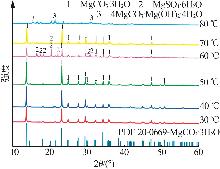
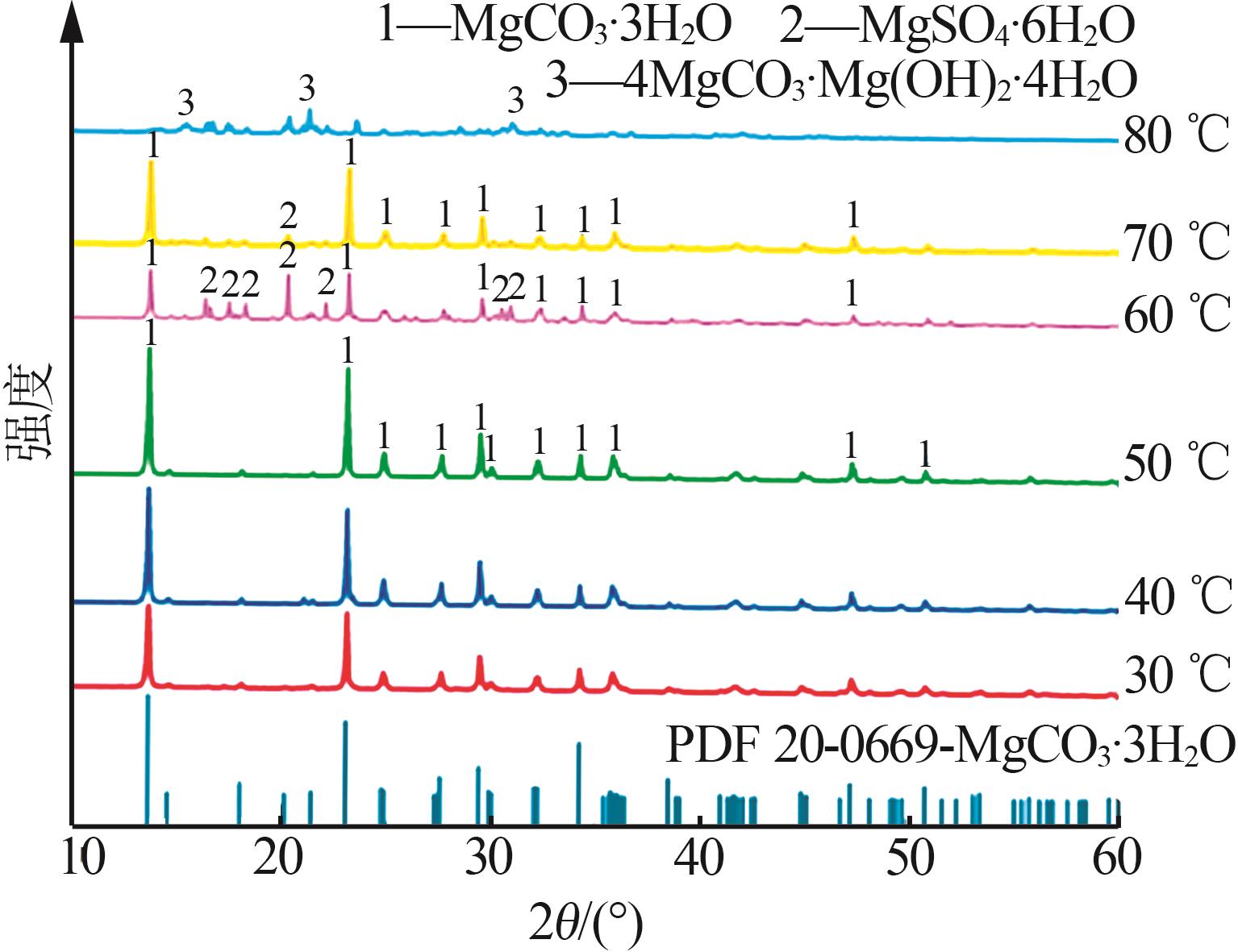
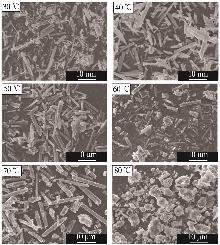
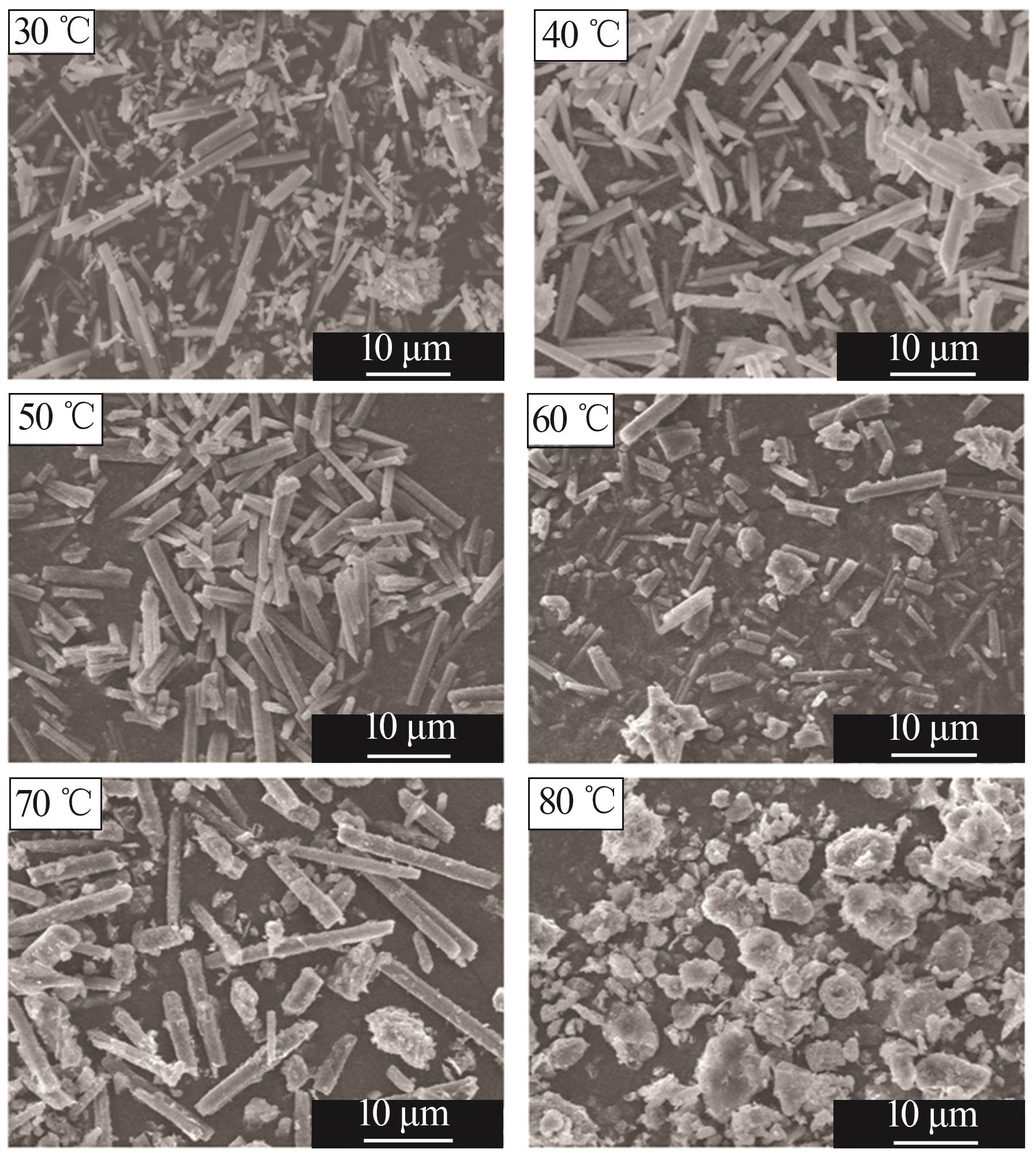
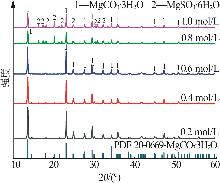
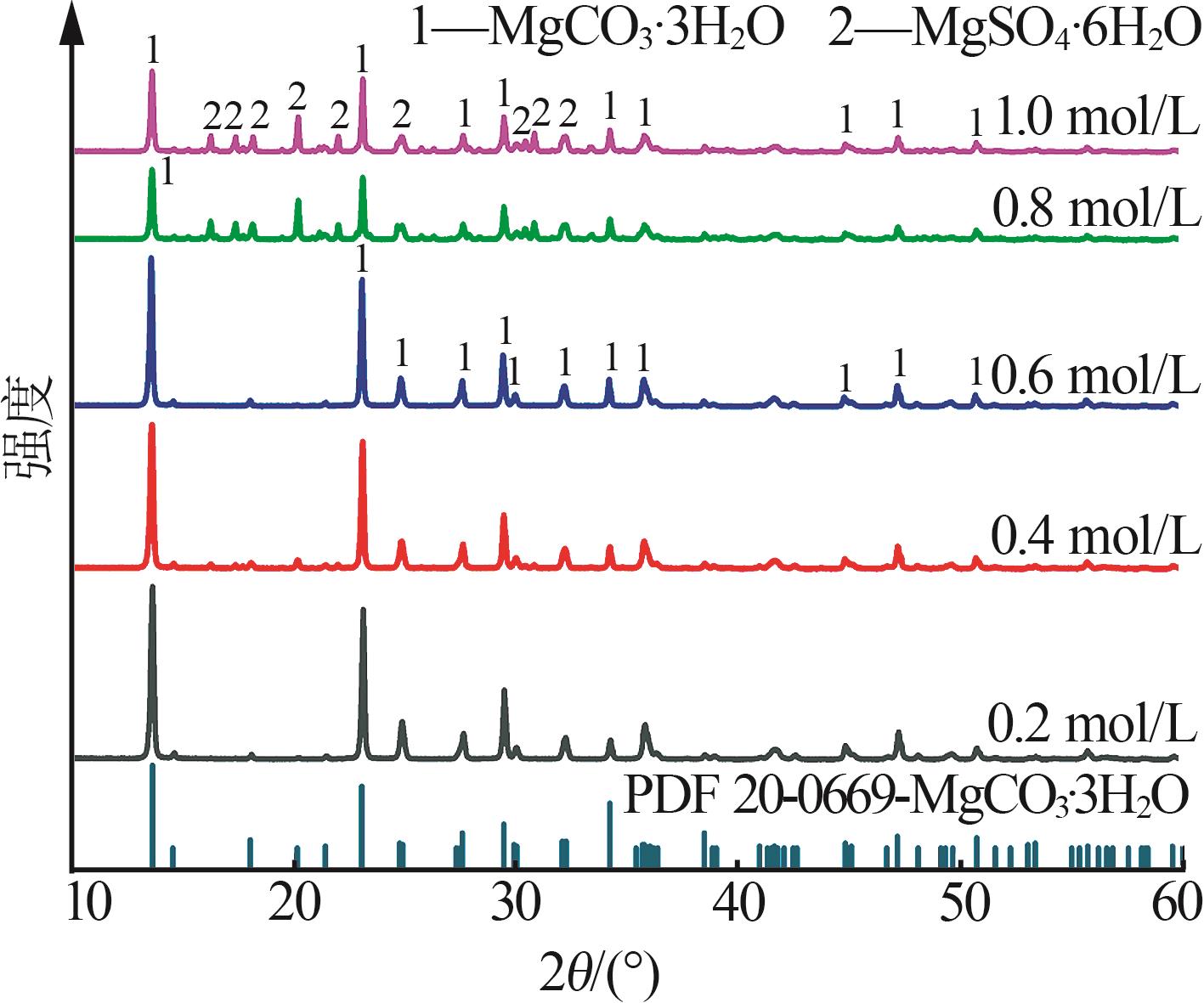
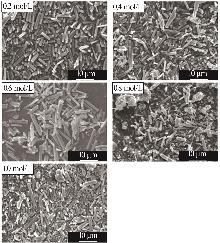
