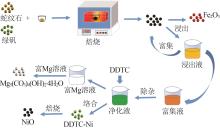
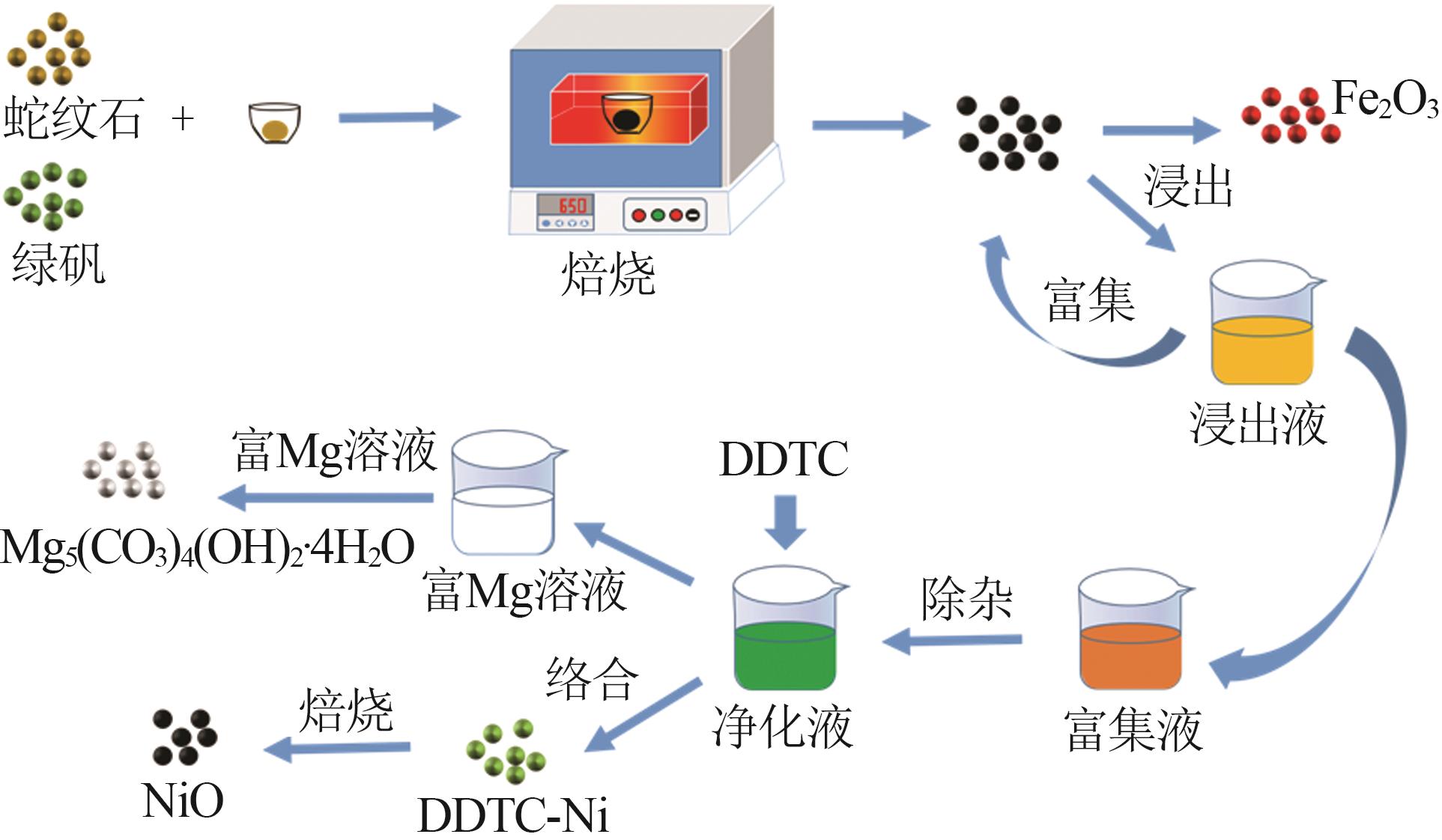
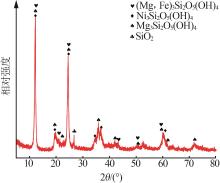
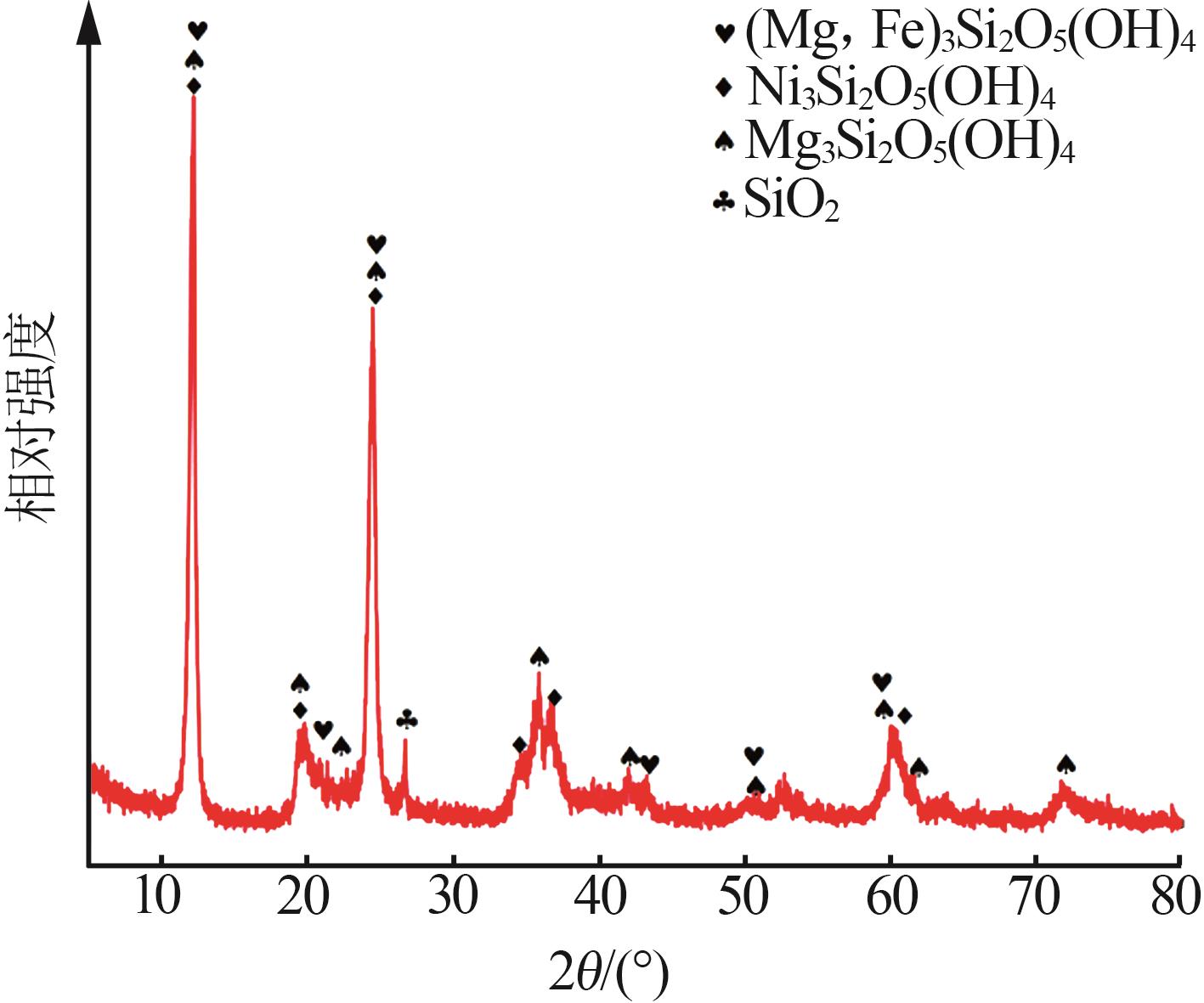
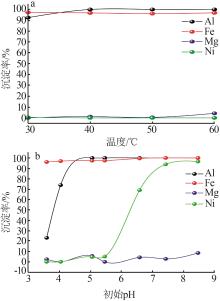
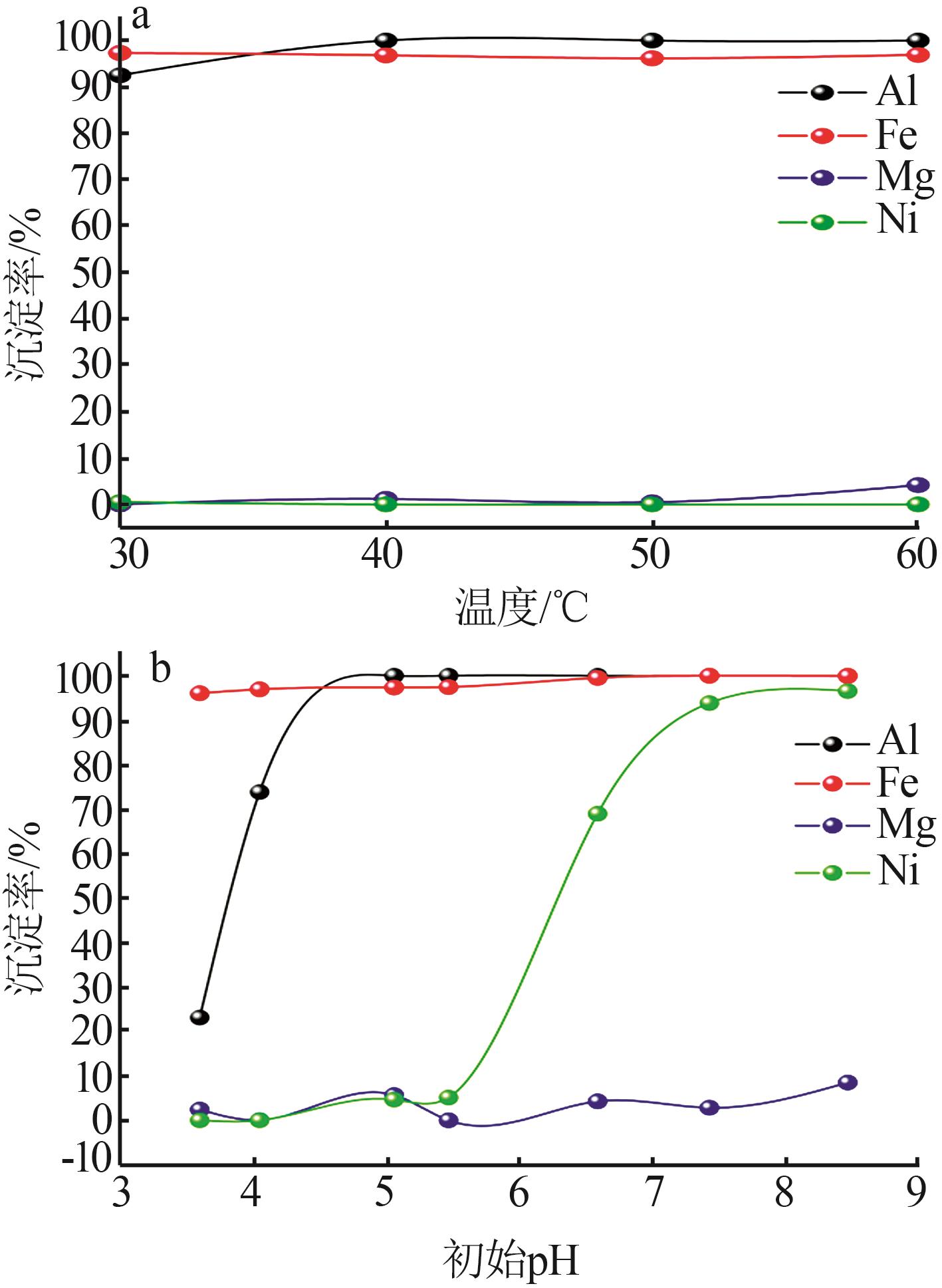
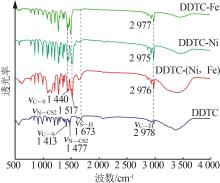
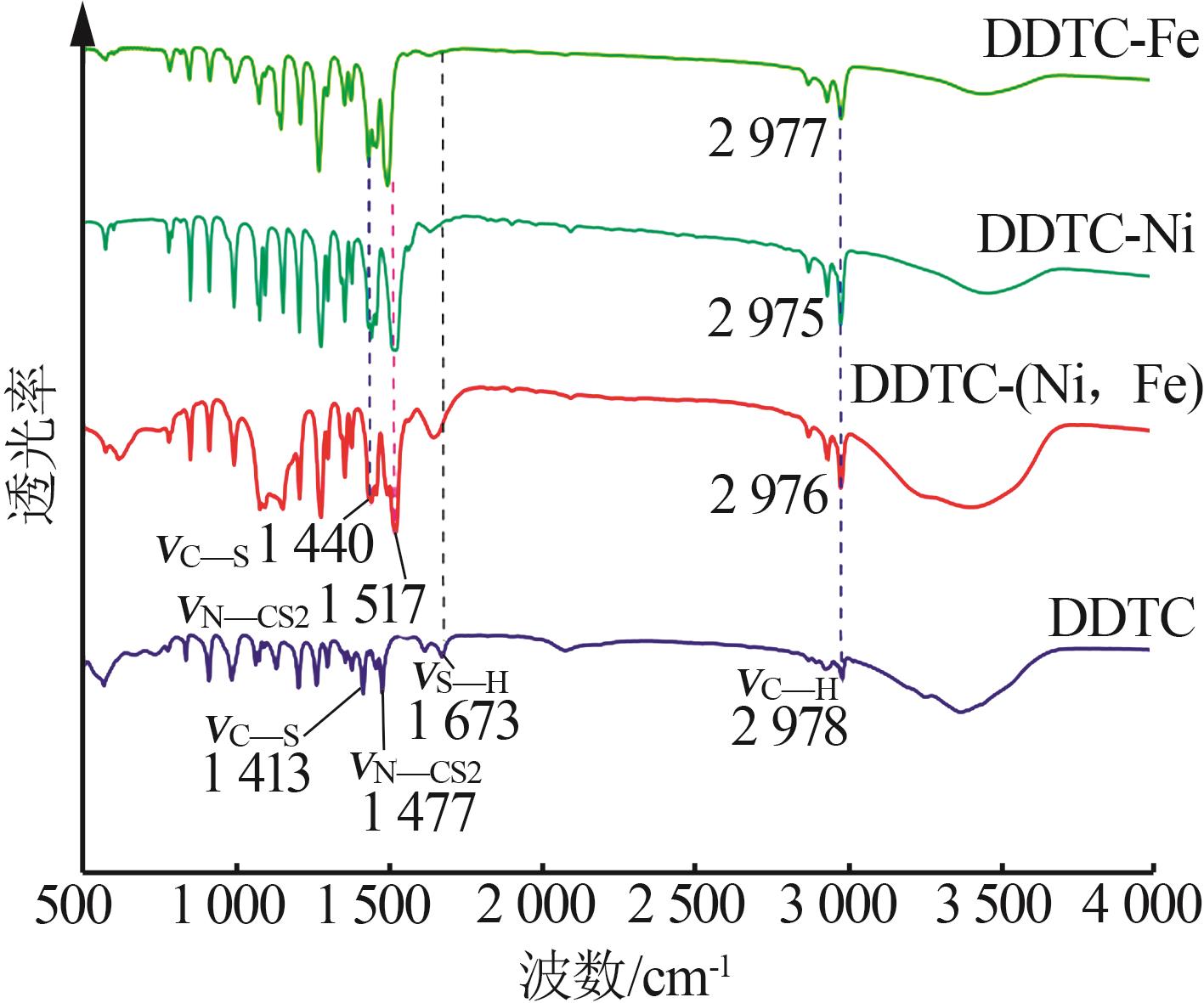
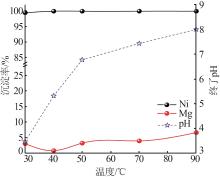
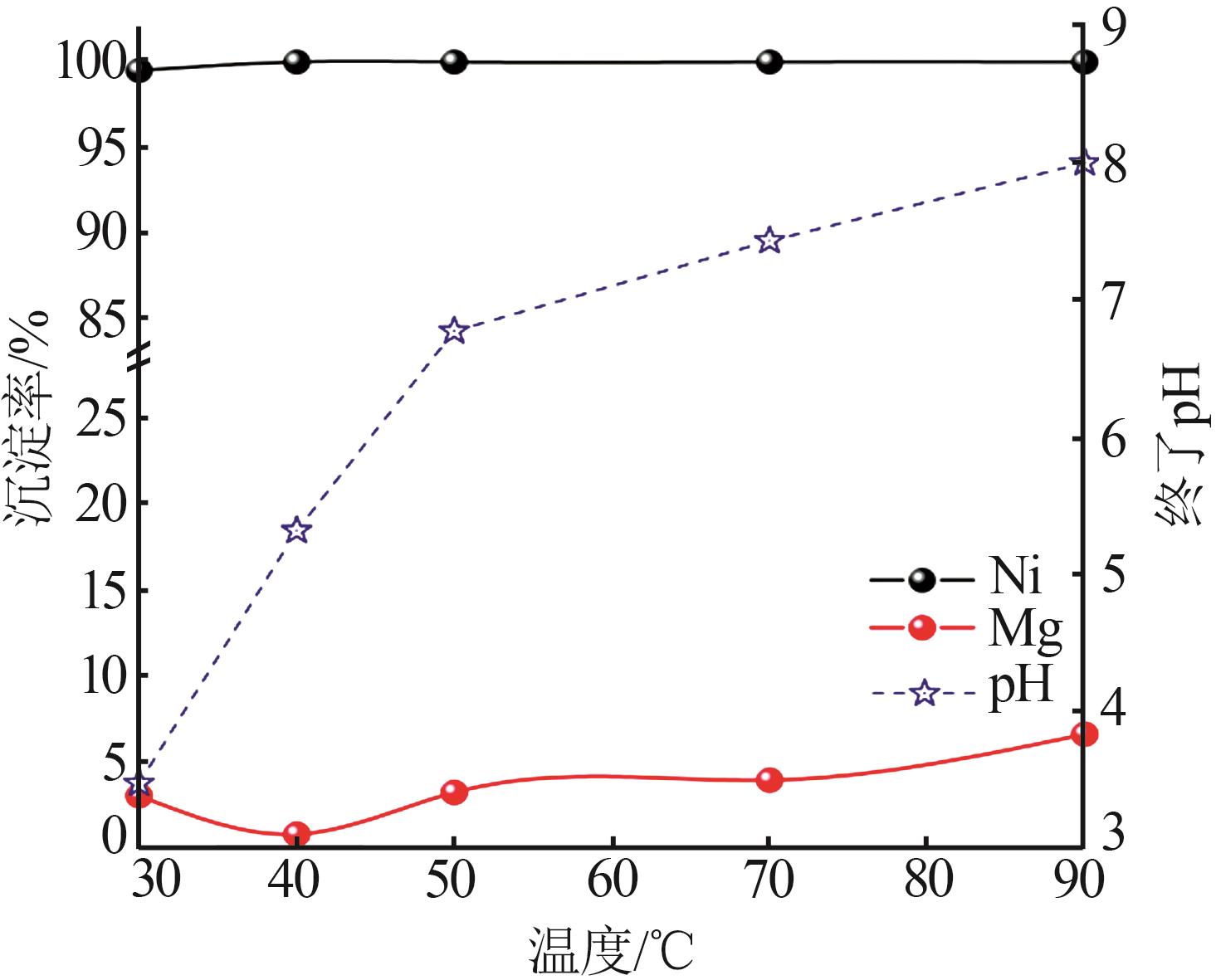
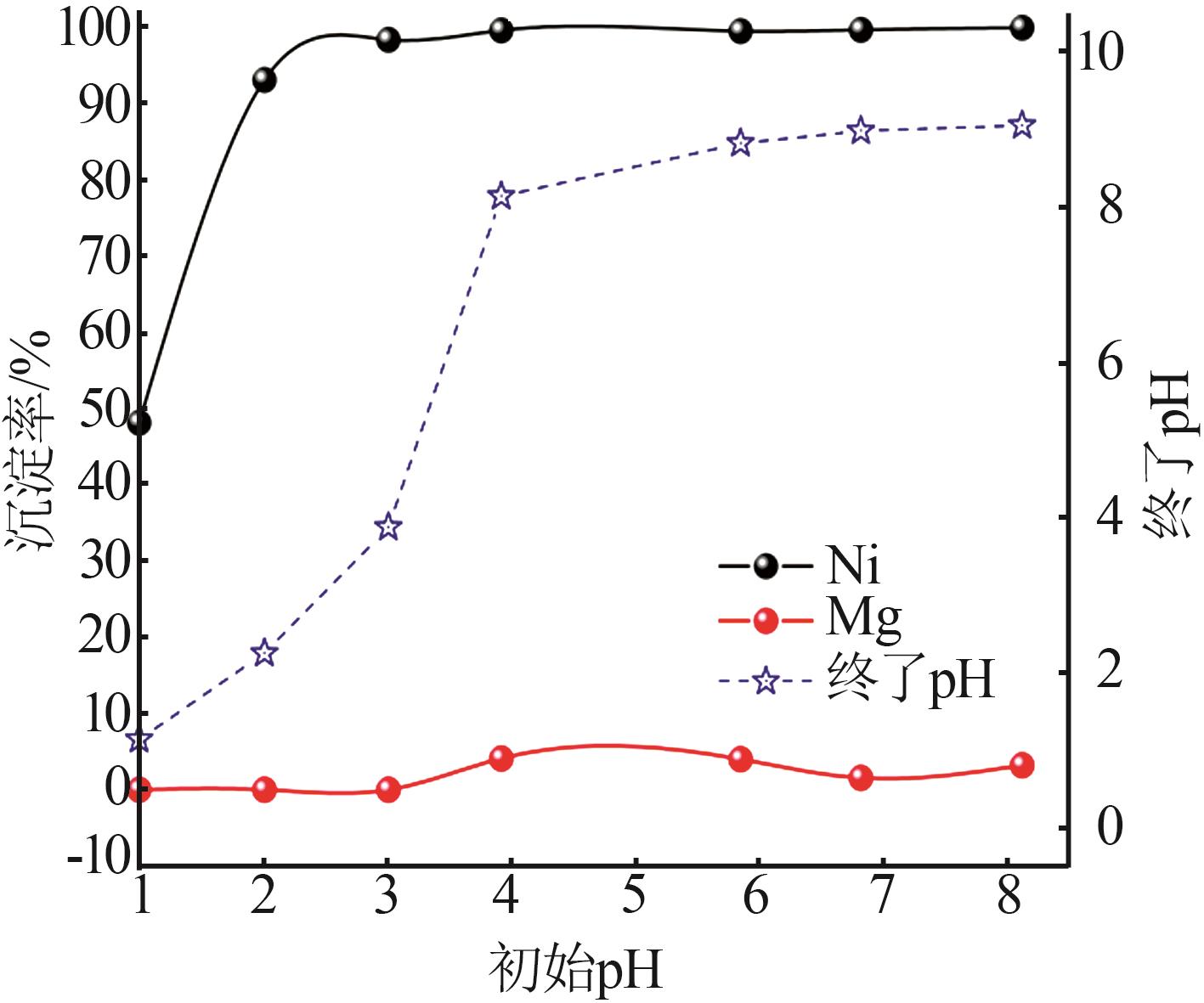
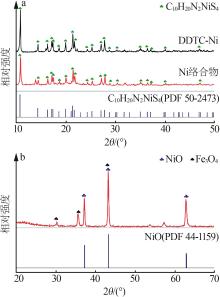
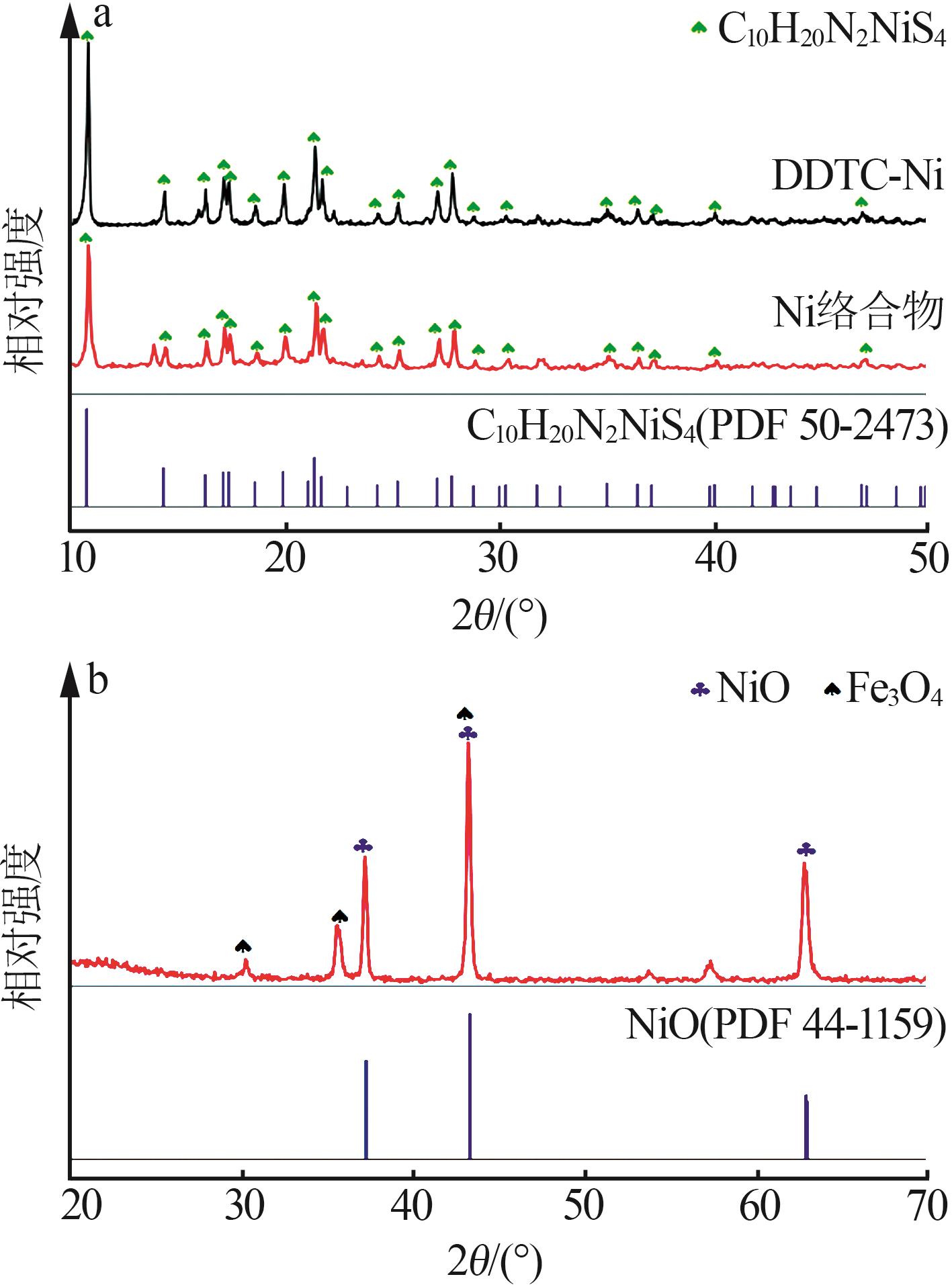
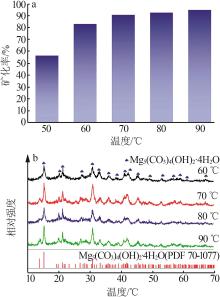
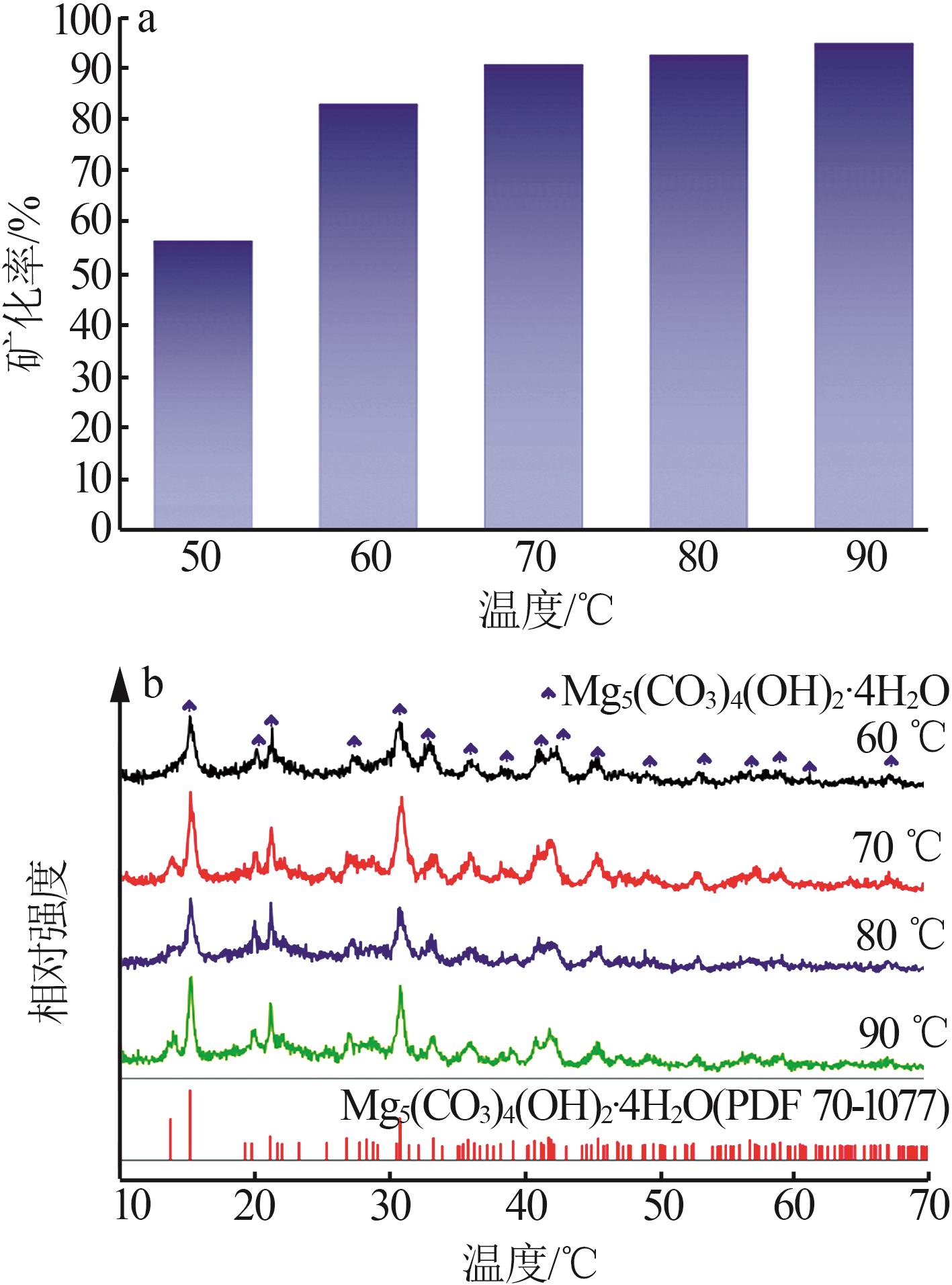
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (1): 93-99.doi: 10.19964/j.issn.1006-4990.2022-0139
