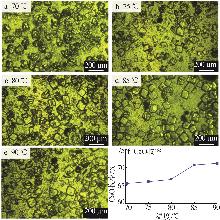
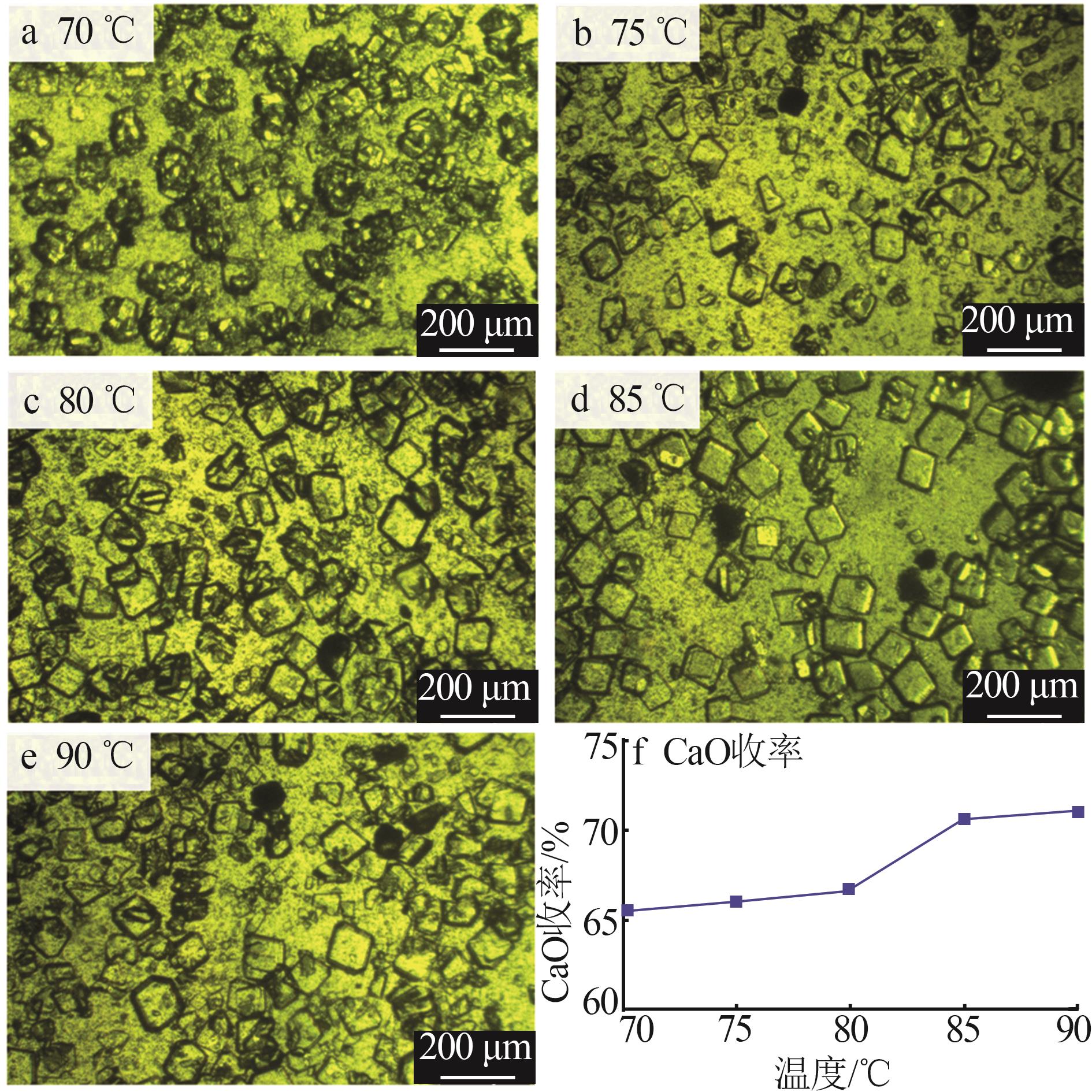
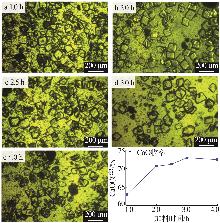
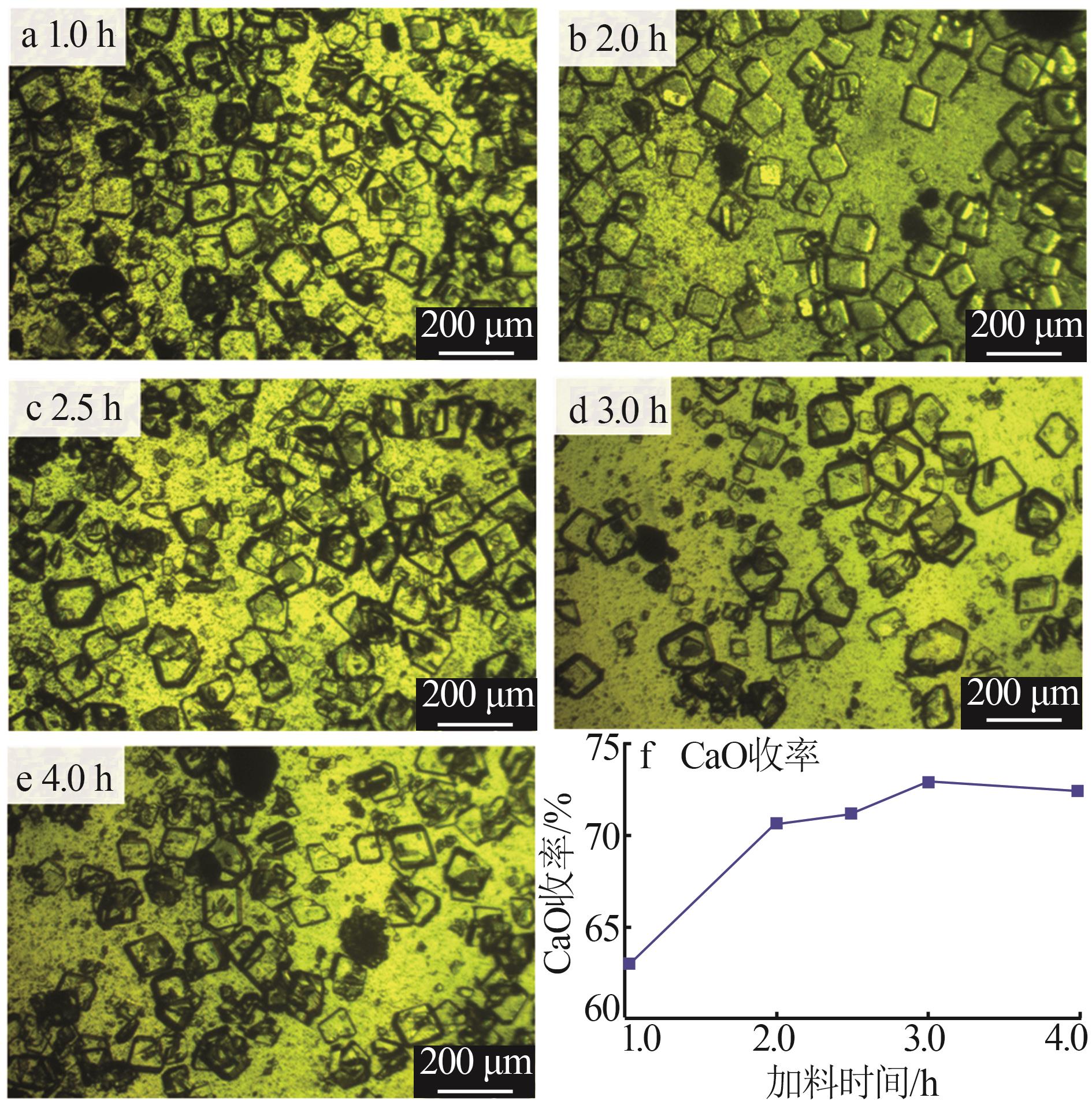
| [1] |
申军.我国芒硝矿产资源及其加工业的现状与发展[J].化工矿物与加工,2003,32(2):1-4.
|
|
SHEN Jun.Status of mirabilite resources and prospect of its processing industry[J].Industrial Minerals and Porocessing,2003,32(2):1-4.
|
| [2] |
陈敏.青海省芒硝资源开发利用现状及前景[J].无机盐工业,2010,42(9):4-5,22.
|
|
CHEN Min.Present status and prospect of development and utilization of mirabilite resources in Qinghai[J].Inorganic Chemicals Industry,2010,42(9):4-5,22.
|
| [3] |
李代荣.中国芒硝矿特征与成因简介[J].矿产勘查,2020,11(3):511-516.
|
|
LI Dairong.Introduction to characteristics and genesis of mirabilite deposits in China[J].Mineral Exploration,2020,11(3):511-516.
