Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (11): 100-107.doi: 10.19964/j.issn.1006-4990.2024-0449
• Environment·Health·Safety • Previous Articles Next Articles
Study on crystal structure control of aragonite prepared by CO2 mineralization from carbide slag
CHANG Jingang1( ), GE Yan2, KOU Junjie1, WANG Jiamian2, YANG Zhiyong1, CAI Lihong2,3(
), GE Yan2, KOU Junjie1, WANG Jiamian2, YANG Zhiyong1, CAI Lihong2,3( )
)
- 1. GD Power Datong Power Generation Co. ,Ltd. ,Datong 037001,China
2. Yuanchu Technology(Beijing)Co. ,Ltd. ,Beijing 100020,China
3. Tsinghua University,Beijing 100084,China
-
Received:2024-08-14Online:2025-11-10Published:2025-07-25 -
Contact:CAI Lihong E-mail:12068421@ceic.com;caili-hong2005@sina.com
CLC Number:
Cite this article
CHANG Jingang, GE Yan, KOU Junjie, WANG Jiamian, YANG Zhiyong, CAI Lihong. Study on crystal structure control of aragonite prepared by CO2 mineralization from carbide slag[J]. Inorganic Chemicals Industry, 2025, 57(11): 100-107.
share this article
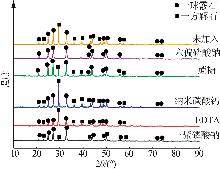
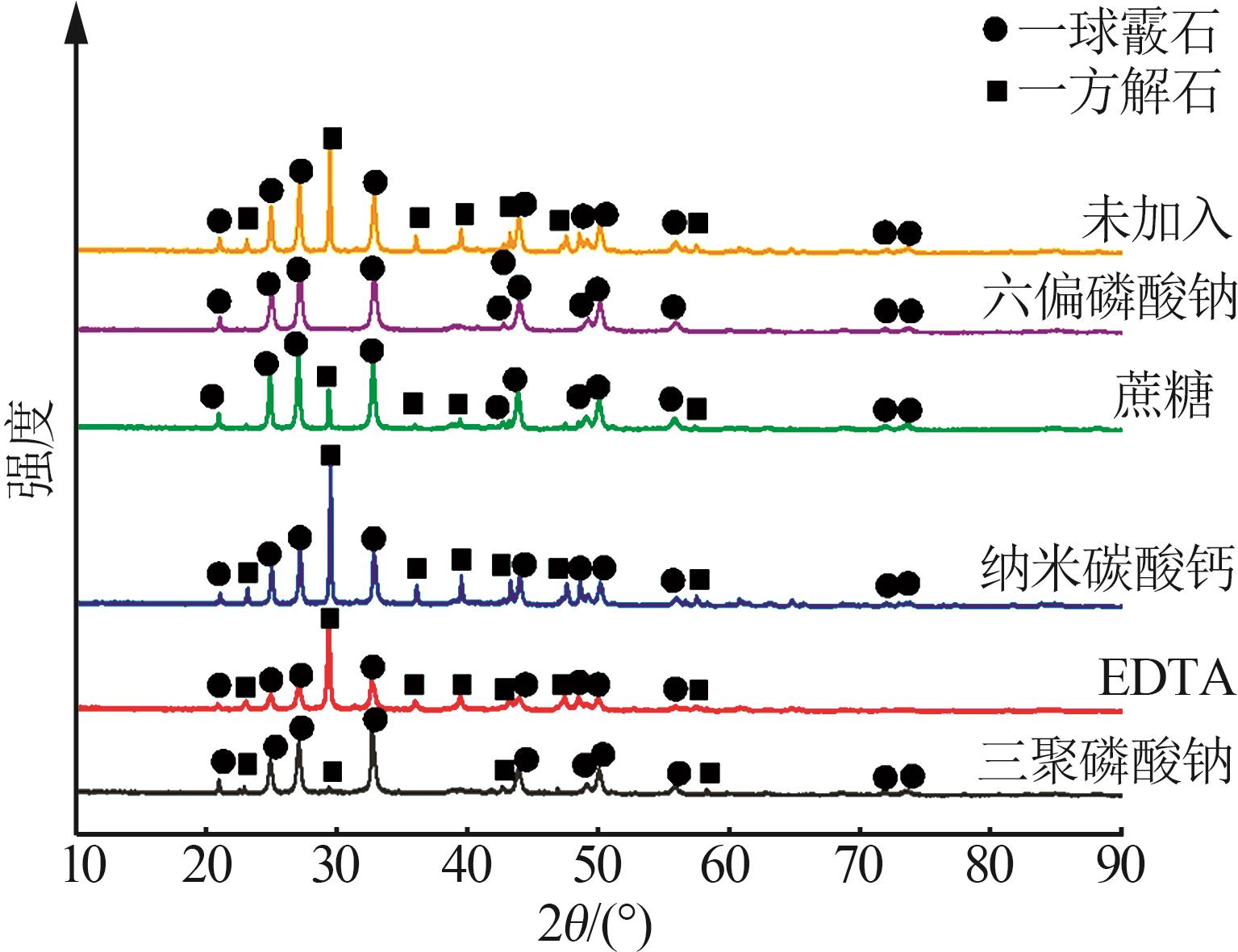
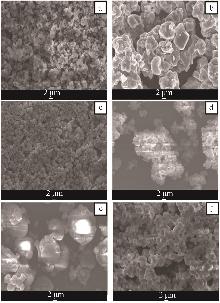
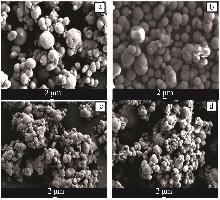
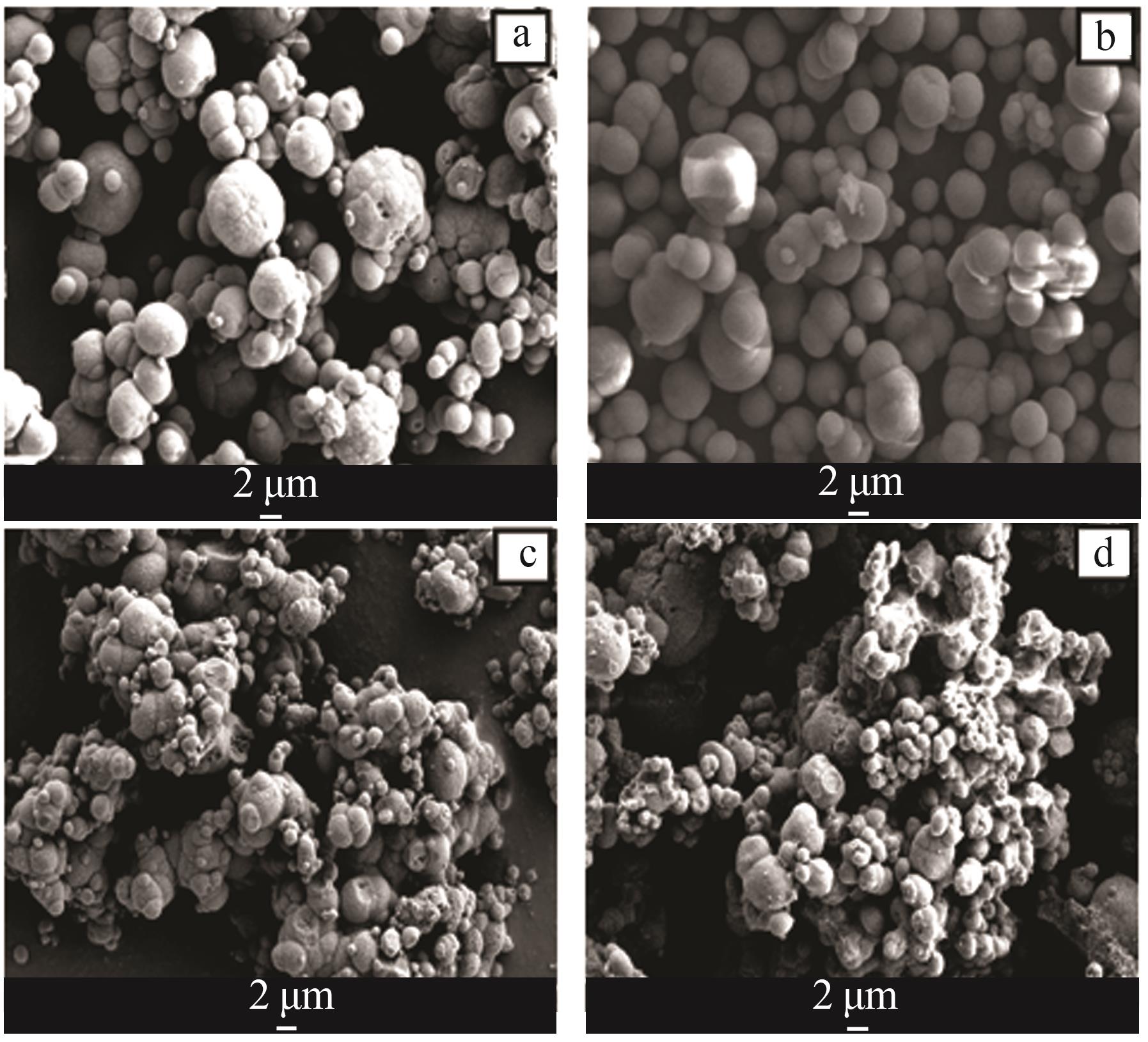
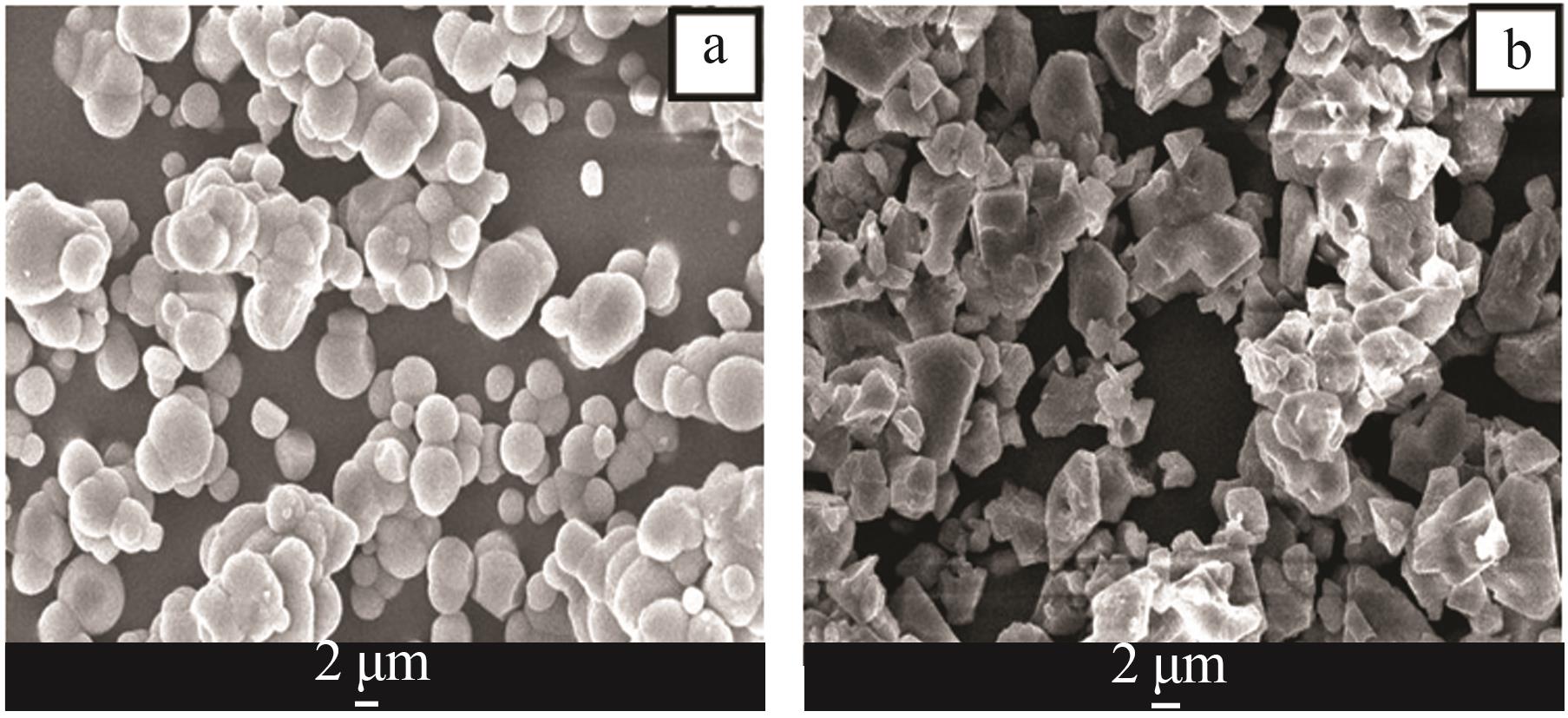
Table 2
Analysis table of whiteness and particle size of calcium carbonate obtained by adding different crystal form control agents"
| 序号 | 晶型 控制剂 | 产品白度 | 粒径D10/μm | 粒径D50 /μm | 粒径D90 /μm | 粒径D97 /μm |
|---|---|---|---|---|---|---|
| a | 未添加 | 97.52 | 2.28 | 14.39 | 27.10 | 33.30 |
| b | (NaPO3)6 | 98.78 | 1.35 | 5.99 | 15.09 | 21.48 |
| c | 蔗糖 | 98.51 | 2.36 | 9.39 | 18.32 | 23.47 |
| d | 纳米碳酸钙 | 98.73 | 1.20 | 6.12 | 16.92 | 22.26 |
