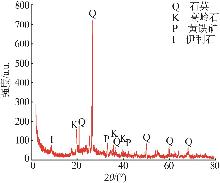
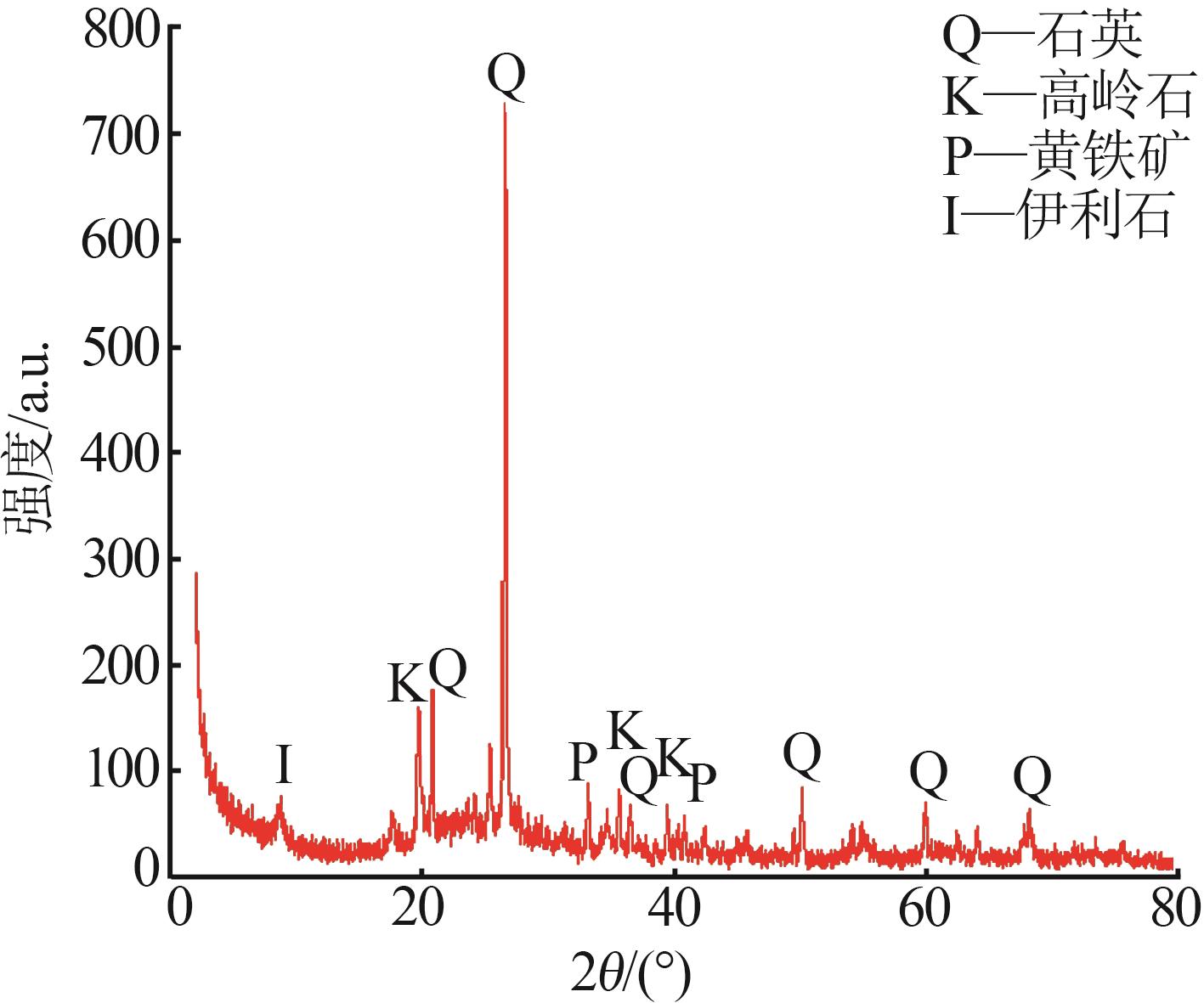
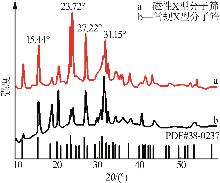
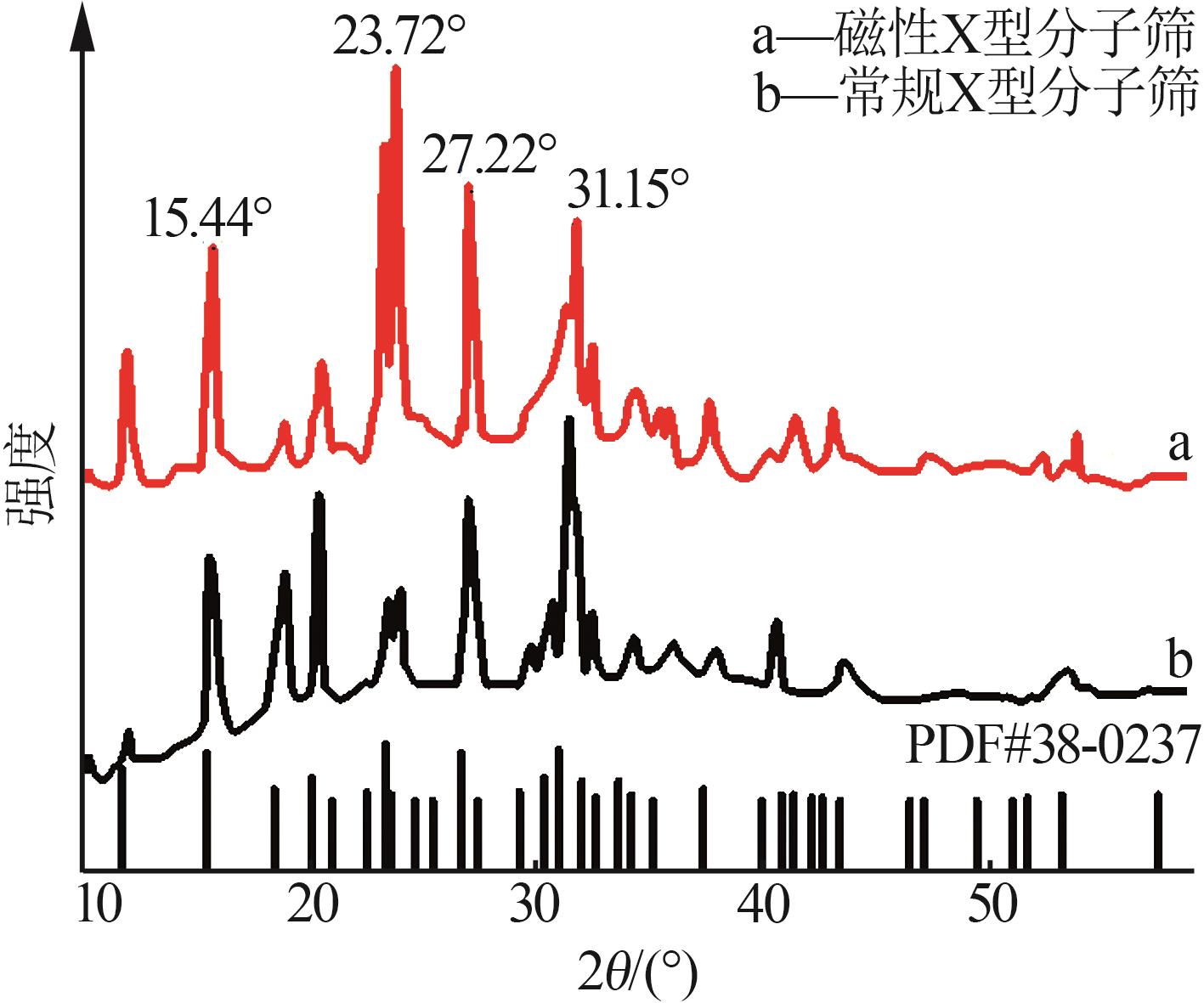
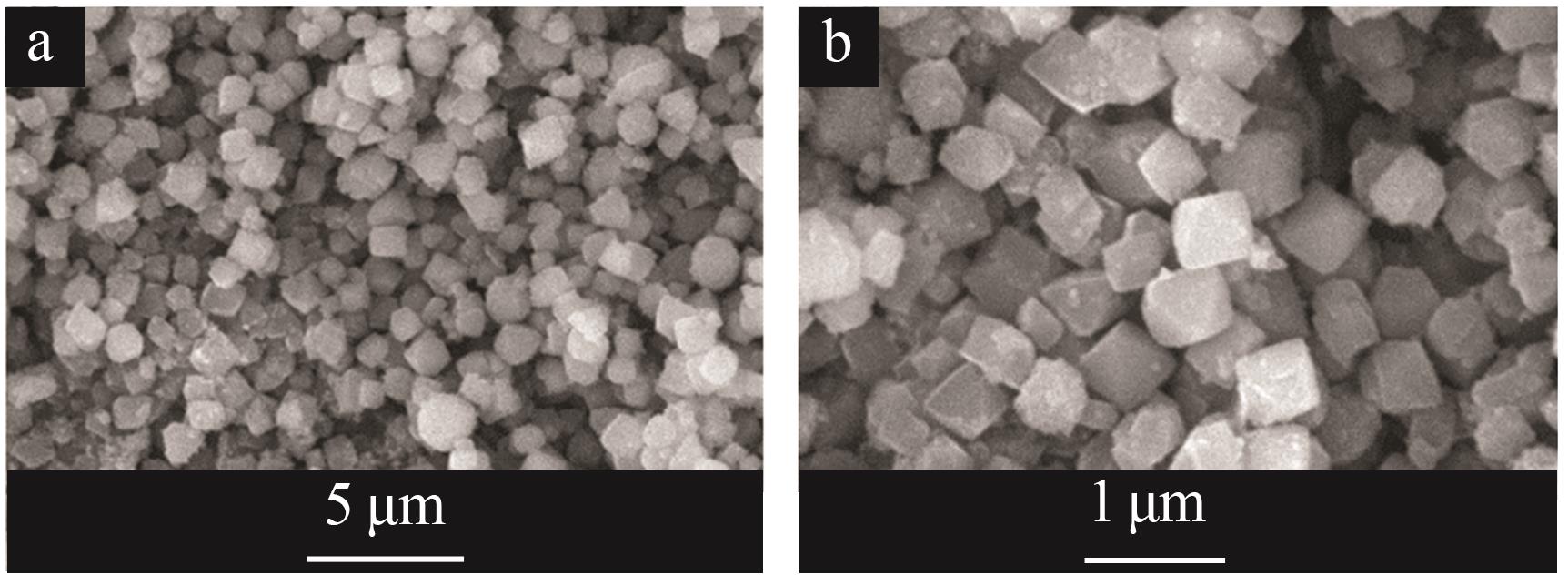
