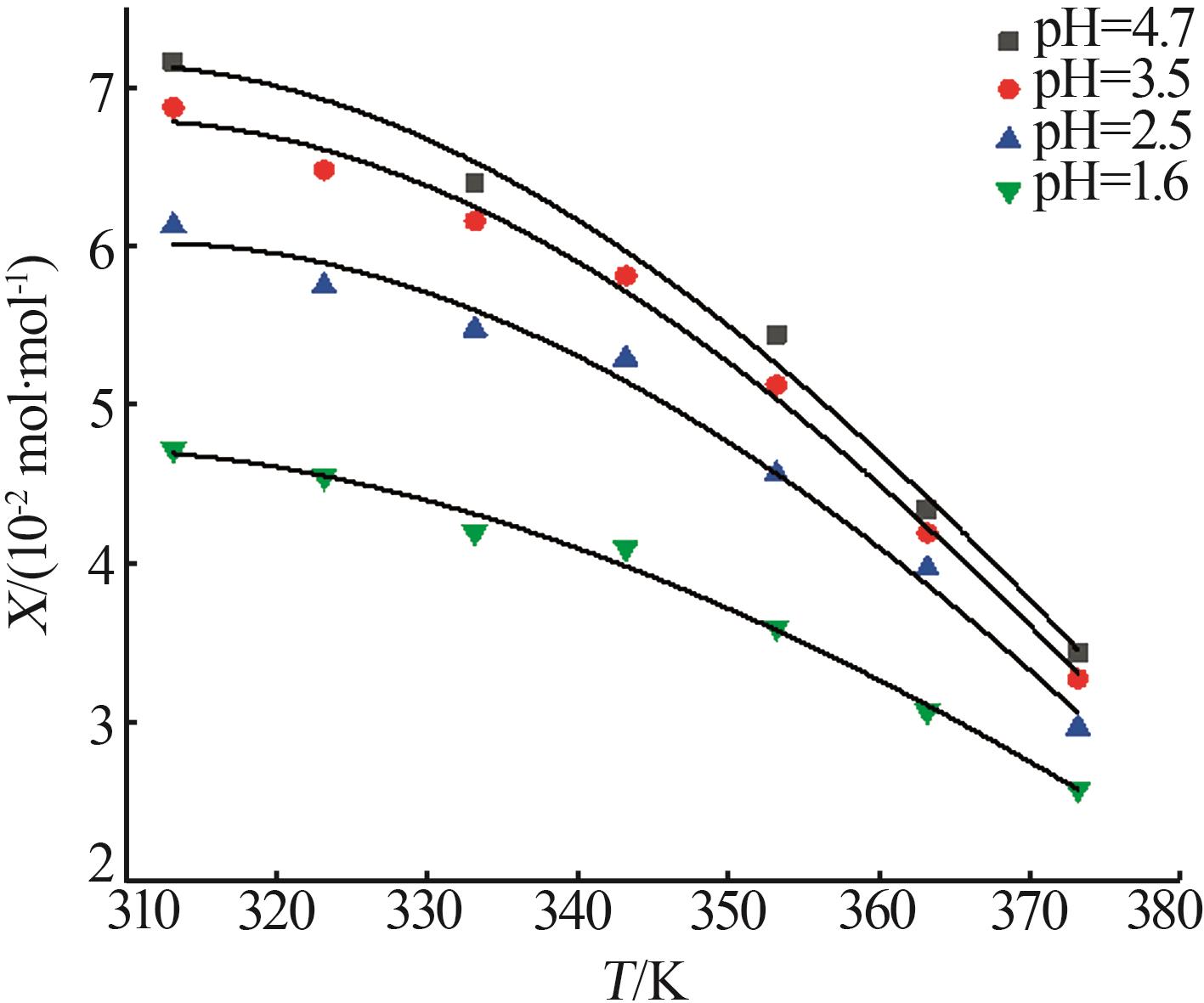
Table 1
Measurement and model calculation values of manganese sulfate solubility"
| T/K | Xcal/ (10-2 mol·mol-1) | Xref[ (10-2 mol·mol-1) | RD/% | Xcal/ (10-2 mol·mol-1) | Xexp/ (10-2 mol·mol-1) | RD/% | Xcal/ (10-2 mol·mol-1) | Xexp/ (10-2 mol·mol-1) | RD/% | Xcal/ (10-2 mol·mol-1) | Xexp/ (10-2 mol·mol-1) | RD/% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH=4.7 | pH=3.5 | pH=2.5 | pH=1.6 | ||||||||||||
| 373.15 | 3.46 | 3.43 | -0.87 | 3.49 | 3.27 | -6.73 | 3.20 | 2.97 | -7.74 | 2.60 | 2.58 | -0.78 | |||
| 363.15 | 4.26 | 4.34 | 1.84 | 4.28 | 4.19 | -2.15 | 3.85 | 3.97 | 3.02 | 3.06 | 3.07 | 0.33 | |||
| 353.15 | 5.11 | 5.44 | 6.07 | 5.13 | 5.12 | 1.54 | 4.54 | 4.56 | 0.44 | 3.54 | 3.59 | 1.39 | |||
| 343.15 | 5.95 | 5.94 | 5.81 | -2.24 | 5.19 | 5.29 | 1.89 | 3.98 | 4.10 | 2.93 | |||||
| 333.15 | 6.63 | 6.39 | -3.76 | 6.60 | 6.15 | -6.45 | 5.72 | 5.47 | -4.57 | 4.36 | 4.19 | -4.06 | |||
| 323.15 | 7.00 | 6.95 | 6.47 | -5.78 | 6.01 | 5.74 | -4.70 | 4.59 | 4.54 | -1.10 | |||||
| 313.15 | 6.93 | 7.15 | 3.08 | 6.86 | 6.86 | -0.59 | 5.97 | 6.12 | 2.45 | 4.64 | 4.71 | 1.49 | |||