| e | EDTA | 97.79 | 3.97 | 17.01 | 30.08 | 37.05 |
| f | Na5P3O10 | 97.68 | 2.03 | 10.73 | 23.31 | 30.42 |
| [1] | 高红.电石渣循环利用 助力绿色低碳发展[J].中国经贸导刊,2021(22):51-52. |
| GAO Hong.Recycling of carbide slag helps green and low-carbon development[J].China Economic & Trade Herald,2021(22):51-52. | |
| [2] | 赵立文,朱干宇,李少鹏,等.电石渣特性及综合利用研究进展[J].洁净煤技术,2021,27(3):13-26. |
| ZHAO Liwen, ZHU Ganyu, LI Shaopeng,et al.Research progress on characteristics and comprehensive utilization of calcium carbide slag[J].Clean Coal Technology,2021,27(3):13-26. | |
| [3] | ZHAO Yuyang, ZHAN Jiayu, LIU Guorui,et al.Evaluation of dioxins and dioxin-like compounds from a cement plant using carbide slag from chlor-alkali industry as the major raw material[J].Journal of Hazardous Materials,2017,330:135-141. |
| [4] | 范可心,郝淑贤,段趁飞,等.探究晶型控制剂对纳米碳酸钙形貌影响的研究进展[J].煤炭与化工,2023,46(2):143-146, 160. |
| FAN Kexin, HAO Shuxian, DUAN Chenfei,et al.The effect of crystal control agent on the morphology of calcium carbonate was investigated[J].Coal and Chemical Industry,2023,46(2):143-146,160. | |
| [5] | 王鑫,韦明,刘琨.球霰石型碳酸钙的调控制备研究进展[J].硅酸盐通报,2022,41(8):2860-2870,2878. |
| WANG Xin, WEI Ming, LIU Kun.Research progress on control and preparation of vaterite-type calcium carbonate[J].Bulletin of the Chinese Ceramic Society,2022,41(8):2860-2870,2878. | |
| [6] | YANG Baojun, SHAO Zongqi, ZHANG Dongxue,et al.A mild route for the preparation of calcium carbonate rod bundles in large scale from carbide slag[J].Micro & Nano Letters,2021,16(3):187-193. |
| [7] | WANG Yongjing, YE Baofang, HONG Zengchun,et al.Uniform calcite mircro/nanorods preparation from carbide slag using recyclable citrate extractant[J].Journal of Cleaner Production,2020,253:119930. |