• Research & Development • Previous Articles Next Articles
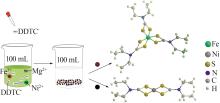
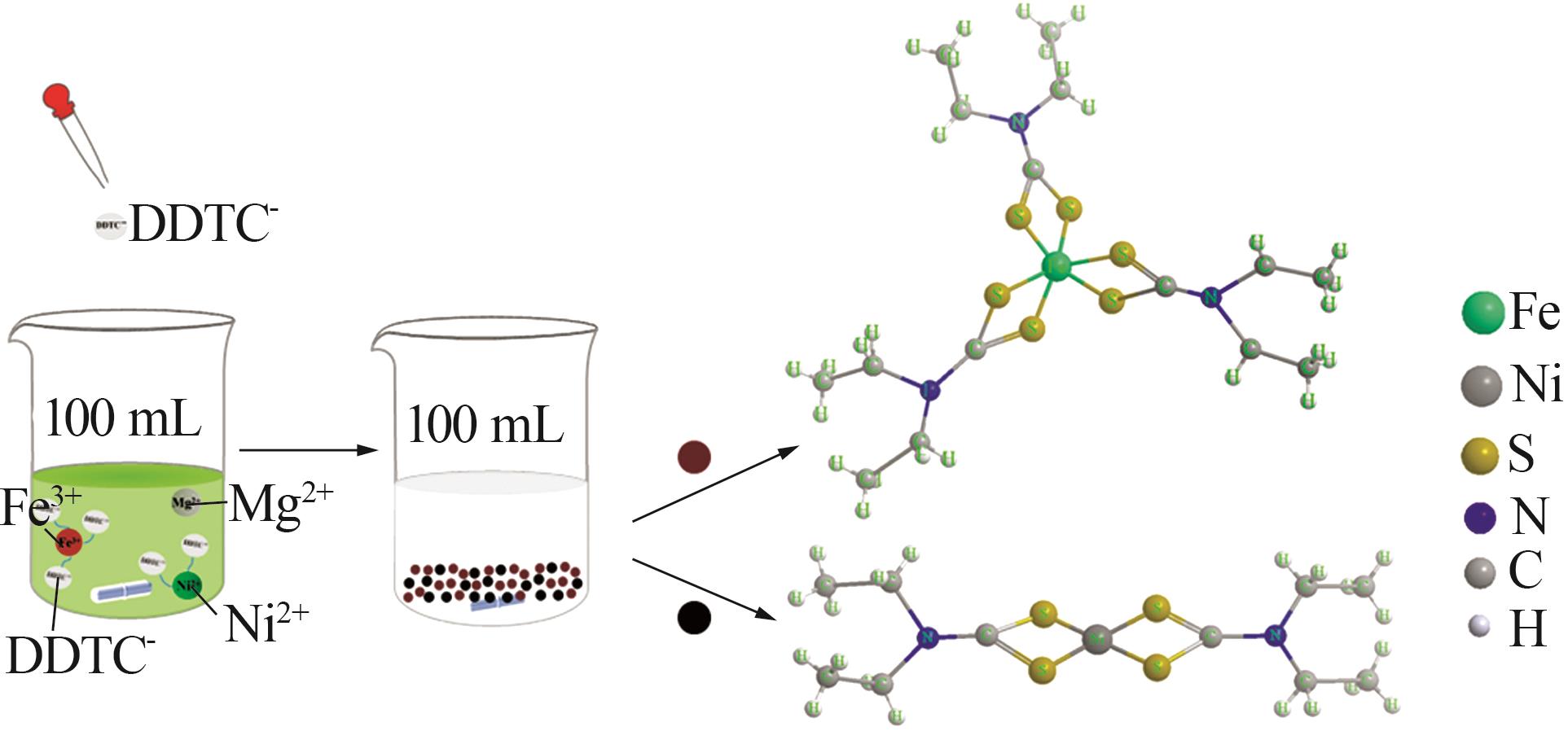
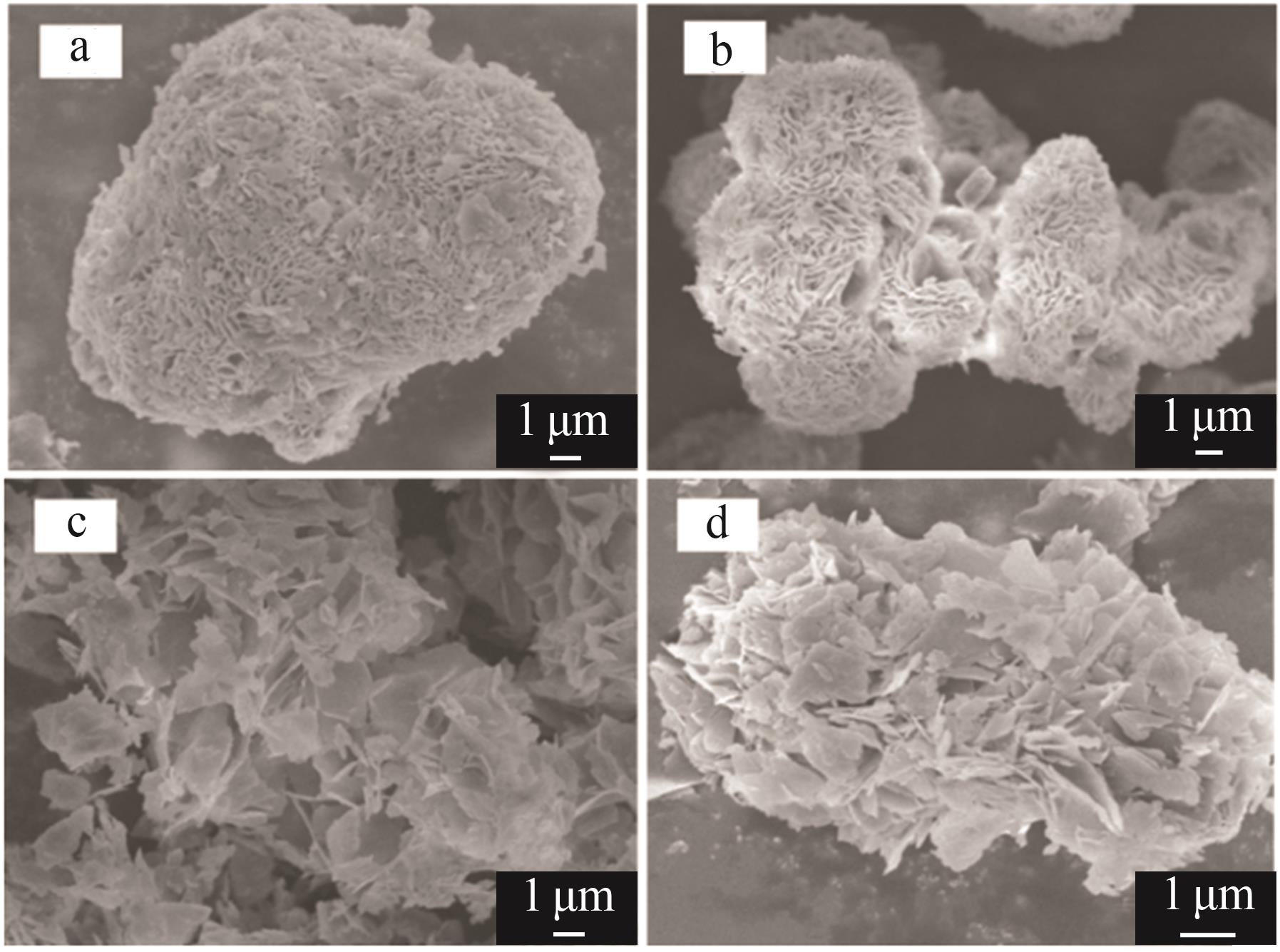
Serpentine coupled with copperas to enrich magnesium solution for simultaneous CO2 mineralization and nickel recovery
WENG Dingsong1( ),WANG Zhenghao1,CHEN Liang1,YIN Rentao1,XIAO Haibing1,LIU Weizao2,LIANG Bin1,LUO Dongmei1(
),WANG Zhenghao1,CHEN Liang1,YIN Rentao1,XIAO Haibing1,LIU Weizao2,LIANG Bin1,LUO Dongmei1( )
)
- 1. School of Chemical Engineering,SiChuan University,Chengdu 610065,China
2. College of materials science and engineering,ChongQing University,Chongqing 400030,China
-
Received:2022-05-06Online:2023-01-10Published:2023-01-17 -
