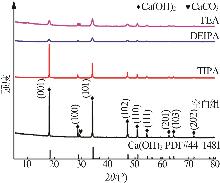
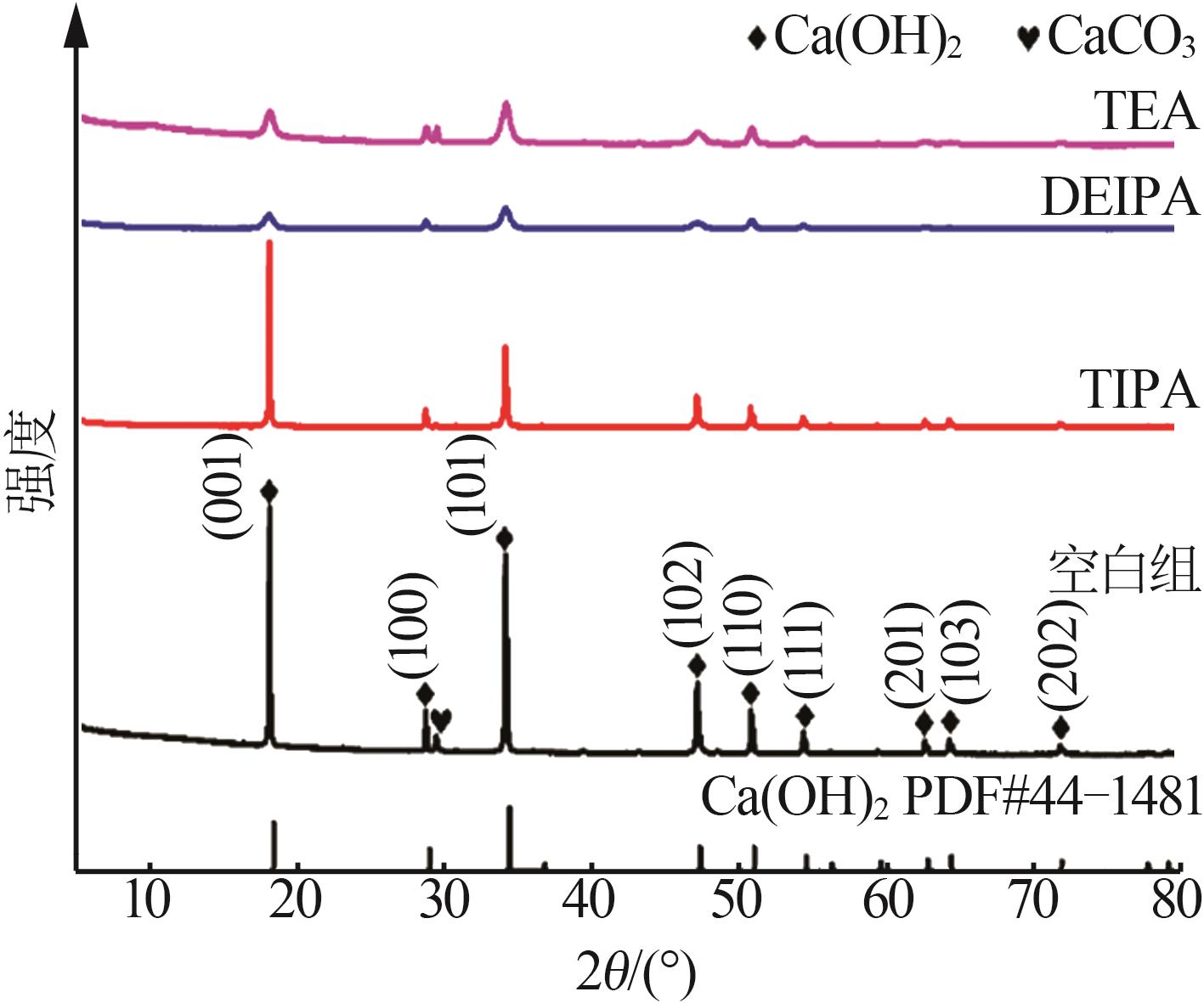
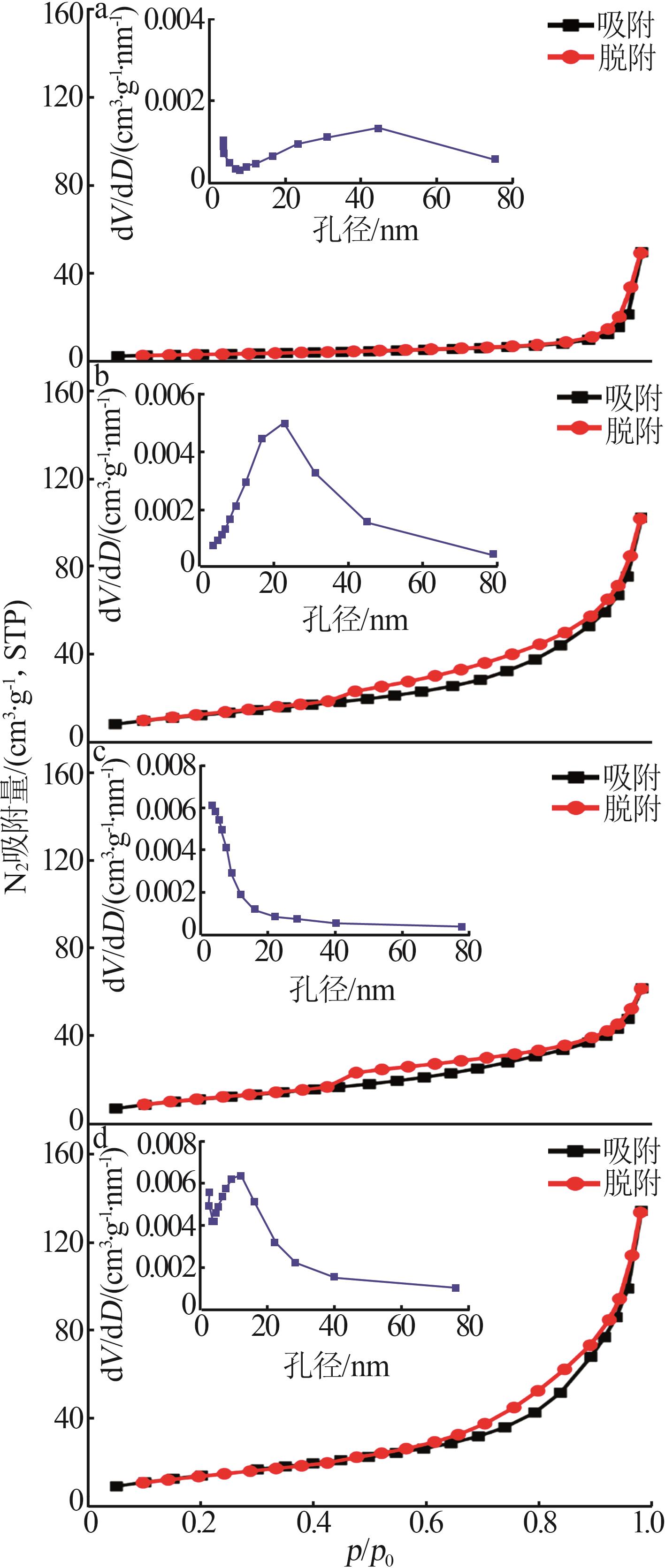
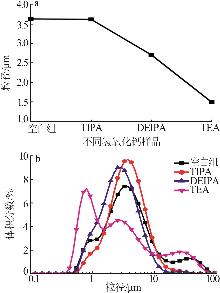
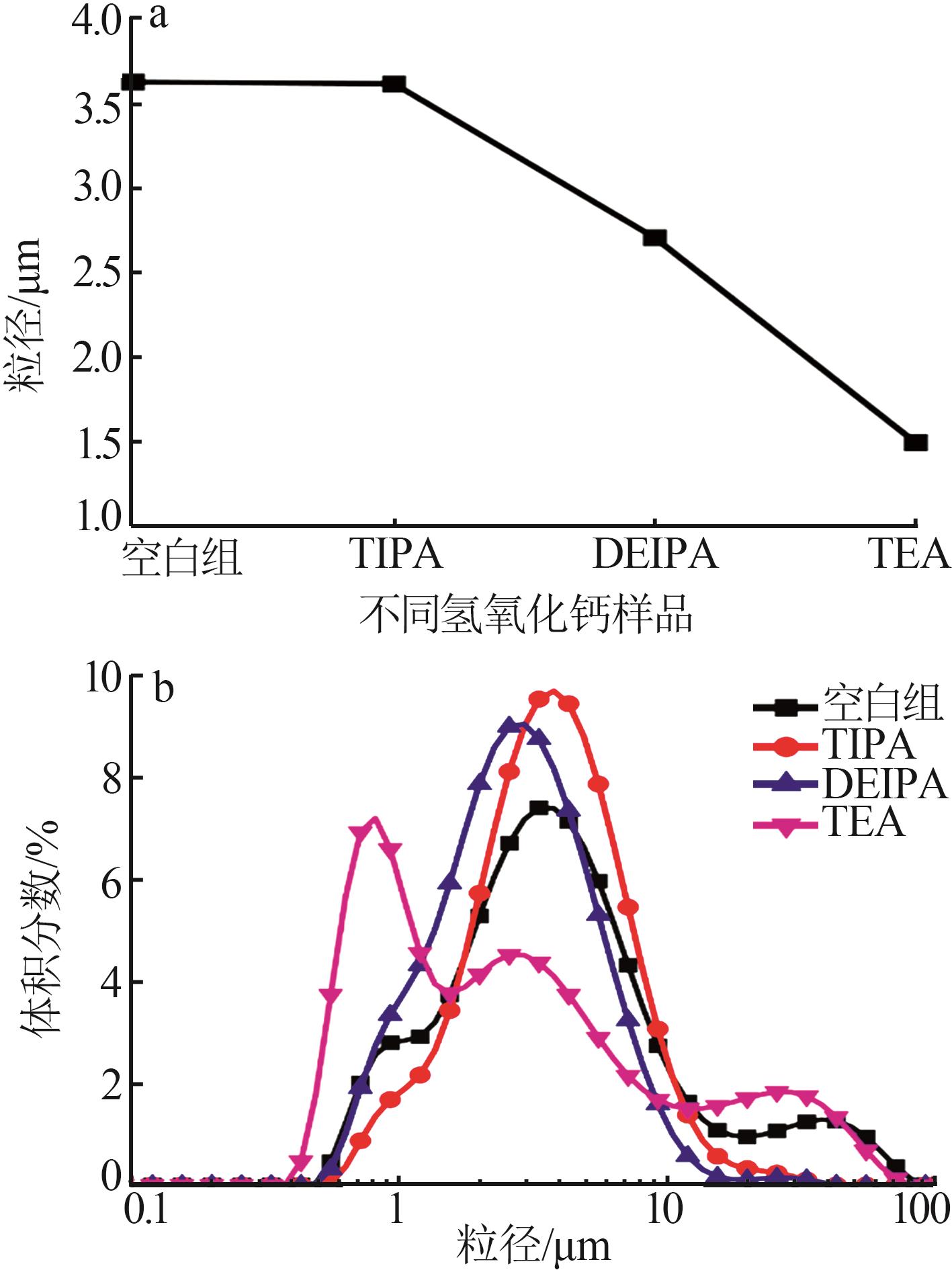
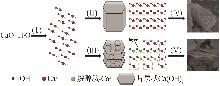
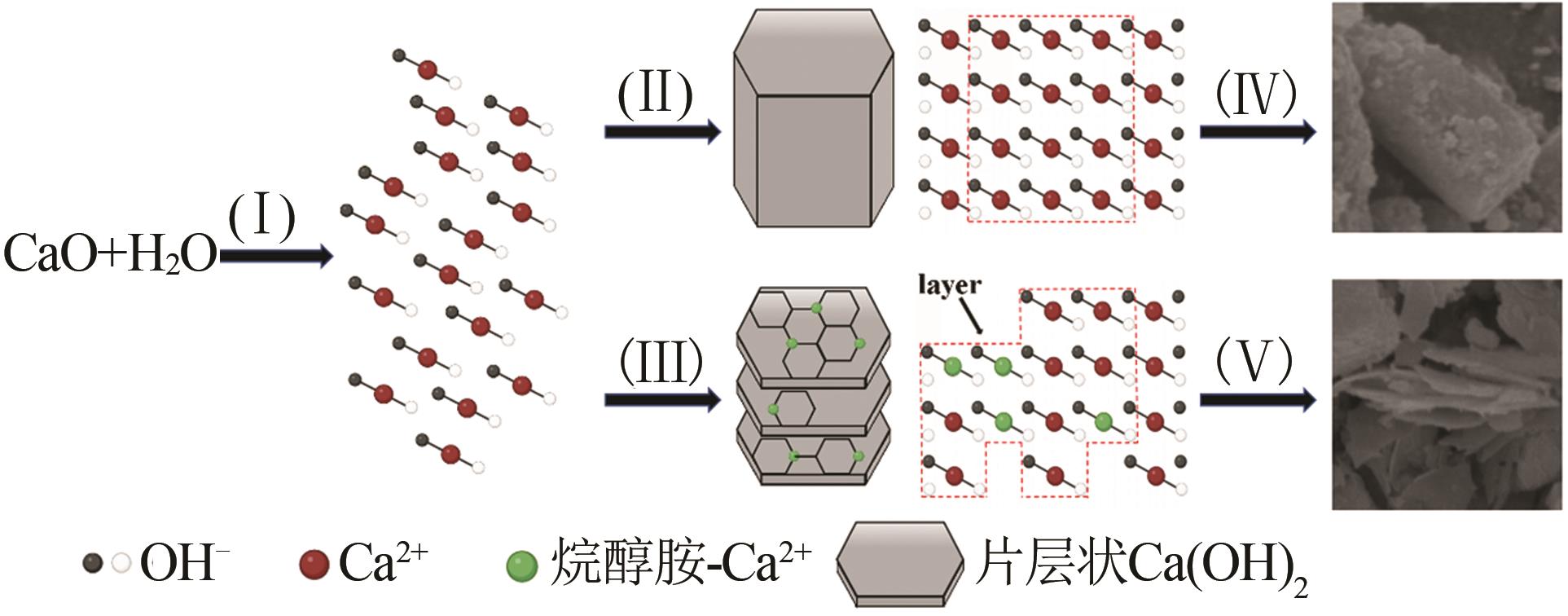