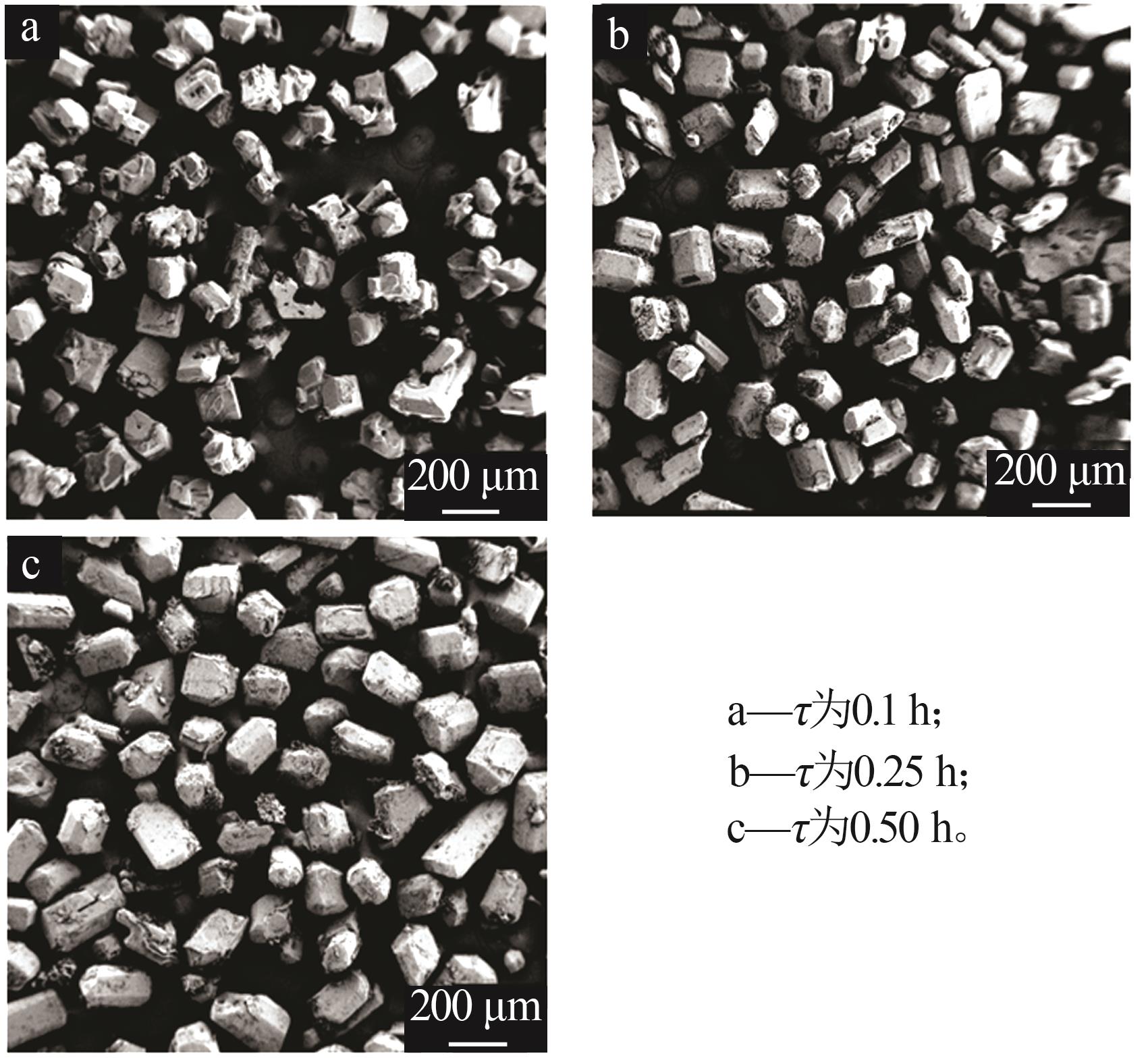
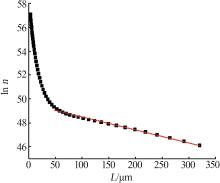
Table 2
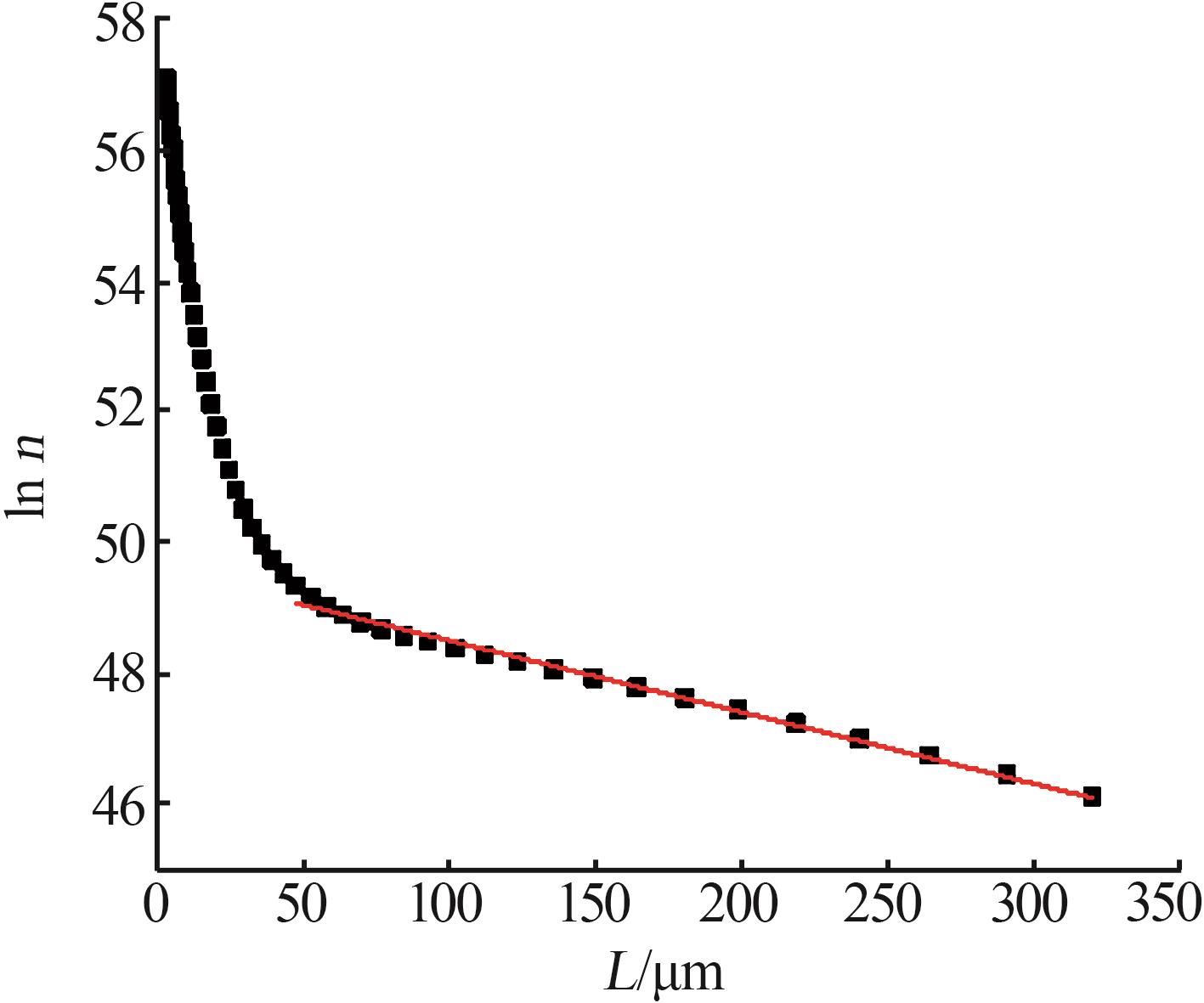
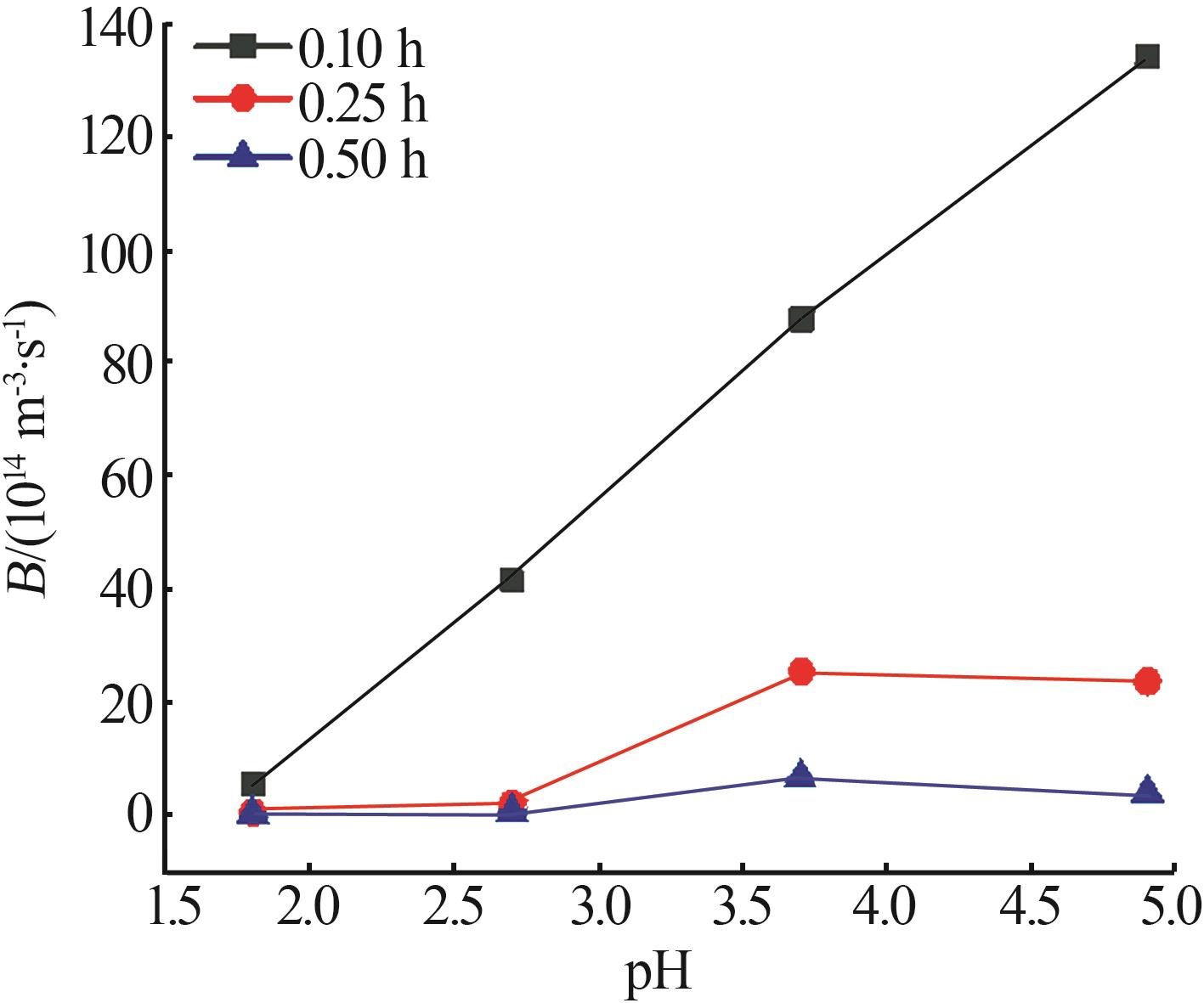
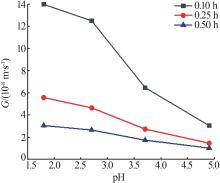
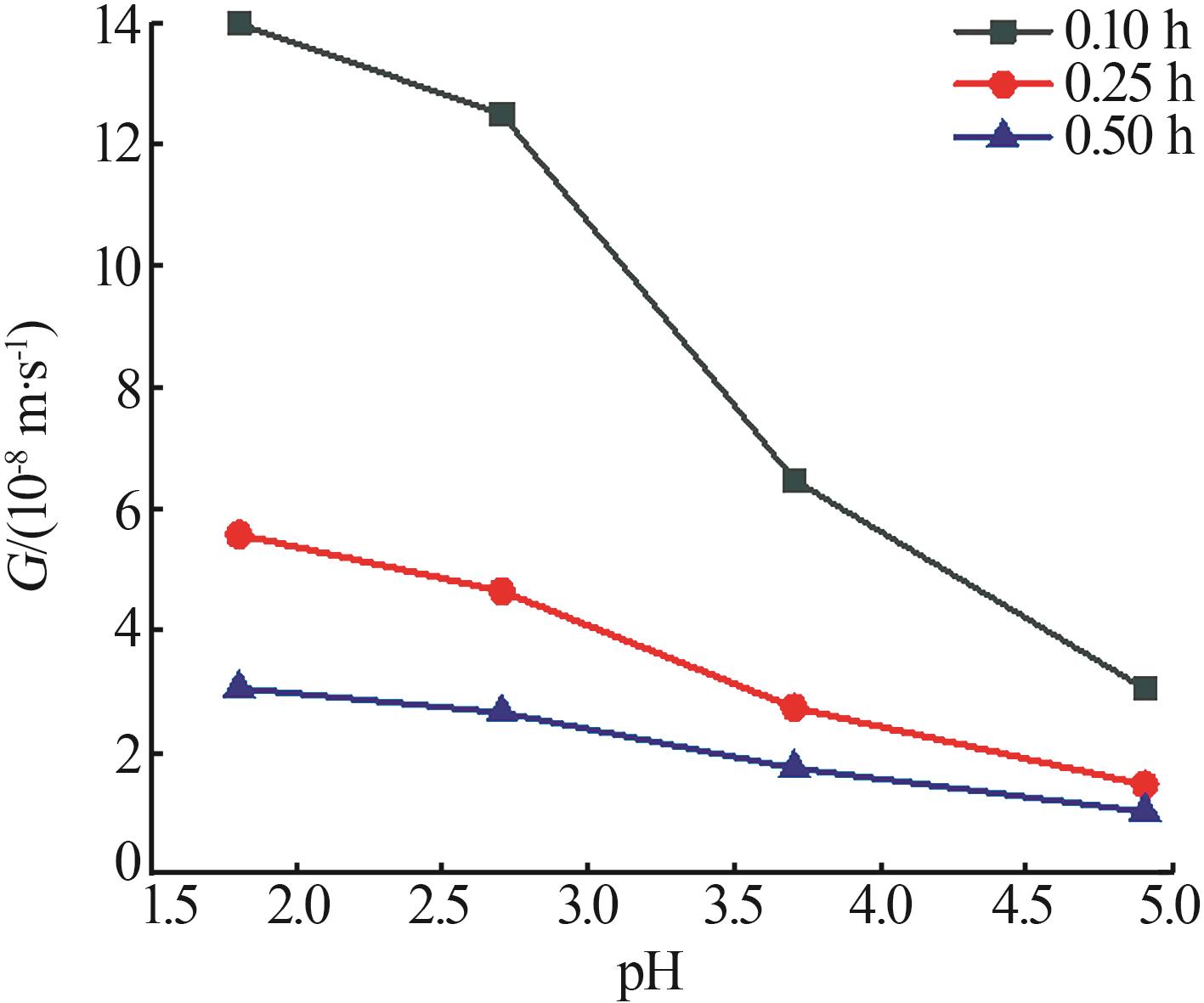
Nucleation and growth rates of manganese sulfate monohydrate with different pH values and residence time"
| pH | 停留时间/ h | 相对过 饱和度 | 粒数密度/ m-4 | 生长速率/ (m·s-1) | 成核速率/ (m³·s)-1 | 生长速率方程G=KGσg | 成核速率方程B=KNσn | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KG | g | KN | n | ||||||||
| 1.6 | 0.10 | 0.041 | 4.02×1021 | 1.40×10-7 | 5.63×1014 | 6.520×10-6 | 1.208 | 1.539×1020 | 3.919 | ||
| 0.25 | 0.022 | 8.64×1020 | 5.59×10-8 | 4.82×1013 | |||||||
| 0.50 | 0.008 | 5.83×1020 | 3.06×10-8 | 1.78×1013 | |||||||
| 2.5 | 0.10 | 0.039 | 1.22×1022 | 1.25×10-7 | 1.53×1015 | 7.823×10-6 | 1.279 | 3.102×1019 | 3.057 | ||
| 0.25 | 0.020 | 4.14×1021 | 4.66×10-8 | 1.93×1014 | |||||||
| 0.50 | 0.009 | 1.69×1021 | 2.66×10-8 | 4.49×1013 | |||||||
| 3.5 | 0.10 | 0.048 | 1.35×1023 | 6.48×10-8 | 8.78×1015 | 1.330×10-6 | 0.995 | 1.077×1018 | 1.583 | ||
| 0.25 | 0.020 | 9.27×1022 | 2.74×10-8 | 2.54×1015 | |||||||