|
| [4] |
赵宏旭,刘磊.硝盐联产技术在元明粉生产中的应用[J].化工设计通讯,2020,46(9):52-53.
|
|
ZHAO Hongxu, LIU Lei.Application of nitrate combined production technology in the production process of yuanmingfen[J].Che-mical Engineering Design Communications,2020,46(9):52-53.
|
| [5] |
戎宽伟,徐素国.钙芒硝矿溶浸开采技术进展与展望[J].矿业研究与开发,2014,34(3):1-3,81.
|
|
RONG Kuanwei, XU Suguo.Progress and prospects for solution mining of glauberite[J].Mining Research and Development,2014,34(3):1-3,81.
|
| [6] |
张秀峰,谭秀民,张利珍.钙芒硝矿石的水浸工艺研究[J].矿产保护与利用,2017,37(4):69-72.
|
|
ZHANG Xiufeng, TAN Xiumin, ZHANG Lizhen.Research on water leaching process of glauberite ore[J].Conservation and Utilization of Mineral Resources,2017,37(4):69-72.
|
| [7] |
杨大涌,周堃.钙芒硝尾矿的综合利用[J].化工矿物与加工,2020,49(12):53-56.
|
|
YANG Dayong, ZHOU Kun.Comprehensive utilization of glauberite tailings[J].Industrial Minerals & Processing,2020,49(12):53-56.
|
| [8] |
JIANG Zheyuan, SUN Xinpo, LUO Yaqiong,et al.Recycling,reusing and environmental safety of industrial by-product gypsum in construction and building materials[J].Construction and Building Materials,2024,432:136609.
|
| [9] |
刘林程,左海滨,许志强.工业石膏的资源化利用途径与展望[J].无机盐工业,2021,53(10):1-9.
|
|
LIU Lincheng, ZUO Haibin, XU Zhiqiang.Resource utilization approach of industrial gypsum and its prospect[J].Inorganic Chemicals Industry,2021,53(10):1-9.
|
| [10] |
彭诗谷,李伟,石晴,等.一种高效的芒硝石膏浮选提纯工艺:中国,113289768A[P].2021-08-24.
|
| [11] |
谭明洋,黄平,张营,等.旋流器在磷石膏预处理中的应用研究[J].磷肥与复肥,2018,33(11):42-43.
|
|
TAN Mingyang, HUANG Ping, ZHANG Ying,et al.Application research of hydrocyclone in pretreatment of phosphogypsum[J].Phosphate & Compound Fertilizer,2018,33(11):42-43.
|
| [12] |
赵红涛,包炜军,孙振华,等.磷石膏中杂质深度脱除技术[J].化工进展,2017,36(4):1240-1246.
|
|
ZHAO Hongtao, BAO Weijun, SUN Zhenhua,et al.Deep removal of impurities from phosphogypsum[J].Chemical Industry and Engineering Progress,2017,36(4):1240-1246.
|
| [13] |
尹会斌,李军,郑卓超.钙芒硝石膏深度提纯工艺的研究[J].无机盐工业,2022,54(11):104-111.
|
|
YIN Huibin, LI Jun, ZHENG Zhuochao.Research on deep purification process of glauberite gypsum[J].Inorganic Chemicals Industry,2022,54(11):104-111.
|
| [14] |
曹涌钢,张子龙,李泽皓,等.工业副产石膏制备α型半水石膏的研究进展[J].化工进展,2025,44(3):1505-1519.
|
|
CAO Yonggang, ZHANG Zilong, LI Zehao,et al.Research progress on preparation of α-hemihydrate gypsum from industrial by-product gypsum[J].Chemical Industry and Engineering Progress,2025,44(3):1505-1519.
|
| [15] |
秦颖,刘新庄,桑海风.工业副产石膏在建材领域的资源化利用现状及展望[J].中国建材科技,2023,32(5):23-25.
|
|
QIN Ying, LIU Xinzhuang, SANG Haifeng.Current situation and prospect of resource utilization of industrial by-product gypsum in the field of building materials[J].China Building Materials Science & Technology,2023,32(5):23-25.
|
| [16] |
POPOVIĆ D, STUPAR G, MILADINOVIĆ J,et al.Solubility in the ternary system CaSO4+Na2SO4+H2O at 298.15 K[J].Russian Journal of Physical Chemistry A,2011,85(13):2349-2353.
|
 ), ZHENG Zhuochao, LI Jun(
), ZHENG Zhuochao, LI Jun( )
)