Inorganic Chemicals Industry ›› 2026, Vol. 58 ›› Issue (1): 99-107.doi: 10.19964/j.issn.1006-4990.2024-0687
• Environment·Health·Safety • Previous Articles Next Articles
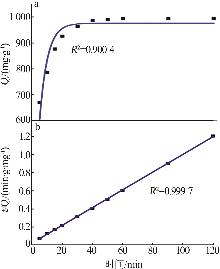
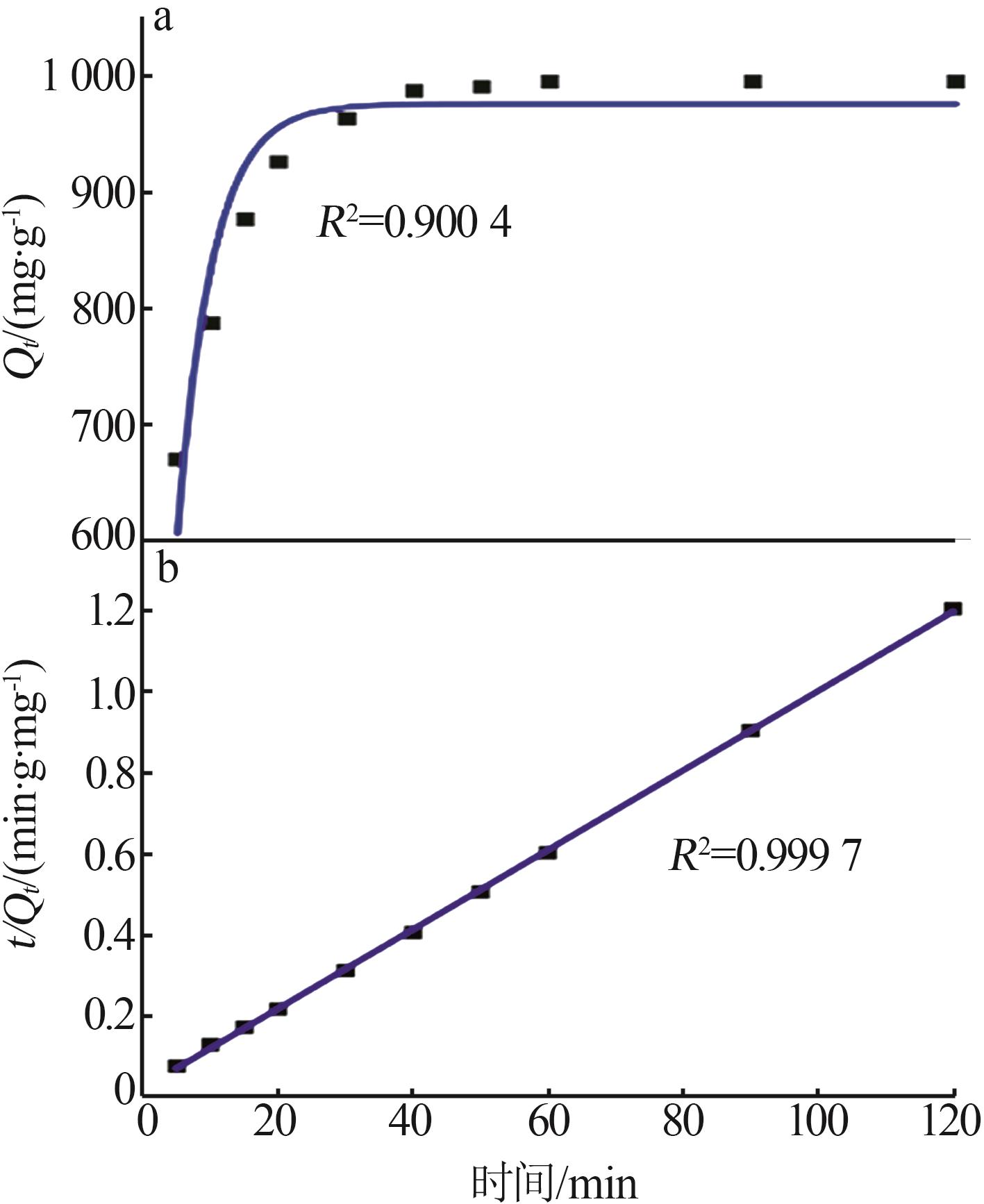
Study on synthesis of high specific surface area calcium hydroxide by quicklime digestion and its adsorption performance for lead