| 0.50 | 0.013 | 3.83×1022 | 1.74×10-8 | 6.66×1014 | |||||||
| 4.7 | 0.10 | 0.042 | 4.41×1023 | 3.05×10-8 | 1.34×1016 | 8.006×10-7 | 1.034 | 1.323×1020 | 2.900 | ||
| 0.25 | 0.023 | 1.61×1023 | 1.47×10-8 | 2.36×1015 | |||||||
| 0.50 | 0.013 | 3.88×1022 | 1.03×10-8 | 3.99×1014 | |||||||
| 1 | 王崇国,刘广龙,金小容,等.锂离子电池正极材料的研究进 展[J].当代化工研究,2023(9):12-14. |
| WANG Chongguo, LIU Guanglong, JIN Xiaorong,et al.Research progress of lithium⁃ion battery cathode material[J].Modern Che⁃Research mical,2023(9):12-14. | |
| 2 | 韩啸,张成锟,吴华龙,等.锂离子电池的工作原理与关键材 料[J].金属功能材料,2021,28(2):37-58. |
| HAN Xiao, ZHANG Chengkun, WU Hualong,et al.Working mechanism and key materials of the lithium ion batteries[J].Metallic Functional Materials,2021,28(2):37-58. | |
| 3 | 刘德新,马腾跃,安金玲,等.锰基钠离子电池正极材料设计及电化学性能研究[J].无机盐工业,2024,56(3):51-55. |
| LIU Dexin, MA Tengyue, AN Jinling,et al.Study on cathode material design and electrochemical properties of manganese⁃based sodium ion battery[J].Inorganic Chemicals Industry,2024,56(3):51-55. | |
| 4 | 中华人民共和国工业和信息化部. 电池用硫酸锰: [S].北京:化工出版社,2016. |
| 5 | MOHAGHEGHI M, ASKARI M.Kinetics of the antisolvent crystallization of manganese sulfate monohydrate from a pregnant leach solution[J].Chemical Papers,2024,78(3):1529-1535. |
