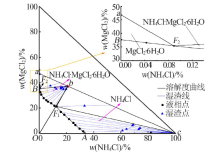
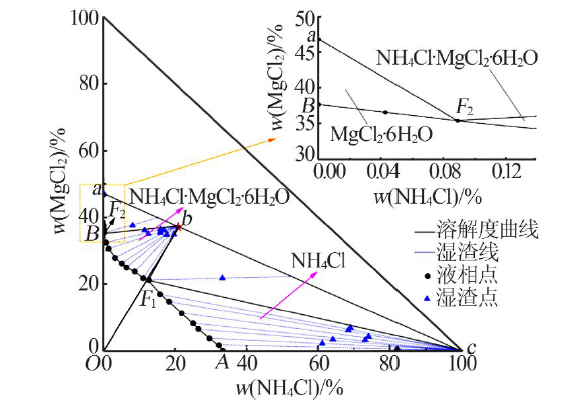
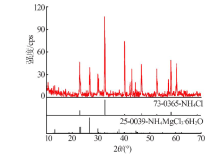
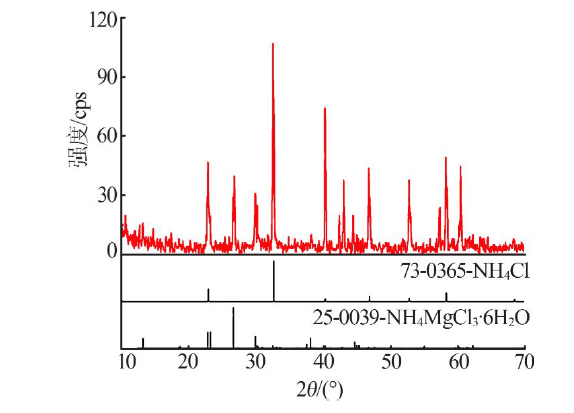
| [1] |
周训, 曹琴, 尹菲, 等. 四川盆地东部高褶带三叠系地层卤水和温泉的地球化学特征及成因[J]. 地质学报, 2015, 89(11):1908-1920.
|
| [2] |
杨家敏, 朱静, 胡雪, 等. 283.15 K下三元KCl-NH4Cl-H2O和KH2PO4-NH4H2PO4-H2O体系固-液相平衡测定与关联[J]. 无机盐工业, 2021, 53(1):30-35.
|
| [3] |
ZHONG Y L, CHEN J X, SU M, et al. Solid-liquid equilibrium,crystal type,solid solubility and thermal stability studies of potassium ammonium chloride solid solution[J]. Fluid Phase Equilibria, 2017, 439:24-30.
doi: 10.1016/j.fluid.2017.02.015
|
| [4] |
于旭东. 川东北普光地区深部卤水多温蒸发析盐规律研究[D]. 北京:中国地质科学院, 2021.
|
| [5] |
李武, 董亚萍, 宋彭生. 盐湖卤水资源开发利用[M]. 北京: 化学工业出版社, 2012.
|
| [6] |
OUYANG H T, ZENG D W, ZHOU H Y, et al. Solubility of the ternary system LiCl+NH4Cl+H2O[J]. Journal of Chemical & Engineering Data, 2011, 56(4):1096-1104.
doi: 10.1021/je101056t
|
| [7] |
YANG H T, ZENG D W, LIANG T Y, et al. Experimental determination and modeling of the solubility phase diagram of the quaternary system MgCl2+LiCl+NH4Cl+H2O at 298.15 K and its applications in industry[J]. Industrial & Engineering Chemistry Research, 2013, 52(48):17057-17063.
doi: 10.1021/ie4027555
|
| [8] |
王硕, 赵斌, 郭宏飞, 等. 0 ℃时NH4Cl-MgCl2-KCl-H2O四元体系相平衡研究及应用[J]. 高校化学工程学报, 2017, 31(3):515-520.
|
| [9] |
赵晓玲, 秦亚欠, 赵斌, 等. 25 ℃时四元体系KCl-MgCl2-NH4Cl-H2O相平衡研究及应用[J]. 高校化学工程学报, 2018, 32(2):266-274.
|
| [10] |
WU J X, ZHANG G C, ZHAO B, et al. Phase diagram of the quaternary system KCl-MgCl2-NH4Cl-H2O at t=60.00 ℃ and their application[J]. Journal of Solution Chemistry, 2017, 46(1):58-69.
doi: 10.1007/s10953-016-0558-7
|
| [11] |
吴强, 胡雪, 朱静, 等. KH2PO4-KCl-H2O、NH4H2PO4-NH4Cl-H2O三元体系283.15 K相平衡研究[J]. 无机盐工业, 2020, 52(11):24-28.
|
| [12] |
YU X D, ZENG Y, ZHANG Z X. Solid-liquid metastable phase equilibria in the ternary systems KCl+NH4Cl+H2O and NH4Cl+MgCl2+H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2012, 57(6):1759-1765.
doi: 10.1021/je300124u
|
| [13] |
YU X D, WANG L, CHEN J, et al. Salt-water phase equilibria in ternary systems K+(Mg2+),NH4+//Cl--H2O at T=273 K[J]. Journal of Chemical & Engineering Data, 2017, 62(4):1427-1432.
doi: 10.1021/acs.jced.6b00981
|
| [14] |
张志翔, 曾英, 于旭东. 三元体系MgCl2+NH4Cl+H2O 298.15 K稳定相平衡[J]. 化学工程, 2012, 40(1):38-42.
|
| [15] |
中国科学院青海盐湖分析室. 卤水和盐的分析方法[M]. 北京: 科学出版社, 1988.
|
 ),YANG Bo1,CHEN Niancu1,LUO Jun1,REN Siying1,ZENG Ying1,2,YU Xudong1,2(
),YANG Bo1,CHEN Niancu1,LUO Jun1,REN Siying1,ZENG Ying1,2,YU Xudong1,2( )
)