CHEN Jianjun1( ), LI Li2, LIU Laibao1, ZHANG Daiyu3, JIANG Chenxi1, ZHU Hanzhen1, WANG Fu1(
), LI Li2, LIU Laibao1, ZHANG Daiyu3, JIANG Chenxi1, ZHU Hanzhen1, WANG Fu1( ), LIAO Qilong1(
), LIAO Qilong1( )
)
- 1. School of Materials and Chemistry,Southwest University of Science and Technology,Mianyang 621010,China
2. Sichuan Jinding Group Co. ,Ltd. ,Emeishan 614200,China
3. School of Environment and Resources,Southwest University of Science and Technology,Mianyang 621010,China
-
Received:2024-12-19Online:2026-01-10Published:2026-01-20 -
Contact:WANG Fu, LIAO Qilong E-mail:2855631454@qq.com;wangfu@swust.edu.cn;liaoqilong@swust.edu.cn
CLC Number:
Cite this article
CHEN Jianjun, LI Li, LIU Laibao, ZHANG Daiyu, JIANG Chenxi, ZHU Hanzhen, WANG Fu, LIAO Qilong. Study on synthesis of high specific surface area calcium hydroxide by quicklime digestion and its adsorption performance for lead[J]. Inorganic Chemicals Industry, 2026, 58(1): 99-107.