| 6 | 张曼曼,张德友,周进,等.离心萃取制备电池级硫酸锰[J].电池,2023,53(4):424-427. |
| ZHANG Manman, ZHANG Deyou, ZHOU Jin,et al.Preparation of battery grade manganese sulfate by centrifugal extraction[J].Battery Bimonthly,2023,53(4):424-427. | |
| 7 | 邹兴.高纯硫酸锰生产技术现状[J].中国锰业,2018,36(6):4-6. |
| ZOU Xing.Present situation of production technology of high purity manganese sulfate[J].China′s Manganese Industry,2018, 36(6):4-6. | |
| 8 | 王天雄,刘京,范玉英.菱锰矿浸出液制备电池级硫酸锰的技术研究[J].中国锰业,2015,33(2):22-25. |
| WANG Tianxiong, LIU Jing, FAN Yuying.A study on preparation of battery grade manganese sulfate with rhodochrosite leachate[J].China′s Manganese Industry,2015,33(2):22-25. | |
| 9 | 杨攀,王家伟,王松,等.蒸发结晶法深度净化硫酸锰工艺研 究[J].矿冶工程,2022,42(3):88-91. |
| YANG Pan, WANG Jiawei, WANG Song,et al.Deep purification of manganese sulfate by evaporation and crystallization[J].Mining and Metallurgical Engineering,2022,42(3):88-91. | |
| 10 | 刘中华.高温结晶法制备硫酸锰的工艺及自动连续装置:中国,107055622[P].2017-06-12. |
| 11 | 何雨林,李富杰,罗志虹,等.工业硫酸锰高温结晶纯化制备电池级硫酸锰的研究[J].矿冶工程,2019,39(3):85-88. |