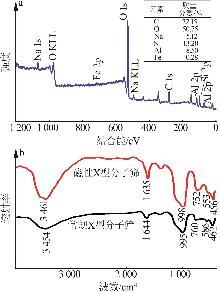
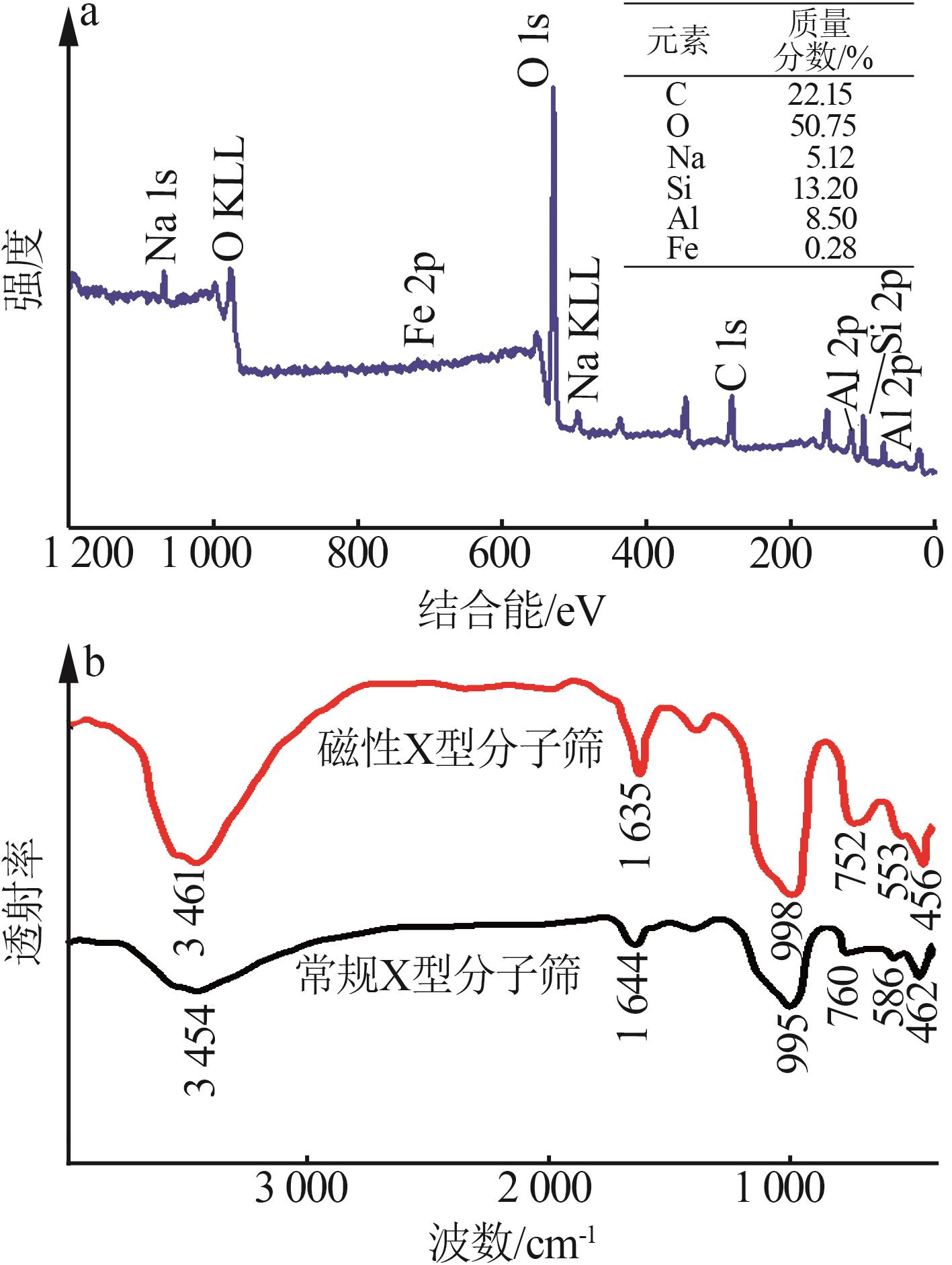
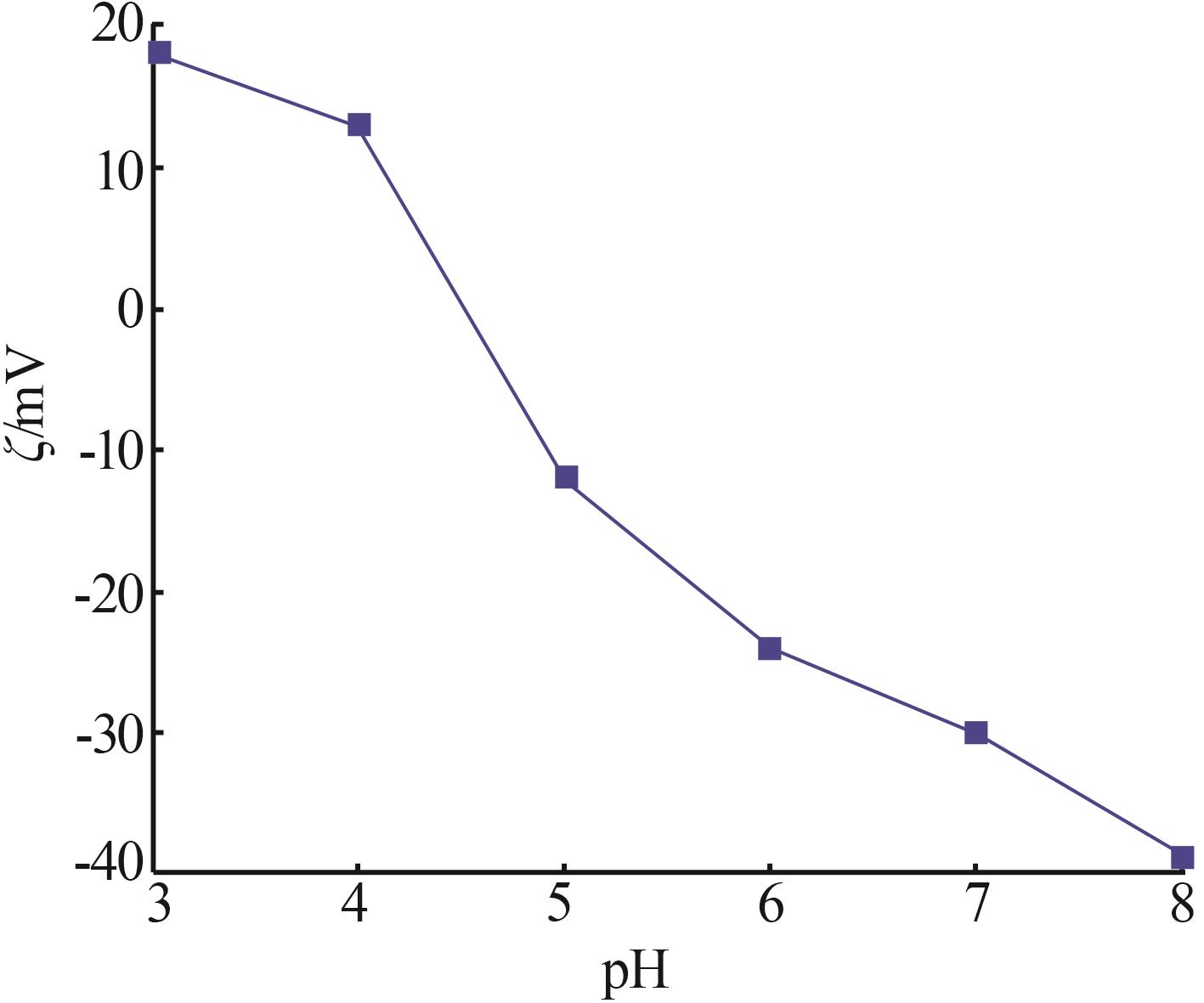
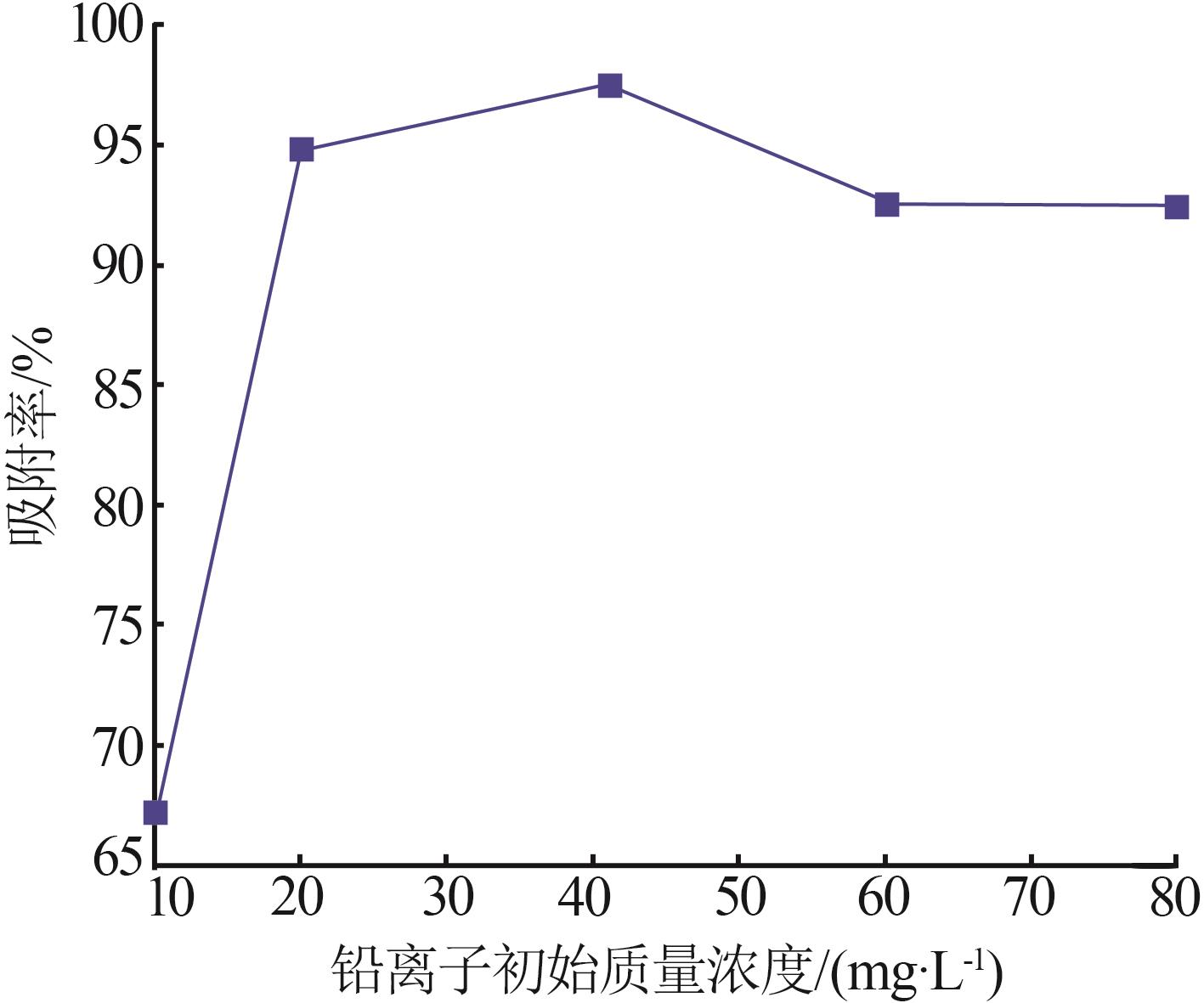
Inorganic Chemicals Industry ›› 2026, Vol. 58 ›› Issue (1): 92-98.doi: 10.19964/j.issn.1006-4990.2025-0042
• Environment·Health·Safety • Previous Articles Next Articles
Study on preparation of magnetic X zeolite from coal gangue and its adsorption properties of Pb2+
PENG Huanling( ), CUI Wei, LEI Hongmei
), CUI Wei, LEI Hongmei
- Xi′an Kedagaoxin University,Xi′an 710109,China
-
Received:2025-01-20Online:2026-01-10Published:2025-09-25
CLC Number:
Cite this article
PENG Huanling, CUI Wei, LEI Hongmei. Study on preparation of magnetic X zeolite from coal gangue and its adsorption properties of Pb2+[J]. Inorganic Chemicals Industry, 2026, 58(1): 92-98.