share this article
| [1] | CHARKIEWICZ A E, BACKSTRAND J R.Lead toxicity and pollution in Poland[J].International Journal of Environmental Research and Public Health,2020,17(12):4385. |
| [2] | TANG Xiaodan, LUAN Yukun, ZHAO Yuyan,et al.Tetrasodium iminodisuccinate modified montmorillonite for Pb and Cd adsorption from water:Characterization and mechanism[J].Journal of Environmental Chemical Engineering,2024,12(5):113953. |
| [3] | 余龙,王德贵,刘天鸿,等.功能化氮掺杂空心碳球的制备及其对Pb2+的吸附特性[J].广东石油化工学院学报,2024,34(4):46-52. |
| YU Long, WANG Degui, LIU Tianhong,et al.Preparation of functionalized N⁃doped hollow carbon spheres and their adsorption properties for Pb2+ [J].Journal of Guangdong University of Petrochemical Technology,2024,34(4):46-52. | |
| [4] | 施玲芳,张润花,谢言兰,等.硫改性生物炭镉铅吸附机制及其对油麦菜的影响[J].河南农业科学,2023,52(2):84-93. |
| SHI Lingfang, ZHANG Runhua, XIE Yanlan,et al.Mechanism of cadmium⁃lead adsorption of sulfur⁃modified biochar and its effect on lettuce[J].Journal of Henan Agricultural Sciences,2023,52(2):84-93. | |
| [5] | SUO Chengyu, XU Dandan, YUAN Rongfang,et al.Synchronous removal of Cd(Ⅱ),Pb(Ⅱ),and Cu(Ⅱ) by coagulation in the presence of polymeric ferric sulfate[J].Desalination and Water Treatment,2020,195:421-434. |
| [6] | CHEN Liang, QIU Yunren.Removal of Cd(Ⅱ) from dilute aqueous solutions by complexation-ultrafiltration using rotating disk membrane and the shear stability of PAA-Cd complex[J].Chinese Journal of Chemical Engineering,2019,27(3):519-527. |
| [7] | MARIN N M, STANCULESCU I.Removal of procainamide and lidocaine on Amberlite XAD7HP resin and of As(V),Pb(Ⅱ) and Cd(Ⅱ) on the impregnated resin for water treatment[J].Materials Chemistry and Physics,2022,277:125582. |
| [8] | XU Damao, FU Rongbing, WANG Junxian,et al.Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade:Available stabilizing materials and associated evaluation methods:A critical review[J].Journal of Cleaner Production,2021,321:128730. |
| [9] | GONG Longda, WANG Jingwen, ABBAS T,et al.Immobilization of exchangeable Cd in soil using mixed amendment and its effect on soil microbial communities under paddy upland rotation system[J].Chemosphere,2021,262:127828. |
| [10] | HOU Qinxuan, HAN Dongya, ZHANG Ying,et al.The bioaccessibility and fractionation of arsenic in anoxic soils as a function of stabilization using low⁃cost Fe/Al⁃based materials:A long⁃term experiment[J].Ecotoxicology and Environmental Safety,2020,191:110210. |
| [11] | CHANG Honghong, CUI Longhui, LI Xing,et al.Preparation and characterization of surface modified powder of calcium hydroxide used as chlorine scavenger[J].Journal of Loss Prevention in the Process Industries,2012,25(6):1028-1032. |
| [12] | JALU R G, CHAMADA T A, KASIRAJAN D R.Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead Pb(Ⅱ) from aqueous solution[J].Environmental Challenges,2021,4:100193. |
| [13] | RAHALI S, AISSA M ALI BEN, MODWI A,et al.Application of mesoporous CaO@g-C3N4 nanosorbent materials for high⁃efficiency removal of Pb(Ⅱ) from aqueous solution[J].Journal of Molecular Liquids,2023,379:121594. |
| [14] | KIM S H, CHUNG H, JEONG S,et al.Identification of pH⁃dependent removal mechanisms of lead and arsenic by basic oxygen furnace slag:Relative contribution of precipitation and adsorpti⁃on[J].Journal of Cleaner Production,2021,279:123451. |
| [15] | 闫东杰,赵佳璇,李灶渊,等.石灰消化条件对高比表面积Ca(OH)2性能的影响[J].中国粉体技术,2023,29(4):46- 60. |
| YAN Dongjie, ZHAO Jiaxuan, LI Zaoyuan,et al.Influence of lime digestion conditions on properties of Ca(OH)2 with high specific surface area[J].China Powder Science and Technology,2023,29(4):46-60. | |