| HE Yulin, LI Fujie, LUO Zhihong,et al.Preparation of battery⁃grade manganese sulfate by purification of industrial⁃grade manganese sulfate with high⁃temperature crystallization method[J].Mining and Metallurgical Engineering,2019,39(3):85-88. | |
| 12 | SAFAEEFAR P, ANG H M, TADE M O,et al.Growth kinetics of manganese sulphate from heating and salting⁃out batch crystallisation[J].Developments in Chemical Engineering and Mineral Processing,2006,14(1/2):303-312. |
| 13 | ZHANG Xueping, LIU Youquan, ZHOU Lang,et al.Experiment and calculation of phase equilibrium in quaternary system NaBr-KBr-CaBr2-H2O at 273.15 K containing in gas field brine of Sichuan Basin[J].Russian Journal of Physical Chemistry A,2023,97(7):1368-1375. |
| 14 | CAO Yun, DU Shichao, KE Xiao,et al.Interplay between thermodynamics and kinetics on polymorphic behavior of vortioxetine hydrobromide in reactive crystallization[J].Organic Process Research & Development,2020,24(7):1233-1243. |
| 15 | LUO Yanghui, WU Guogan, SUN Baiwang.Antisolvent crystallization of biapenem:Estimation of growth and nucleation kineti⁃cs[J].Journal of Chemical & Engineering Data,2013,58(3):588-597. |
| 16 | 张得江.磷酸钠间歇及连续结晶过程的研究[D].天津:天津大学,2018. |