share this article
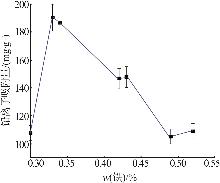
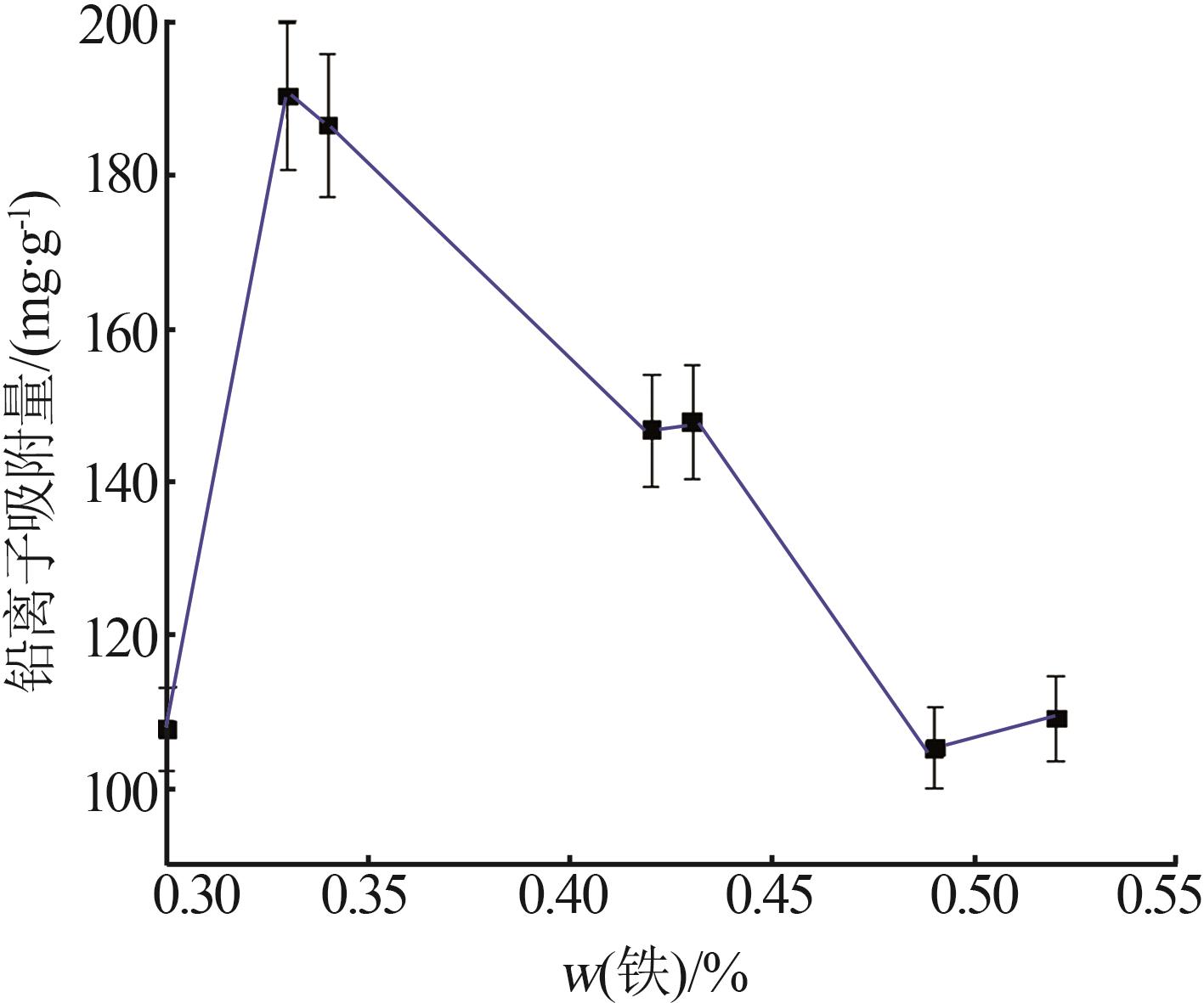
Table 3
Experimental results and analysis of orthogonal test"
| 样品号 | 因素 | 吸附量/ (mg·g-1) | ||
|---|---|---|---|---|
A 晶化时间/h | B 晶化温度/℃ | C n(H2C2O4)/ n(Fe3+) | ||
| 1 | 6 | 75 | 4 | 107.75 |
| 2 | 6 | 85 | 5 | 105.26 |
| 3 | 6 | 95 | 6 | 109.11 |
| 4 | 8 | 75 | 5 | 146.81 |
| 5 | 8 | 85 | 6 | 147.80 |
| 6 | 8 | 95 | 4 | 146.71 |
| 7 | 10 | 75 | 6 | 184.22 |
| 8 | 10 | 85 | 4 | 186.47 |
| 9 | 10 | 95 | 5 | 190.24 |
| K1 | 322.11 | 438.78 | 440.93 | |
| K2 | 441.32 | 544.79 | 442.31 | |
| K3 | 560.93 | 446.06 | 441.13 | |
| 极差R | 238.82 | 106.01 | 1.38 | |
| 主次顺序 | A>B>C | |||
| 最优水平 | A3 | B2 | C2 | |
| 最优组合 | A3B2C2 | |||
| [1] | 田宇红,杨荣添,张李超,等.煤矸石复合吸附材料制备及脱硫性能[J].化学工业与工程,2024,41(6):120-127. |
| TIAN Yuhong, YANG Rongtian, ZHANG Lichao,et al.Preparation and desulfurization performance of coal gangue composite adsorption material[J].Chemical Industry and Engineering,2024,41(6):120-127. | |
| [2] | 张伟,李宇,段向杰,等.烧结气氛对煤矸石陶粒烧结过程的影响[J].冶金能源,2024,43(6):25-31. |
| ZHANG Wei, LI Yu, DUAN Xiangjie,et al.Effect of sintering atmosphere on sintering process of coal gangue ceramsite[J].Energy for Metallurgical Industry,2024,43(6):25-31. | |
| [3] | 张晓婉,李巧玲.混合塑料与改性煤矸石模板制备多孔碳材料及其吸附性能的研究[J].现代化工,2020,40(5):194-198. |
| ZHANG Xiaowan, LI Qiaoling.Preparation of porous carbon material from mixed plastics/modified gangue template and study on its adsorptive properties[J].Modern Chemical Industry,2020,40(5):194-198. | |
| [4] | 张福旺.煤矸石环境污染及资源化再利用研究[J].资源节约与环保,2020(6):124-125. |
| ZHANG Fuwang.Study on environmental pollution and resource reuse of coal gangue[J].Resources Economization & Environmental Protection,2020(6):124-125. | |
| [5] | 胡恬,张琨.陕西澄合矿区煤矸石绿化基质研究[J].西安科技大学学报,2022,42(4):709-715. |
| HU Tian, ZHANG Kun.Research on greening substrates of coal gangue in chenghe mining area of shaanxi province[J].Journal of Xi′an University of Science and Technology,2022,42(4):709- 715. | |
| [6] | 方晨阳.高铝煤矸石制备NaA型分子筛的机理及工艺研究[D].呼和浩特:内蒙古工业大学,2024. |
| FANG Chenyang.Study on the mechanism and process of preparing NaA molecular sieve from high alumina coal gangue[D].Hohhot:Inner Mongolia University of Technology,2024. | |
| [7] | 柳黄飞,张莉,刘涛.分子筛快速合成技术研究进展[J].无机盐工业,2025,57(2):36-43. |
| LIU Huangfei, ZHANG Li, LIU Tao.Research progress of fast synthesis technologies of zeolites[J].Inorganic Chemicals Industry,2025,57(2):36-43. | |
| [8] | 詹新妮.稻壳制备活性炭联产X型分子筛的研究[D].长春:吉林大学,2023. |
| ZHAN Xinni.Study on preparation of activated carbon from rice husk and co⁃production of zeolite X[D].Changchun:Jilin University,2023. | |
| [9] | 刘红,徐积昀,殷萌,等.13X分子筛/凹凸棒土颗粒型复合材料吸附Zn2+的研究[J].武汉科技大学学报,2019,42(6):442-448. |
| LIU Hong, XU Jiyun, YIN Meng,et al.Adsorption of Zn2+ by 13X molecular sieve/attapulgite granular composite[J].Journal of Wuhan University of Science and Technology,2019,42(6):442-448. | |