| [16] | KUMAR P, SINGHAL R, SHARMA A K,et al.Structural,optical,and morphological study of iron⁃nickel Co⁃doped calcium hydroxide nanoparticles[J].Open Ceramics,2024,18:100600. |
| [17] | ALNATHEER Y, DEVANESAN S, ALGHAMDI O G,et al.Calcium hydroxide nanoparticles synthesized with medicinal plants extract as surface coating for titanium alloy bone implants using a simple method:Characterization and biomechanical evaluati⁃on[J].Biocatalysis and Agricultural Biotechnology,2024,58:103165. |
| [18] | TANG Ruijian, LI Changming, GONG Zijun,et al.The effect of magnesium content and roasting process on the structure and desulfurization activity of slaked lime derived from limestone[J].Journal of Environmental Chemical Engineering,2024,12(4):113195. |
| [19] | 王杰,赵旭波,汤勇,等.生石灰湿法消化制备高比表面积氢氧化钙及结构表征[J].无机盐工业,2024,56(12):104-112. |
| WANG Jie, ZHAO Xubo, TANG Yong,et al.Preparation and structural characterization of high specific surface area calcium hydroxide by wet digestion of quicklime[J].Inorganic Chemicals Industry,2024,56(12):104-112. | |
| [20] | DU Yucheng, MENG Qi, HOU Ruiqin,et al.Fabrication of nano⁃sized Ca(OH)2 with excellent adsorption ability for N2O4 [J].Particuology,2012,10(6):737-743. |
| [21] | WANG Jian, KONG Xiangming, YIN Jianhao,et al.Impacts of two alkanolamines on crystallization and morphology of calcium hydroxide[J].Cement and Concrete Research,2020,138:106250. |
| [22] | CHEN Na, TENG Jie, JIAO Feipeng,et al.Preparation of triethanolamine functionalized carbon nanotube for aqueous removal of Pb(Ⅱ)[J].Desalination and Water Treatment,2017,71:191-200. |
| [23] | CHAUDHARY M, MAITI A.Defluoridation by highly efficient calcium hydroxide nanorods from synthetic and industrial wastewater[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2019,561:79-88. |
| [24] | HARISH H, KUMAR P, KUMARI J,et al.Influence of impurity on the properties of chemically synthesized calcium hydroxide[J].Journal of Nano⁃ and Electronic Physics,2021,13(1):01029. |
| [25] | 刘越,郑强,邢佳斌,等.石灰石干法制备高性能氢氧化钙的工艺及应用研究[J].无机盐工业,2023,55(10):42-49,85. |
| LIU Yue, ZHENG Qiang, XING Jiabin,et al.Study on process and application of high⁃performance calcium hydroxide prepared by dry digestion from limestone[J].Inorganic Chemicals Industry,2023,55(10):42-49,85. | |
| [26] | ZHU Jinmeng, ZHANG Peiyao, DING Jinghan,et al.Nano Ca(OH)2:A review on synthesis,properties and applications[J].Journal of Cultural Heritage,2021,50:25-42. |
| [27] | 熊爽,严金生,周洲,等.生石灰消化反应条件对氢氧化钙特性影响[J].无机盐工业,2023,55(12):50-58. |
| XIONG Shuang, YAN Jinsheng, ZHOU Zhou,et al.Effect of reaction conditions of quicklime digestion on properties of calcium hydroxide[J].Inorganic Chemicals Industry,2023,55(12):50-58. | |
| [28] | ZHENG Entao, FENG Guo, JIANG Feng,et al.Effect of process parameters on the synthesis and lead ions removal performance of novel porous hydroxyapatite sheets prepared via non⁃aqueous precipitation method[J].Ceramics International,2024,50(7):10897-10905. |
| [29] | WU Jiawen, SUN Xiaonan, WU Junting,et al.Eggshell⁃enhanced biochar with in situ formed CaO/Ca(OH)2 for efficient removal of Pb2+ and Cd2+ from wastewater:Performance and mechanistic insights[J].Separation and Purification Technology,2025,354:129352. |
| [30] | YANG Shuangjian, YANG Liyun, GAO Mengdan,et al.Synthesis of zeolite⁃geopolymer composites with high zeolite content for Pb(Ⅱ) removal by a simple two⁃step method using fly ash and metakaolin[J].Journal of Cleaner Production,2022,378:134528. |