Contact:LUO Dongmei E-mail:songvae17@163.com;dmluo@scu.edu.cn
CLC Number:
Cite this article
WENG Dingsong,WANG Zhenghao,CHEN Liang,YIN Rentao,XIAO Haibing,LIU Weizao,LIANG Bin,LUO Dongmei. Serpentine coupled with copperas to enrich magnesium solution for simultaneous CO2 mineralization and nickel recovery[J]. Inorganic Chemicals Industry, 2023, 55(1): 93-99.
share this article
| 1 | 张本曰, 刘丹, 郭锐, 等. 含镍蛇纹石的综合利用现状[J].矿产综合利用, 2020(4):13-20. |
| ZHANG Benyue, LIU Dan, GUO Rui, et al. Comprehensive utilization status of nickel-containing serpentine[J].Multipurpose Utilization of Mineral Resources, 2020(4):13-20. | |
| 2 | 肖景波, 夏娇彬, 陈居玲. 由蛇纹石制备高纯氧化铁的研究[J].无机盐工业, 2014, 46(11):30-34. |
| XIAO Jingbo, XIA Jiaobin, CHEN Juling. Preparation of high purity ferric oxide from serpentine[J].Inorganic Chemicals Industry, 2014, 46(11):30-34. | |
| 3 | 刘文刚, 李志明, 吴冠达, 等. 高温焙烧对含蛇纹石尾矿亚甲基蓝吸附性能的影响[J].矿产保护与利用, 2021, 41(3):89-93. |
| LIU Wengang, LI Zhiming, WU Guanda, et al. Effect of high temperature roasting on methylene blue adsorption of serpentine tailings[J].Conservation and Utilization of Mineral Resources, 2021, 41(3):89-93. | |
| 4 | FAGERLUND J, NDUAGU E, ROMÃO I, et al. A stepwise process for carbon dioxide sequestration using magnesium silicates[J].Frontiers of Chemical Engineering in China, 2010, 4(2):133- 141. |
| 5 | FAGERLUND J, NDUAGU E, ROMÃO I, et al. CO2 fixation using magnesium silicate minerals part 1:Process description and performance[J].Energy, 2012, 41(1):184-191. |