| [8] | 王超,杨保俊,周金刚,等.由电石渣制备高分散纳米碳酸钙[J].化工进展,2017,36(S1):346-352. |
| WANG Chao, YANG Baojun, ZHOU Jingang,et al.Preparation of highly dispersed nano calcium carbonate from calcium carbide residue[J].Chemical Industry and Engineering Progress,2017,36(S1):346-352. | |
| [9] | 陈华雄,宋永才.文石晶须制备中氯化镁的影响[J].硅酸盐学报,2003,31(10):940-944. |
| CHEN Huaxiong, SONG Yongcai.Effect of magnesium dichloride on aragonite whiskers crystallization[J].Journal of the Chinese Ceramic Society,2003,31(10):940-944. | |
| [10] | SONG K, KIM W, BANG J H,et al.Polymorphs of pure calcium carbonate prepared by the mineral carbonation of flue gas desulfurization gypsum[J].Materials & Design,2015,83:308-313. |
| [11] | 王倩倩.碳酸钙矿物的晶型调控试验研究[D].包头:内蒙古科技大学,2020. |
| WANG Qianqian.Experimental study on crystal structure control of calcium carbonate minerals[D].Baotou:Inner Mongolia University of Science and Technology,2020. | |
| [12] | 邵宗奇.由电石渣制备多种形貌碳酸钙的工艺条件研究[D].合肥:合肥工业大学,2020. |
| SHAO Zongqi.Research on the process conditions for preparing various morphologies of calcium carbonate from carbide slag[D].Hefei:Hefei University of Technology,2020. | |
| [13] | 赵美霞,于海燕,吴秀梅,等.三聚磷酸钠对草酸钙晶体生长的影响及其防石机理[J].暨南大学学报(自然科学与医学版),2006,27(5):705-709. |
| ZHAO Meixia, YU Haiyan, WU Xiumei,et al.Effect of sodium tripolyphosphate on crystallization of calcium oxalate and its mechanism on prevention of urinary stones[J].Journal of Jinan University(Nature Science and Medicine Edition),2006,27(5):705-709. | |
| [14] | 徐大瑛,耿文娟,钱德全,等.几种糖类物质对纳米碳酸钙结晶的影响[J].无机盐工业,2021,53(11):77-80. |
| XU Daying, GENG Wenjuan, QIAN Dequan,et al.Influence of several carbohydrates on crystallization of nano calcium carbonate[J].Inorganic Chemicals Industry,2021,53(11):77-80. | |