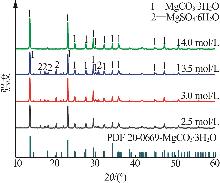
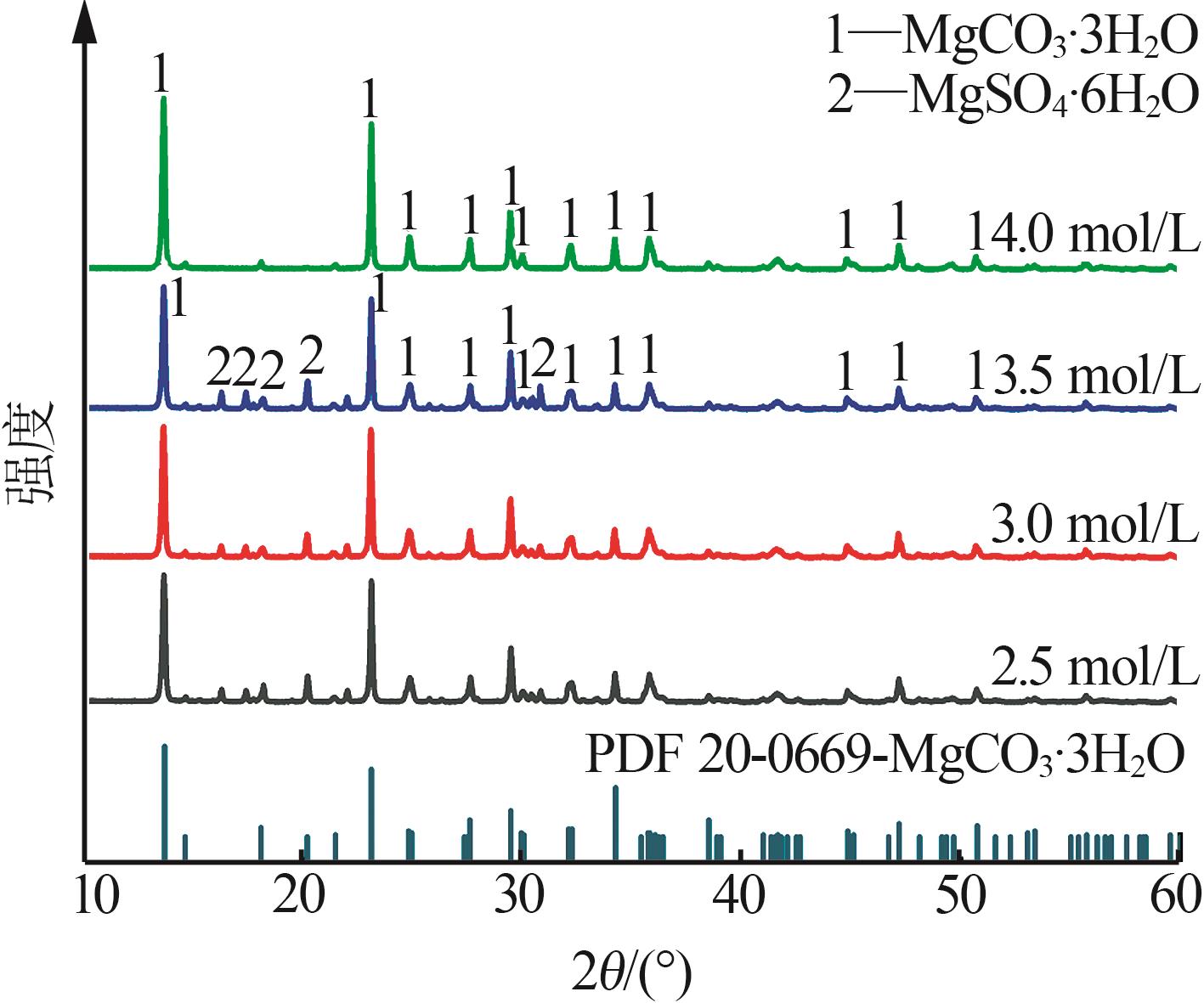
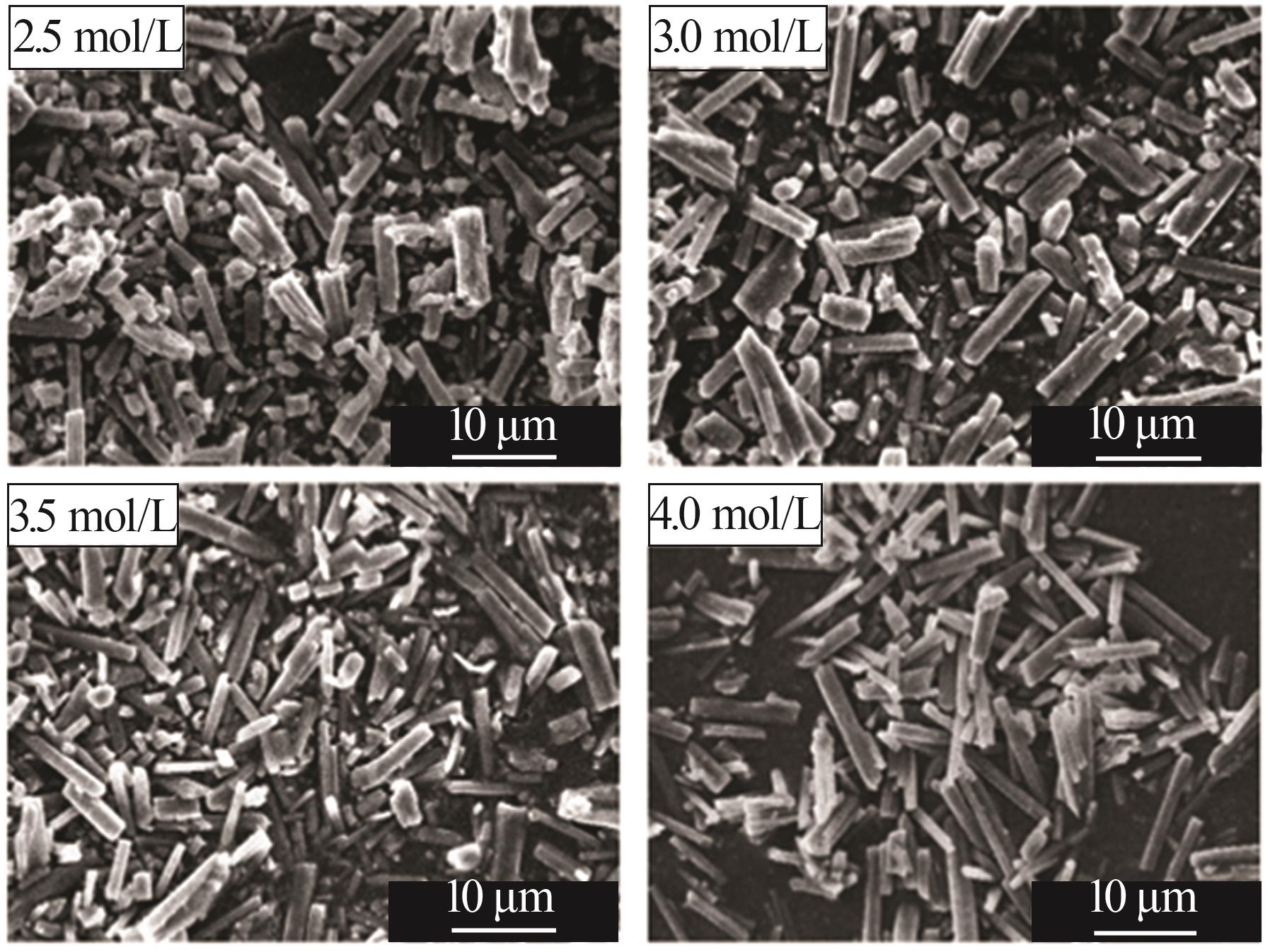
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (5): 26-31.doi: 10.19964/j.issn.1006-4990.2024-0410