| [10] | GAO Jida, LIN Qianji, YANG Tingzhi,et al.Preparation and characterization of ZSM-5 molecular sieve using coal gangue as a raw material via solvent⁃free method:Adsorption performance tests for heavy metal ions and methylene blue[J].Chemosphere,2023,341:139741. |
| [11] | YONG Xiaojing, SU Hui, ZHAO Nana,et al.Xylene and n-hexane adsorption performance of a waste methanol⁃to⁃propylene catalyst under acid⁃base treatment[J].Catalysts,2022,12(9):1028. |
| [12] | LEE Weihao, LIN Yawen, LIN K L.Optimization of synthesis parameters for the preparation of zeolite by reusing industrialwaste as raw material:Sandblasting waste and solar panel waste glass[J].Solid State Sciences,2023,143:107277. |
| [13] | HEERIBOUT L, SEMMER V, BATAMACK P,et al.Brønsted acid strength of solids studied by 1H NMR:Establishing the scale;influence of Lewis acid sites[J].Studies in Surface Science and Catalysis,1996,101:831-840. |
| [14] | BORDIGA S, GROPPO E, AGOSTINI G,et al.Reactivity of surface species in heterogeneous catalysts probed by in situ X⁃ray absorption techniques[J].Chemical Reviews,2013,113(3):1736-1850. |
| [15] | 国家市场监督管理总局,中国国家标准化管理委员会. 分子筛静态水吸附测定方法: [S].北京:中国标准出版社,2021. |
| [16] | 杨建利,杨小刚,李刚,等.超声法制备磁性分子筛及其性能研究[J].应用化工,2019,48(5):1099-1102. |
| YANG Jianli, YANG Xiaogang, LI Gang,et al.Preparation of magnetic molecular sieve by ultrasonic method and its properti⁃es[J].Applied Chemical Industry,2019,48(5):1099-1102. | |
| [17] | 刘红,张靖,刘冰雪,等.Y型分子筛嵌载纳米零价铁对水中Pb2+的去除机理研究[J].武汉科技大学学报,2022,45(2):120-126. |
| LIU Hong, ZHANG Jing, LIU Bingxue,et al.Removal mechanism of Pb2+ from aqueous solution by Y zeolite⁃embedded nanoscale zero⁃valent iron[J].Journal of Wuhan University of Science and Technology,2022,45(2):120-126. | |
| [18] | YIN Zhenzhou, WANG Gang, WANG Lu,et al.An effective way to utilize solid waste resources:The application of modified fly ash in removing Cu2+ and Pb2+ in wastewater[J].Separation and Purification Technology,2024,350:127948. |
| [19] | RAJ A F P A M, KRAJNC S, BAUMAN M,et al.Removal of Pb2+,Cr3+ and Hg2+ ions from aqueous solutions using SiO2 and amino⁃functionalized SiO2 particles[J].Journal of Sol-Gel Science and Technology,2022,103(1):290-308. |
| [20] | CUI Hongbiao, YU Wenli, LI Shuai,et al.Alkali⁃modified biomass ash enhances the adsorption capacities of Cu2+,Cd2+,and Pb2+ and their immobilization in soil[J].Journal of Environmental Chemical Engineering,2024,12(5):113490. |
| [1] | HAN Yingming, SUN Ruize, SUN Yining, SONG Xingfei. Study on properties of modified magnesia sulfide cement by calcined coal gangue powder [J]. Inorganic Chemicals Industry, 2025, 57(4): 97-104. |
| [2] | MAO Shize. Study on microwave roasting-activated coal gangue to improve sulfate corrosion resistance of concrete [J]. Inorganic Chemicals Industry, 2025, 57(3): 86-93. |