| [15] | 叶育倩.纳米碳酸钙的制备及其生长过程的研究[D].上海:华东理工大学,2011. |
| YE Yuqian.Research on the preparation and growth process of nano calcium carbonate[D].Shanghai:East China University of Science and Technology,2011. | |
| [16] | 刘浩然,周高燕,刘骏彦,等.晶种介导强化化学沉淀去除造纸废水中的钙[J].环境污染与防治,2022,44(1):27-32,78. |
| LIU Haoran, ZHOU Gaoyan, LIU Junyan,et al.Seed crystal mediation enhanced chemical precipitation for removal of calcium from papermaking wastewater[J].Environmental Pollution & Control,2022,44(1):27-32,78. | |
| [17] | 吴刚,章守权,葛秀涛,等.六偏磷酸钠对碳酸钙矿化的影响[J].化学研究与应用,2012,24(12):1853-1856. |
| WU Gang, ZHANG Shouquan, GE Xiutao,et al.Research on crystal morphology and shape of CaCO3 adjusted by sodium hexa-metaphosphate[J].Chemical Research and Application,2012,24(12):1853-1856. | |
| [18] | 魏月.电石渣制备球霰石碳酸钙工艺研究[J].纯碱工业,2024(3):27-30. |
| WEI Yue.Research on the preparation process of vaterite calcium carbonate using carbide slag[J].Soda Industry,2024(3):27-30. | |
| [19] | YUE Wenfei, FAN Chuigang, SONG Wenli,et al.Influence of additives on CaCO3 precursors and multicycle CO2 capture performance of CaO sorbents[J].Journal of Environmental Chemical Engineering,2022,10(3):107440. |
| [20] | 郑天文,陈雪梅.球霰石碳酸钙微球的合成及其机理[J].材料科学与工程学报,2018,36(3):358-364. |
| ZHENG Tianwen, CHEN Xuemei.Synthesis of vaterite calcium carbonate microspheres and the crystal growth mechanism[J].Journal of Materials Science and Engineering,2018,36(3):358-364. | |
| [21] | 李昱蓓,刘松辉,朱建平,等.电石渣制备球霰石碳酸钙的工艺及机理[J].建筑材料学报,2023,26(8):939-948. |
| LI Yubei, LIU Songhui, ZHU Jianping,et al.Process and mechanism of vaterite calcium carbonate preparation from calcium carbide slag[J].Journal of Building Materials,2023,26(8):939-948. | |