| 6 | 张克宇, 吴鉴, 周朝金, 等. 结晶法提纯钛白副产硫酸亚铁[J].有色金属工程, 2017, 7(2):10-15. |
| ZHANG Keyu, WU Jian, ZHOU Chaojin, et al. Purification of ferrous sulfate byproduct from titanium dioxide by crystallization process[J].Nonferrous Metals Engineering, 2017, 7(2):10-15. | |
| 7 | 唐舒扬, 郭宇峰, 郑富强, 等. 钛白粉的制备方法现状及展望[J].无机盐工业, 2022, 54(7):27-34. |
| TANG Shuyang, GUO Yufeng, ZHENG Fuqiang, et al. Present situation and prospects of preparation methods of titanium dioxide[J].Inorganic Chemicals Industry, 2022, 54(7):27-34. | |
| 8 | WANG Lin, LIU Weizao, HU Jingpeng, et al. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3 [J].Chinese Journal of Chemical Engineering, 2018, 26(3):583-592. |
| 9 | LIU Weizao, YIN Shu, LUO Dongmei, et al. Optimising the recovery of high-value-added ammonium alum during mineral carbonation of blast furnace slag[J].Journal of Alloys and Compounds, 2019, 774:1151-1159. |
| 10 | XU Xingliang, LIU Weizao, CHU Guanrun, et al. Energy-efficient mineral carbonation of CaSO4 derived from wollastonite via a roasting-leaching route[J].Hydrometallurgy, 2019, 184:151- 161. |
| 11 | YIN Shu, ALDAHRI T, ROHANI S, et al. Insights into the roasting kinetics and mechanism of blast furnace slag with ammonium sulfate for CO2 mineralization[J].Industrial & Engineering Che- mistry Research, 2019, 58(31):14026-14036. |
| 12 | LI Jinhui, CHEN Zhifeng, SHEN Bangpo, et al. The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate[J].Journal of Cleaner Production, 2017, 140:1148-1155. |
| 13 | RIBEIRO P P M, NEUMANN R, SANTOS I D D, et al. Nickel carriers in laterite ores and their influence on the mechanism of nickel extraction by sulfation-roasting-leaching process[J].Minerals Engineering, 2019, 131:90-97. |
| 14 | CUI Fuhui, MU Wenning, WANG Shuai, et al. Sodium sulfate activation mechanism on co-sulfating roasting to nickel-copper sulfide concentrate in metal extractions,microtopography and kinetics[J].Minerals Engineering, 2018, 123:104-116. |
| 15 |
CHEN Yangfan, TENG Wenxin, FENG Xin, et al. Efficient extraction and separation of zinc and iron from electric arc furnace dust by roasting with FeSO4·7H2O followed by water leaching[J].Separation and Purification Technology, 2022, 281.Doi:10.1016/j.seppur.2021.119936.