• High-value utilization of magnesium resources • Previous Articles Next Articles
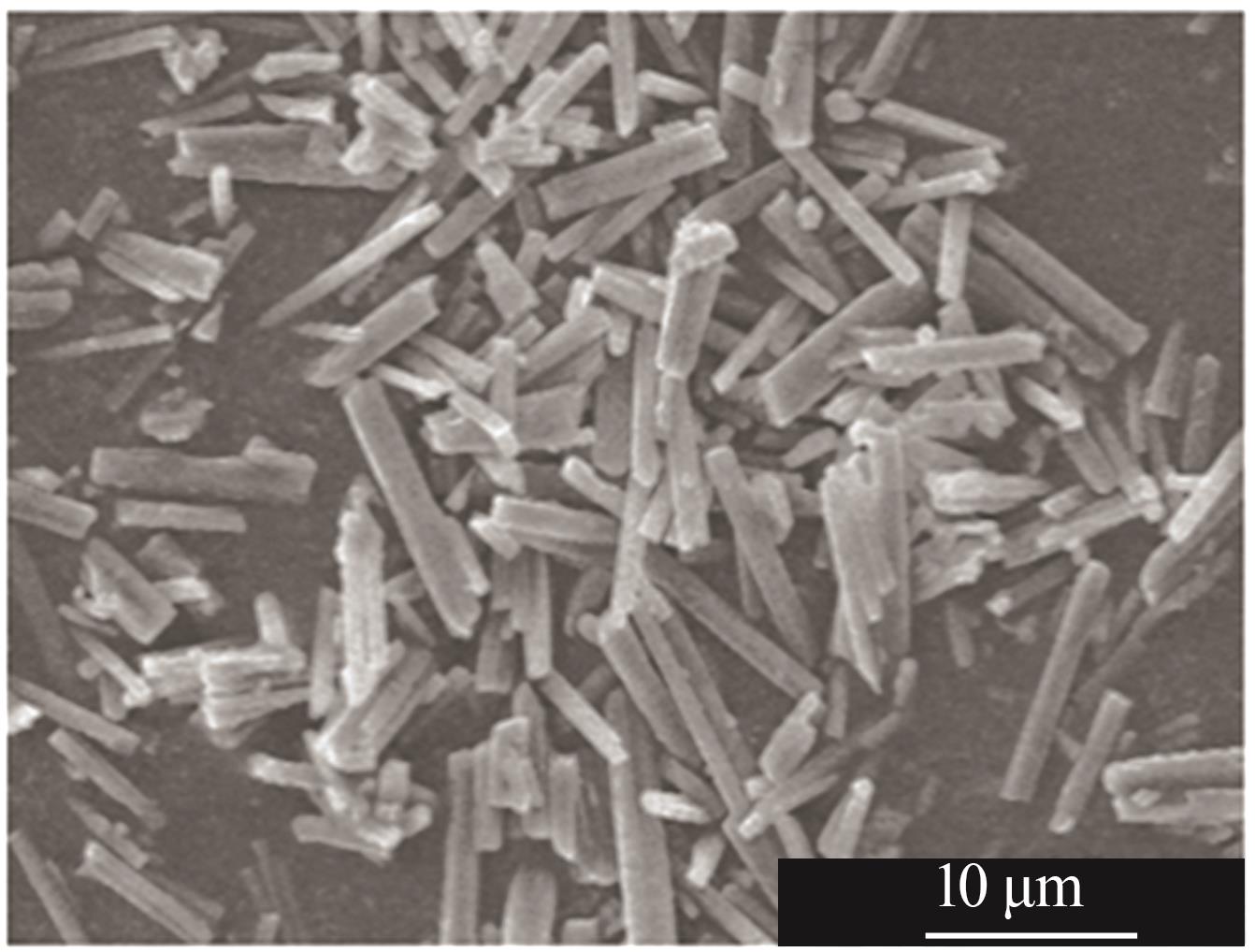
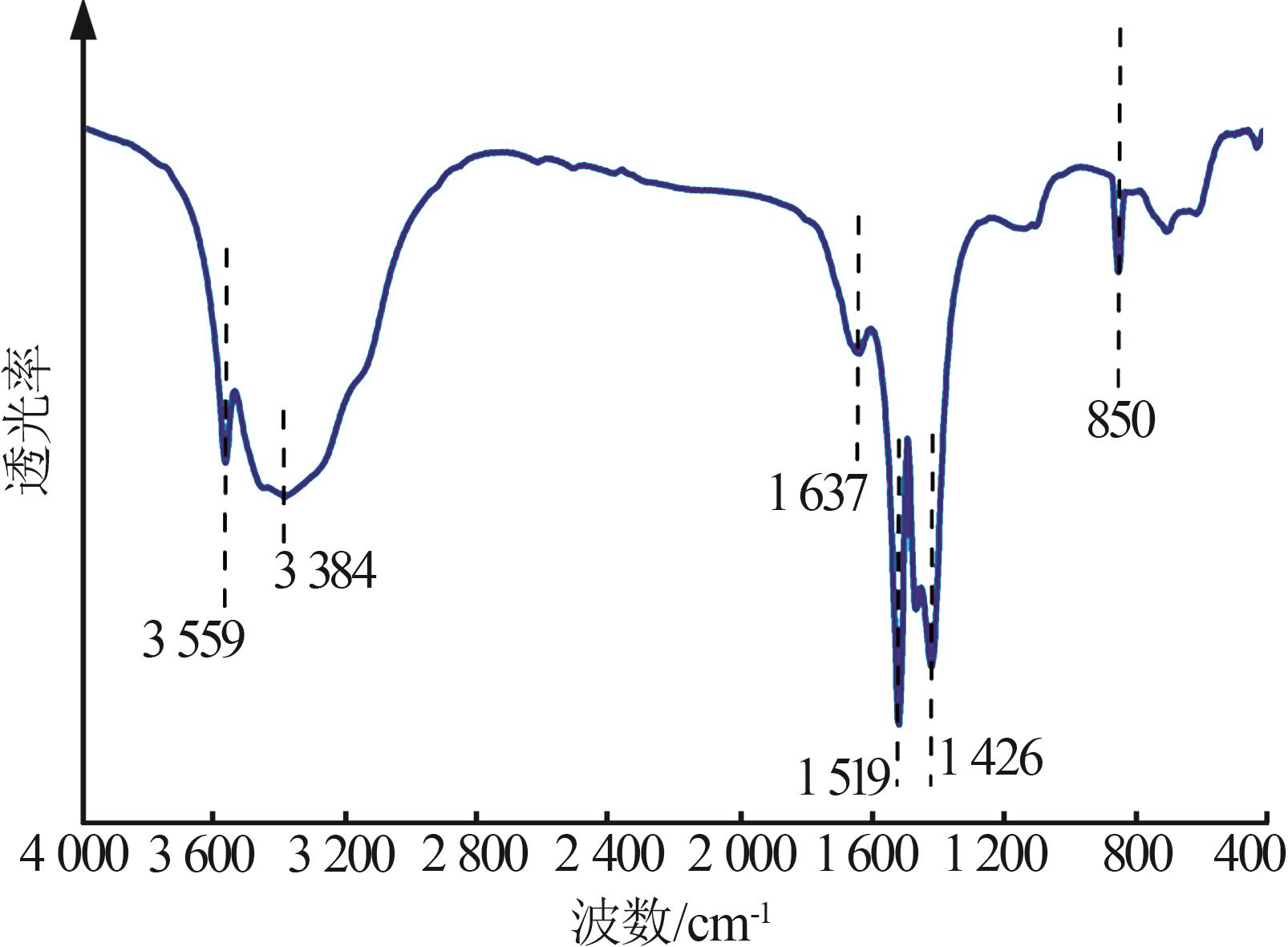
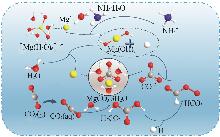
Study on morphology control of magnesium carbonate trihydrate prepared by CO2 mineralization of magnesium sulfate
MA Chuxuan1( ), CHENG Huaigang1,2(
), CHENG Huaigang1,2( ), CHENG Wenting1, MA Zhuohui1
), CHENG Wenting1, MA Zhuohui1
- 1. Institute of Resources and Environmental Engineering,Shanxi University,Engineering Research Center for CO2 Emission Reduction and Resource Utilization,Ministry of Education,Taiyuan 030006,China
2. College of Chemical Engineering,Qinghai University,Xining 810016,China
-
Received:2024-07-18Online:2025-05-10Published:2025-06-05 -
Contact:CHENG Huaigang E-mail:1242710163@qq.com;chenghg@sxu.edu.cn
CLC Number:
Cite this article
MA Chuxuan, CHENG Huaigang, CHENG Wenting, MA Zhuohui. Study on morphology control of magnesium carbonate trihydrate prepared by CO2 mineralization of magnesium sulfate[J]. Inorganic Chemicals Industry, 2025, 57(5): 26-31.