| [22] | 童张法,胡超,李立硕,等.间歇鼓泡碳化法制备立方形纳米碳酸钙工艺条件优化[J].广西科学,2015,22(1):53-59. |
| TONG Zhangfa, HU Chao, LI Lishuo,et al.Optimization of processing conditions for the preparation of cubic nano-sized calcium carbonate by intermittent bubbling carbonation[J].Guangxi Sciences,2015,22(1):53-59. | |
| [23] | KONOPACKA-ŁYSKAWA D, KOŚCIELSKA B, ŁAPIŃSKI M.Precipitation of spherical vaterite particles via carbonation route in the bubble column and the gas-lift reactor[J].JOM,2019,71(3):1041-1048. |
| [24] | 伊昌,朱银燕,安学勤.蔗糖、柠檬酸及其复配对合成纳米碳酸钙的影响[J].功能材料,2011,42(4):589-592. |
| YI Chang, ZHU Yinyan, AN Xueqin.Effect of sucrose,citric acid and their mixture on formation of nano-sized calcium carbona-te[J].Journal of Functional Materials,2011,42(4):589-592. | |
| [25] | 周高燕.晶种介导强化化学沉淀法除钙技术研究[D].杭州:浙江大学,2021. |
| ZHOU Gaoyan.Research on calcium removal technology by seed mediated enhanced chemical precipitation[D].Hangzhou:Zhejiang University,2021. | |
| [26] | YE Yuqian, CHEN Xuemei.The role of crystal control agent in the formation of needlelike calcium carbonate nano particles[J].Advanced Materials Research,2011,236/237/238:2150-2159. |
| [27] | 朱海洋,陆用海.三聚磷酸钠对碳酸钙结晶的影响[J].日用化学工业,1998,28(1):9-11. |
| ZHU Haiyang, LU Yonghai.The effects of sodium tripolyphosphate on the crystallization of calcium carbonate[J].China Surfactant Detergent & Cosmetics,1998,28(1):9-11. | |
| [28] | 陈义雯.不同形貌轻质碳酸钙的制备工艺研究[D].合肥:合肥工业大学,2021. |
| CHEN Yiwen.Research on the preparation process of lightweight calcium carbonate with different morphologies[D].Hefei:Hefei University of Technology,2021. |