| [3] | HAN Wei, SONG Yongming, LIU Qi, XU Jinling, XU Rong, LI Chunquan, YIN Shuaijun, SUN Zhiming. Study on performance of calcium chloride assisted thermal activation of coal gangue and its peroxymonosulfate activation toward benzo(a)pyrene degradation [J]. Inorganic Chemicals Industry, 2025, 57(1): 103-112. |
| [4] | CHAI Chunjing, FENG Zhengjun, WU Haibin, SHI Xiaokai, ZHANG Junjie, SONG Huiping. Effect of coal gangue-based porous matrix on soil solute transport [J]. Inorganic Chemicals Industry, 2024, 56(9): 107-116. |
| [5] | LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan. Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash [J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104. |
| [6] | LIU Yichang, XIE Zhipeng, LIU Yunfeng, ZHANG Da, LIANG Feng. Study on impedance matching strategy of enhancing microwave absorption performance of nitrogen-doped single-walled carbon nanohorns [J]. Inorganic Chemicals Industry, 2024, 56(12): 29-34. |
| [7] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [8] | FU Minglian, CEN Jianmei, CHEN Zhangxu. Study on preparation of magnetic SiO2/chitosan composite aerogel and its adsorption for Cu2+ [J]. Inorganic Chemicals Industry, 2023, 55(6): 70-77. |
| [9] | WANG Lijuan, YAN Kezhou, GUO Zhiqiang, ZHAO Zhonghe, GUO Yanxia, CHENG Fangqin. Preparation of poly-aluminum chloride from acid leaching liquor of red mud-coal gangue activated by sodium salt [J]. Inorganic Chemicals Industry, 2023, 55(4): 76-83. |
| [10] | ZHANG Chenhu, MA Yi, ZHU Shan, CHEN Peng, WANG Chengyong, LI Ziwen. Study on adsorption of heavy metal ions in mineral processing wastewater by chelating modified coal gangue [J]. Inorganic Chemicals Industry, 2023, 55(4): 97-103. |
| [11] | MAO Xinyu,WANG Yubin,WANG Wenwen,LI Shuqin,MA Xiaoxiao. Effect of alternating external magnetic field on the dissolution behavior of calcium sulfate scale and its mechanism [J]. Inorganic Chemicals Industry, 2022, 54(3): 97-101. |
| [12] | LI Xiaoqi,SUN Yongjun,CHEN Aowen,YU Yuanyuan,SUN Wenquan,ZHOU Jun. Study on preparation of magnetic heavy metal capture flocculant [J]. Inorganic Chemicals Industry, 2022, 54(2): 81-84. |
| [13] | WANG Xiaohuan,LI Shenghao,SHI Zhiming,WANG Jun,XINBA Yaer,LIU Liang. Research status of FeTiO3 materials [J]. Inorganic Chemicals Industry, 2022, 54(1): 12-17. |
| [14] | Wang Lixia,Ren Dongmei,Wang Qiong,Chang Chun. Stduy on preparation of magnetic metal-organic frame materials and their adsorption properties [J]. Inorganic Chemicals Industry, 2021, 53(9): 46-50. |
| [15] | Wang Yongqin,Huang Yangze,Fu Yu. Research progress of doped metal oxide semiconductor materials [J]. Inorganic Chemicals Industry, 2021, 53(4): 1-7. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||