doi: 10.1016/j.seppur.2021.119936 |
| 16 |
ZHANG Xiufeng, CHEN Zhichao, ROHANI S, et al. Simultaneous extraction of lithium,rubidium,cesium and potassium from lepidolite via roasting with iron(Ⅱ) sulfate followed by water leaching[J].Hydrometallurgy, 2022, 208.Doi:10.1016/j.hydro-met.2022.105820.
doi: 10.1016/j.hydro-met.2022.105820 |
| 17 | 牟文宁, 崔富晖, 黄志鹏, 等. 红土镍矿硫酸焙烧-浸出溶液中铁、镍回收的研究[J].矿产综合利用, 2018(1):22-25. |
| MU Wenning, CUI Fuhui, HUANG Zhipeng, et al. Research on the recovery of iron and nickel from leaching liquid of laterite nickel ore treated by sulfuric acid roasting-water leaching[J].Multipurpose Utilization of Mineral Resources, 2018(1):22-25. | |
| 18 | 严苹方. 巯基捕集剂及自制磁性巯基胺型树脂对微量络合铜镍的去除特性研究[D].广州:广东工业大学, 2017. |
| YAN Pingfang. Study on the characteristics of trace complex copper and nickel removal by the thiol metal capture agent and the self-made magnetic mercapto amine resin[D].Guangzhou:Gu-angdong University of Technology, 2017. | |
| 19 | XIAO Xiao, YE Maoyou, YAN Pingfang, et al. Disodium N,N-bis-(dithiocarboxy)ethanediamine:Synthesis,performance,and mechanism of action toward trace ethylenediaminetetraacetic acid copper(Ⅱ)[J].Environmental Science and Pollution Research International, 2016, 23(19):19696-19706. |
| 20 | YANG Xin, PENG Xianjia, KONG Linghao, et al. Removal of Ni(Ⅱ) from strongly acidic wastewater by chelating precipitation and recovery of NiO from the precipitates[J].Journal of Environmental Sciences, 2021, 104:365-375. |
| 21 | 严苹方, 孙水裕, 叶茂友, 等. 巯基重金属捕集剂脱除电镀废水中低浓度Ni的效能及机理研究[J].环境科学学报, 2015, 35(9):2833-2839. |
| YAN Pingfang, SUN Shuiyu, YE Maoyou, et al. Research on the efficiency and mechanism of low-concentration Ni removal from electroplating wastewater by the heavy metal capturing agent with thiol[J].Acta Scientiae Circumstantiae, 2015, 35(9):2833-2839. |
| [1] | JIANG Bowen, LI Yanpei, RUI Yichuan, ZHANG Zefang. Study on structure⁃performance relationship of complex agent in CMP of 6063 aluminum alloy [J]. Inorganic Chemicals Industry, 2024, 56(8): 47-53. |
| [2] | LI Xiao,ZHAO Yuxiang,LI Bo,ZOU Xingwu,WANG Shuxuan. Study on controllable synthesis of spherical strontium fluoride [J]. Inorganic Chemicals Industry, 2022, 54(5): 67-71. |
| [3] | Zhang Shangqiang,Sun Guanhua,Sun Yanmin,Zhu Jinjian,Nan Jun,Xiao Han,Zhang Jingcheng,Song Guoliang. Study on preparation of CNTs-Al2O3 supported Pd catalyst and its performance [J]. Inorganic Chemicals Industry, 2021, 53(6): 194-198. |
| [4] | Du Xiaowang,Zhong Jianchu,Wang Xiaotian. Study on removal of silicon from high-silicon brucite by sodium roasting method [J]. Inorganic Chemicals Industry, 2020, 52(10): 92-95. |
| [5] | TANG Shu-Zhen, ZHANG Rong-Liang, SHE Yuan-Yuan. Synthesis of nanometer PbS by heterogeneous reaction [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 22-. |
| [6] | XIAO Jing-Bo, XIA Jiao-Bin, CHEN Ju-Ling. Preparation of high purity ferric oxide from serpentine [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(11): 30-. |
| [7] | SONG Li-Ying, HU Qing-Fu- , LIU Bao-Shu- , HU Xiao-Xiang. Development and comprehensive utilization of serpentine [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 5-. |
| [8] | LI Hao, LIAO Jin-Yun, ZHANG Xi-Bin, LIAO Wei-Wen, WEN Li-Li, YANG Jing-Bo. Preparation of wheat-like cobalt-nickel alloy microcrystals [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(2): 20-. |
| [9] | LU Shuang-Jia, ZHONG Jian-Chu, WANG Hong-Zhi. Study on purification and impurity removing in preparing silicon-steel grade magnesium oxide with carbonization method [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 23-. |
| [10] | PAN Xin-Ai, YE Jun-Wei, WANG Hao-Yu, GONG Wei-Tao, LIN Yuan, NING Gui-Ling. Preparation of high purity boric acid by complex crystallization [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(10): 18-. |
| [11] | Li Hao;Zhang Xibin. Effect of complexing agents/surfactants on flowery Co microcrystals prepared by solvothermal method [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(6): 0-0. |
| [12] | Hu Zhen;Zhang Li;Yu Hailian. Study on removing of basic nitrogen compounds from diesel oil with complexant ferric trichloride [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(1): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||