| ZHANG Dejiang.Research on batch and continuous crystallization processes of sodium phosphate[D].Tianjin:Tianjin University,2018. | |
| 17 | 周桓,张闯,白晓琴,等.KCl-MgCl2-H2O体系25 ℃盐析光卤石的等温热力学与结晶规律[J].天津科技大学学报,2014, 29(6):46-51. |
| ZHOU Huan, ZHANG Chuang, BAI Xiaoqin,et al.Isothermal thermodynamics and crystallization behavior of carnallite salt⁃out in KCl-MgCl2-H2O system at 25 ℃[J].Journal of Tianjin University of Science & Technology,2014,29(6):46-51. | |
| 18 | APELBLAT A, MANZUROLA E.Solubilities of o-acetylsalicylic,4-aminosalicylic,3,5-dinitrosalicylic,and p-toluic acid,and magnesium-DL-aspartate in water from T=(278 to 348) K[J].The Journal of Chemical Thermodynamics,1999,31(1):85-91. |
| 19 | 刘光启,马连湘,项曙光.化学化工物性数据手册-无机卷[M].2版.北京:化学工业出版社,2013. |
| 20 | RANDOLPH A D, LARSON M A.Theory of particulate proces⁃ses⁃chapter 1[J].Theory of Particulate Processes,1971,47(9):1-11. |
| 21 | OREHEK J, TESLIĆ D, LIKOZAR B.Continuous crystallization processes in pharmaceutical manufacturing:A review[J].Organic Process Research & Development,2021,25(1):16-42. |