| [1] | MA Chuxuan, CHENG Huaigang, CHENG Wenting, MA Zhuohui. Study on morphology control of magnesium carbonate trihydrate prepared by CO2 mineralization of magnesium sulfate [J]. Inorganic Chemicals Industry, 2025, 57(5): 26-31. |
| [2] | ZHANG Jinjun, GUO Linlin, MIAO Chengpeng, LI Xingyu, PANG Yaheng, YANG Rongkai, YU Yasen. Study on preparation of spherical calcium carbonate for coating fillers based on carbide slag as raw materials [J]. Inorganic Chemicals Industry, 2025, 57(2): 113-119. |
| [3] | WU Jie, WU Zewen, WANG Feng, ZHANG Qi, TANG Zhongfeng. Research progress on modification and preparation of carbide slag based CO2 adsorbents [J]. Inorganic Chemicals Industry, 2025, 57(11): 1-9. |
| [4] | ZHANG Jianle, CAO Yapeng, ZU Minghua, ZHANG Zhikun, LIU Yumin, HAN Jilong. Study on morphology of calcium carbonate controlled by sodium dodecyl sulfate and amino acid double template [J]. Inorganic Chemicals Industry, 2025, 57(1): 58-63. |
| [5] | YAN Xin, LIU Hailu, LIU Baolin, LIU Yi, LIU Yanyang. Research on key technologies and mechanisms of green nano calcium carbonate production [J]. Inorganic Chemicals Industry, 2025, 57(1): 71-76. |
| [6] | QI Xingzhao, WANG Feng, WU Jie, ZHANG Qi, TANG Zhongfeng. Study on pyrolysis behavior and mechanism of calcium carbide slag/magnesium carbonate system [J]. Inorganic Chemicals Industry, 2024, 56(10): 118-126. |
| [7] | ZHANG Jinjun, GUO Linlin, LIU Bojing, FENG Shuang, SHI Qi. Study on preparation of needle-like shaped CaCO3 from calcium carbide slag [J]. Inorganic Chemicals Industry, 2023, 55(7): 103-108. |
| [8] | YANG Yue, ZHU Ganyu, ZHANG Jianbo, MENG Ziheng, LIU Xinhui, YANG Jing, YAN Kun, PENG Zonggui, WANG Qiujian, LI Huiquan. Study on preparation of desulfurizer and byproduct gypsum from calcium carbide slag by cyclone separation [J]. Inorganic Chemicals Industry, 2023, 55(5): 78-84. |