| [1] | LIU Hongzhen, ZHENG Zhuochao, LI Jun. Study on purification of glauberite gypsum by crystalline phase transition method [J]. Inorganic Chemicals Industry, 2025, 57(12): 77-82. |
| [2] | TANG Ziling, LIANG Meina, TANG Hongjie, PENG Yuxuan, JIANG Zhuocheng, WANG Dunqiu, LI Juhao. Study on preparation and morphology of nano-calcium carbonate based on snail shell biomass [J]. Inorganic Chemicals Industry, 2025, 57(12): 83-91. |
| [3] | LI Zhixin, XU Kaidong, CHANG Shizhuo, WANG Jina, XUE Kaiwang, WU Mengyu, YANG Yilong, LIU Bingqian, YE Zhicheng, ZHU Longlong, HE Jiale. Effect of activators on properties of carbide slag-steel slag composite cementitious materials [J]. Inorganic Chemicals Industry, 2025, 57(12): 107-113. |
| [4] | WU Tuoxiu, DENG Rongdong, WU Siyuan, ZHU Qinqin, ZHAO Ruiqi. Effect of sulfuric acid addition sequence on flotation separation of calcite and apatite [J]. Inorganic Chemicals Industry, 2025, 57(12): 41-47. |
| [5] | YIN Yujiao, WU Fei, ZHOU Wenqiang, HE Dianwei. Preparation of C-S-H/PCE nanocomposites and its effect on early hydration of cement [J]. Inorganic Chemicals Industry, 2025, 57(9): 66-72. |
| [6] | YAN Xin, LIU Hailu, LIU Baolin, LIU Yi, LIU Yanyang. Research on key technologies and mechanisms of green nano calcium carbonate production [J]. Inorganic Chemicals Industry, 2025, 57(1): 71-76. |
| [7] | ZHANG Jianle, CAO Yapeng, ZU Minghua, ZHANG Zhikun, LIU Yumin, HAN Jilong. Study on morphology of calcium carbonate controlled by sodium dodecyl sulfate and amino acid double template [J]. Inorganic Chemicals Industry, 2025, 57(1): 58-63. |
| [8] | WANG Jie, ZHAO Xubo, TANG Yong, QIN Lingyi, CHEN Xiaopeng, LIAO Dankui, TONG Zhangfa, WANG Linlin. Preparation and structural characterization of high specific surface area calcium hydroxide by wet digestion of quicklime [J]. Inorganic Chemicals Industry, 2024, 56(12): 104-112. |
| [9] | LUO Ya, ZHOU Rong, LÜ Li, YANG Jie, TANG Shengwei, ZHANG Tao. Study on crystallization and filtration properties of calcium sulfate for treating industrial waste sulfuric acid with calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(12): 127-133. |
| [10] | YANG Yuqing, LUO Aiwen, WANG Jie, WU Chenjie, QU Jun, XU Zhigao, DU Dongyun, CAO Yanmin. Study on solidification of soluble phosphorus in phosphogypsum by Ca/Al/La LDH [J]. Inorganic Chemicals Industry, 2024, 56(12): 134-141. |
| [11] | LI Xuelian, TANG Zihan, XU Jie, LI Xiong. Study on performance of calcium sulfate whisker/SBS composite modified asphalt [J]. Inorganic Chemicals Industry, 2024, 56(9): 82-89. |
| [12] | LI Yuxing, ZHANG Jincai, CHENG Fangqin. Research progress of preparation and growth mechanism of various crystalline nano-calcium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(5): 1-10. |
| [13] | CHEN Feng, FENG Kang, LI Ming, SHEN Haojie, TIAN Chengtao, TANG Yuan, LI Zhili, HE Dongsheng. Application of organically modified calcium sulfate whiskers in asphalt modification [J]. Inorganic Chemicals Industry, 2024, 56(3): 125-130. |
| [14] | XIONG Shuang, YAN Jinsheng, ZHOU Zhou, ZHOU Baodi, CHEN Xiaopeng, TONG Zhangfa. Effect of reaction conditions of quicklime digestion on properties of calcium hydroxide [J]. Inorganic Chemicals Industry, 2023, 55(12): 50-58. |
| [15] | GUO Ze, ZHANG Pengfei, YANG Fan, ZHANG Hanquan, LU Manman. Preparation of β-hemihydrate gypsum by phosphogypsum roasting [J]. Inorganic Chemicals Industry, 2023, 55(10): 106-113. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||