| 22 | KOUGOULOS E, JONES A G, WOOD-KACZMAR M W.Estimation of crystallization kinetics for an organic fine chemical using a modified continuous cooling mixed suspension mixed product removal(MSMPR) crystallizer[J].Journal of Crystal Growth,2005,273(3/4):520-528. |
| 23 | 叶铁林.化工结晶过程原理及应用[M].北京:北京工业大学出版社,2006. |
| 24 | 丁绪淮,谈遒.工业结晶[M].北京:化学工业出版社,1985. |
| 25 | 汪镇安.化工工艺设计手册.上册[M].北京:化学工业出版社,2003. (上接第39页) |
| [1] | YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K [J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35. |
| [2] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [3] | FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K [J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. |
| [4] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [5] | LI Yan,WANG Yansong,CHENG Huaigang,KANG Jin,LI Enze,LÜ Hongzhou,LIU Qian. Rapid quality inspection of high-purity lithium carbonate based on determination of magnesium by water-soluble probe method [J]. Inorganic Chemicals Industry, 2023, 55(1): 87-92. |
| [6] | CHEN Shuai,YANG Bo,CHEN Niancu,LUO Jun,REN Siying,ZENG Ying,YU Xudong. Study on phase equilibria of ternary system NH4Cl+MgCl2+H2O at 323.2 K [J]. Inorganic Chemicals Industry, 2022, 54(4): 100-103. |
| [7] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [8] | ZHANG Huanhuan,CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen. Determination and correlation of solubility of potassium dihydrogen phosphate in acetonitrile-water solvent [J]. Inorganic Chemicals Industry, 2022, 54(1): 83-85. |
| [9] | Wang Bin,Deng Xiaochuan,Shi Yifei,Dong Chaochao,Fan Faying,Zhu Chaoliang,Fan Jie. Online determination of the solubility of lithium carbonate in water and NaCl-KCl solution system [J]. Inorganic Chemicals Industry, 2021, 53(7): 73-79. |
| [10] | CHEN Yunfei,MAO Lili,LIU Dan,WANG Haizeng. Measurement and correlation of solubility of magnesium chloride hexahydrate in six polyols [J]. Inorganic Chemicals Industry, 2021, 53(12): 85-90. |
| [11] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [12] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [13] | Xu Wei,Li Mei,Zhang Dongliang,Gao Kai,Li Jianfei. Study on preparation of calcium sulfate whisker by atmospheric pressure acidification using rare earth gypsum [J]. Inorganic Chemicals Industry, 2020, 52(8): 66-71. |
| [14] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [15] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||