| [9] | ZHENG Meiqi, MA Da, LIU Tingting, LIN Yanjun, KONG Xianggui, DUAN Xue. Application progress of magnesium-based layered double hydroxides in heavy metal pollution remediation [J]. Inorganic Chemicals Industry, 2023, 55(4): 1-5. |
| [10] | YANG Fengling,ZHAI Min,REN Lei,ZHANG Yuanyuan,CHENG Fangqin,DONG Hongyu. Influencing factors of crystallization products in wet desulfurization of carbide slag [J]. Inorganic Chemicals Industry, 2023, 55(2): 92-98. |
| [11] | WENG Dingsong,WANG Zhenghao,CHEN Liang,YIN Rentao,XIAO Haibing,LIU Weizao,LIANG Bin,LUO Dongmei. Serpentine coupled with copperas to enrich magnesium solution for simultaneous CO2 mineralization and nickel recovery [J]. Inorganic Chemicals Industry, 2023, 55(1): 93-99. |
| [12] | YAN Xin,WEI Yilan. Present situation and prospect of comprehensive utilization of waste residue containing calcium and magnesium [J]. Inorganic Chemicals Industry, 2022, 54(1): 7-11. |
| [13] | Hu Caixia,Hu Chenxin,Peng Jianlong,Zhong Xing. Preparation of porous spherical calcium carbonate with soluble starch as crystallization controller [J]. Inorganic Chemicals Industry, 2021, 53(5): 51-55. |
| [14] | Fan Tianbo,Jia Xiaohui,Han Dongxue,Chen Si,Jiang Yu,Hu Tingting,Guo Hongfan,Li Li,Liu Yunyi. Study on preparation of vaterite from dolomite by ammonia-alkali method and its mechanism [J]. Inorganic Chemicals Industry, 2021, 53(5): 56-60. |
| [15] | Ye Weihui,Lü Qi,Long Changjiang. Study on rapid preparation process of high-density spherical basic nickel carbonate [J]. Inorganic Chemicals Industry, 2021, 53(5): 69-72. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||