share this article
| 1 | 张苏江,张琳,姜爱玲,等.中国盐湖资源开发利用现状与发展建议[J].无机盐工业,2022,54(10):13-21. |
| ZHANG Sujiang, ZHANG Lin, JIANG Ailing,et al.Current situation and development suggestions of development and utilization of salt lake resources in China[J].Inorganic Chemicals Industry,2022,54(10):13-21. | |
| 2 | 邓良明,蒋志税,李一凰.盐湖水氯镁石复合精制实验研究[J].无机盐工业,2022,54(10):52-56. |
| DENG Liangming, JIANG Zhishui, LI Yihuang.Experimental study on compound refining of salt lake bischofite[J].Inorganic Chemicals Industry,2022,54(10):52-56. | |
| 3 | CHENG Danxu, AN Dong, CHENG Huaigang,et al.The microstructural investigation and the temperature⁃changing separation of brine with a high Mg/Li ratio[J].Applied Sciences,2024,14(4):1333. |
| 4 | LIU Xiuquan, WEN Jing, CHANG Chenggong,et al.Effects of calcination process of salt lake magnesium slag on the properties of magnesium oxysulfide cement[J].Advances in Cement Research,2022,34(5):225-234. |
| 5 | 王彦霞,程文婷,方莉,等.MgCO3·3H2O晶须的制备及其生长机制的研究[J].无机盐工业,2020,52(5):22-26. |
| WANG Yanxia, CHENG Wenting, FANG Li,et al.Study on the preparation and growth mechanism of MgCO3·3H2O whiskers[J].Inorganic Chemicals Industry,2020,52(5):22-26. | |
| 6 | YAO Jin, SUN Haoran, HAN Fang,et al.Enhancing selectivity of modifier on magnesite and dolomite surfaces by pH control[J].Powder Technology,2020,362:698-706. |
| 7 | 崔万顺,文伟翔,高玉娟,等.L-天门冬氨酸镁制备高热稳定性三水碳酸镁晶体[J].北京化工大学学报(自然科学版),2023,50(2):63-69. |
| CUI Wanshun, WEN Weixiang, GAO Yujuan,et al.Preparation of nesquehonite crystals with high thermal stability from magnesium L-aspartate[J].Journal of Beijing University of Chemical Technology(Natural Science Edition),2023,50(2):63-69. | |
| 8 | 刘珈伊,王余莲,时天骄,等.菱镁矿气泡模板法制备三水碳酸镁晶体及其生长机理[J].矿产保护与利用,2022,42(2):114-119. |
| LIU Jiayi, WANG Yulian, SHI Tianjiao,et al.Preparation and growth mechanism of nesquehonite crystal crystals by bubble template method with magnesite[J].Conservation and Utilization of Mineral Resources,2022,42(2):114-119. | |
| 9 | 毕秋艳,党力,曹海莲,等.青海盐湖镁资源开发与利用研究进展[J].盐湖研究,2022,30(1):101-109. |
| BI Qiuyan, DANG Li, CAO Hailian,et al.Development and utilization of magnesium resources in Qinghai salt lakes[J].Journal of Salt Lake Research,2022,30(1):101-109. | |
| 10 | 赵斌,贾志丹,邵朵朵.硫酸镁为原料制备三水碳酸镁晶须的研究[J].人工晶体学报,2017,46(1):18-25. |
| ZHAO Bin, JIA Zhidan, SHAO Duoduo.Preparation of nesquehonite whisker using magnesium sulfate as material[J].Journal of Synthetic Crystals,2017,46(1):18-25. | |
| 11 | GENG Xi, LV Li, LI Chun,et al.The kinetics of CO2 indirect mineralization of MgSO4 to produce MgCO3·3H2O [J].Journal of CO2 Utilization,2019,33:64-71. |
| 12 | CAO Xuepu, ZHANG Ruilei, ZHAO Hua,et al.Preparation and characterization of anhydrous magnesium carbonate:Effect of different anions of the magnesium sources[J].Journal of Crystal Growth,2024,635:127583. |
| 13 | 付梦源,程芳琴,程文婷,等.沉淀结晶法制备不同形貌的碳酸镁水合物[J].盐业与化工,2015,44(12):24-29. |
| FU Mengyuan, CHENG Fangqin, CHENG Wenting,et al.Preparation of magnesium carbonate hydrates with different morphology by precipitation method[J].Journal of Salt and Chemical Industry,2015,44(12):24-29. | |
| 14 | 吴丹,王玉琪,武海虹,等.三水碳酸镁合成与形貌演变过程研究[J].人工晶体学报,2014,43(3):606-613. |
| WU Dan, WANG Yuqi, WU Haihong,et al.Research on preparation and morphology evolution of magnesium carbonate tri⁃hydrate[J].Journal of Synthetic Crystals,2014,43(3):606-613. | |
| 15 | 时天骄,王余莲,刘珈伊,等.共沉淀法制备三水碳酸镁晶体及生长机理[J].中国粉体技术,2021,27(5):120-127. |
| SHI Tianjiao, WANG Yulian, LIU Jiayi,et al.Preparation and growth mechanism of nesquehonite crystals with co⁃precipitation method[J].China Powder Science and Technology,2021,27(5):120-127. | |
| 16 | 张建伟,邹显泓,董鑫,等.撞击流-共沉淀法制备三水碳酸镁的工艺优化及结晶动力学[J].过程工程学报,2023,24(5):533-545. |
| ZHANG Jianwei, ZOU Xianhong, DONG Xin,et al.Process optimization and crystallization kinetics of magnesium carbonate trihydrate prepared by impinging stream coprecipitation method[J].China Industrial Economics,2023,24(5):533-545. | |
| 17 | 王丽,孙宝昌,周海军,等.超重力法制备三水碳酸镁晶须[J].北京化工大学学报(自然科学版),2014,41(2):13-18. |
| WANG Li, SUN Baochang, ZHOU Haijun,et al.The preparation of nesquehonite whiskers by high gravity technology[J].Journal of Beijing University of Chemical Technology(Natural Science Edition),2014,41(2):13-18. | |
| 18 | 王余莲,印万忠,张夏翔,等.大长径比三水碳酸镁晶须的制备及晶体生长机理[J].硅酸盐学报,2018,46(7):938-945. |
| WANG Yulian, YIN Wanzhong, ZHANG Xiaxiang,et al.Preparation and growth mechanism of nesquehonite whiskers with large aspect ratio[J].Journal of the Chinese Ceramic Society,2018,46(7):938-945. | |
| 19 | 郭彦彦.三水碳酸镁晶体形貌的调控研究[J].盐科学与化工,2023,52(4):9-13. |
| GUO Yanyan.Study on the regulation of crystal morphology of magnesium carbonate trihydrate [J].Journal of Salt and Chemical Industry,2023,52(4):9-13. | |
| 20 | JIANG Wenjun, HUA Xiao, HAN Qiaofeng,et al.Preparation of lamellar magnesium hydroxide nanoparticles via precipitation method[J].Powder Technology,2009,191(3):227-230. |
| 21 | 张翠钰,方莉,廖洪强,等.三水碳酸镁在氢氧化镁-二氧化碳-水体系中的沉淀结晶[J].无机盐工业,2018,50(6):36- 41. |
| ZHANG Cuiyu, FANG Li, LIAO Hongqiang,et al.Crystallization of MgCO3·3H2O in Mg(OH)2-CO2-H2O system[J].Inorganic Chemicals Industry,2018,50(6):36-41. | |
| 22 | 朱子雯,成怀刚,程丹续,等.基于碳酸化溶液结构研究的盐湖卤水镁锂分离[J].盐湖研究,2023,31(2):55-62. |
| ZHU Ziwen, CHENG Huaigang, CHENG Danxu,et al.The Mg-Li separation of salt lake brine based on the investigation of carbonated solution structure[J].Journal of Salt Lake Research,2023,31(2):55-62. |
| [1] | YANG Minhang, XU Kai, CHENG Huaigang, CHENG Wenting, SONG Huiping. Regular study on effect of impurity ions in brine on morphology of magnesium sulfate crystallized by halogenation-cold precipitation [J]. Inorganic Chemicals Industry, 2025, 57(4): 22-30. |
| [2] | JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun. Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate [J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81. |
| [3] | LI Yunfei, LIU Shuqing, TANG Shengwei, ZHANG Tao. Preparation of α-hemihydrate gypsum in Mg-Na mixed salt solution system under atmospheric pressure [J]. Inorganic Chemicals Industry, 2023, 55(11): 100-106. |
| [4] | LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao. Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92. |
| [5] | WANG Bingjian,QI Daozheng,CHEN Deng. Degradation processes and mechanism of tricalcium aluminate in different sulfate environment [J]. Inorganic Chemicals Industry, 2022, 54(8): 119-124. |
| [6] | YANG Zhuoying,YANG Fan,YI Meigui,XIANG Lan. Rule of hydrothermal hydrolysis of Mg/Al-bearing TiOSO4 solution [J]. Inorganic Chemicals Industry, 2021, 53(12): 113-116. |
| [7] | Hao Jiantang. Study on producing anhydrous hydrogen fluoride by fluorosilicic acid and magnesium oxide and combined production of high quality magnesium sulfate [J]. Inorganic Chemicals Industry, 2019, 51(8): 40-43. |
| [8] | Feng Yali,Liao Shengde,Li Haoran,Wu Hao,Wang Yuan,Wang Junjie,Zhang Gongliang. Research status of magnesium desulphurization and diversified utilization of desulfurization products [J]. Inorganic Chemicals Industry, 2019, 51(3): 1-6. |
| [9] | Zhao Yuxiang1,2,3,Huang Peijin1,Ma Zhenying4,Li Xiao1,2,3,Li Bo1,2,Zou Xingwu1,2,Wang Shuxuan1,2. Preparation of high purity magnesium fluoride by magnesium sulfate composite refining and fluorination [J]. Inorganic Chemicals Industry, 2019, 51(12): 23-25. |
| [10] | GUO Tao, YANG Hong-Jian, HE Zhen-Hui, CAI Xiao-Sen. Research progress in preparation and modification of magnesium oxysulfate whisker [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 1-. |
| [11] | BI Si-Feng, CUI Xiang-Mei. Freezing conversion of brine in Yiliping salt lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 26-. |
| [12] | SHANG Fang-Yu, SU Xiao-Hong, HU Fang. Study on application of magnesium oxide in flue gas desulphurization in alkali sulfide industry [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 50-. |
| [13] | WU Shi-Qin, LI Jun, JIN Yang, MA Yu-Jing, CHEN Xi. Effect of Fe3+, Mg2+, and Al3+ on phase transformation process of CaSO4·2H2O to CaSO4·0.5H2O in CaCl2-HCl-H2O electrolyte solution [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 30-. |
| [14] | XIE Shao-Lei, ZHANG Chao, JI 律, CHEN Gao-Qi, JING Yan. Research on freezing crystalization behavior of magnesium sulfate subtypes brine at low temperature [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 35-. |
| [15] | LI Guo-Yong, CUI Yi-Shun, FU Ling-Jie. Study on preparation conditions of calcium sulphate whiskers from phosphogypsum [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(8): 59-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||