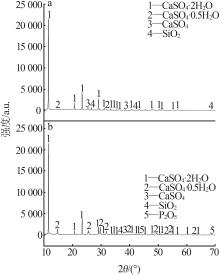
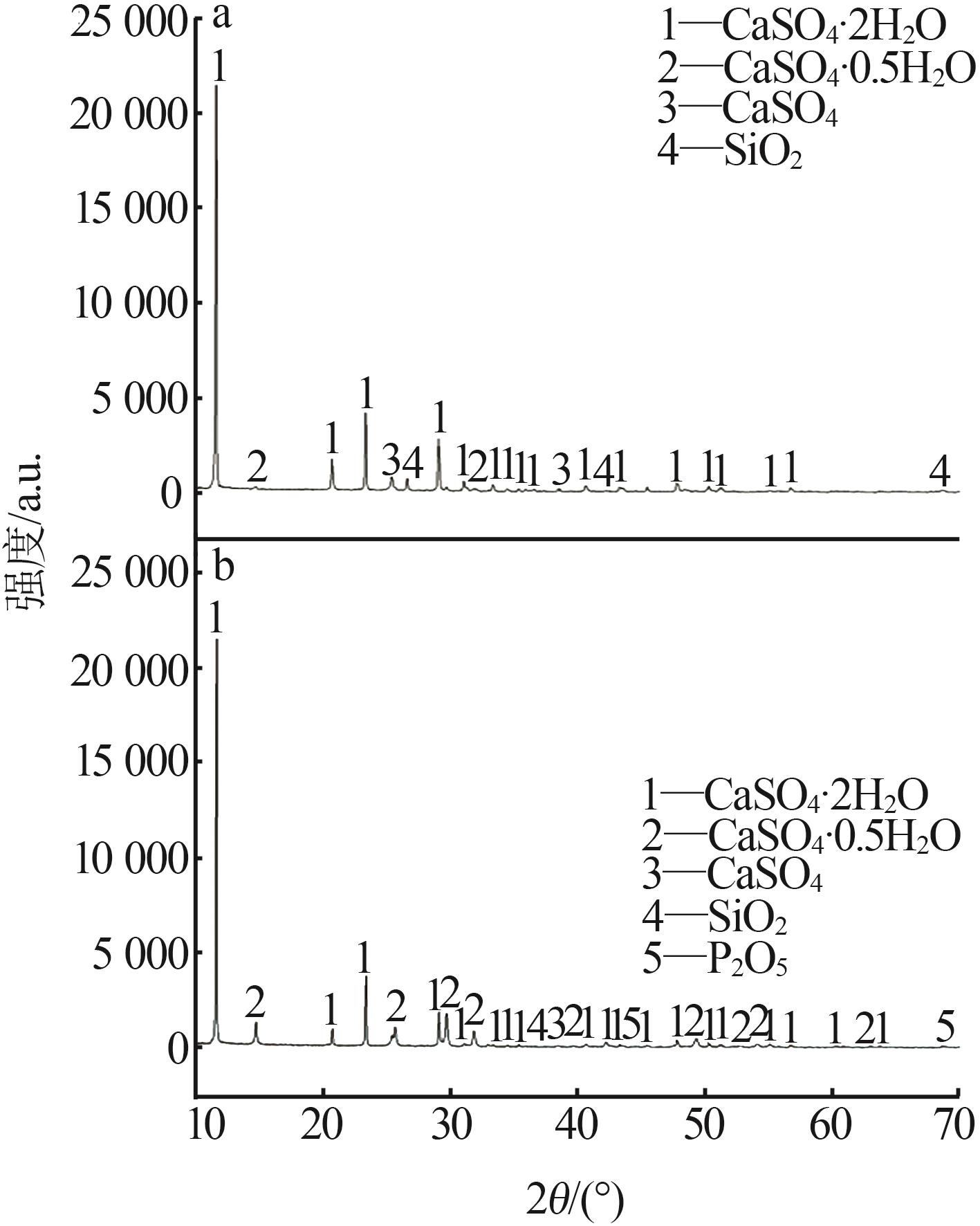
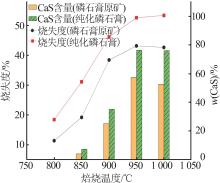
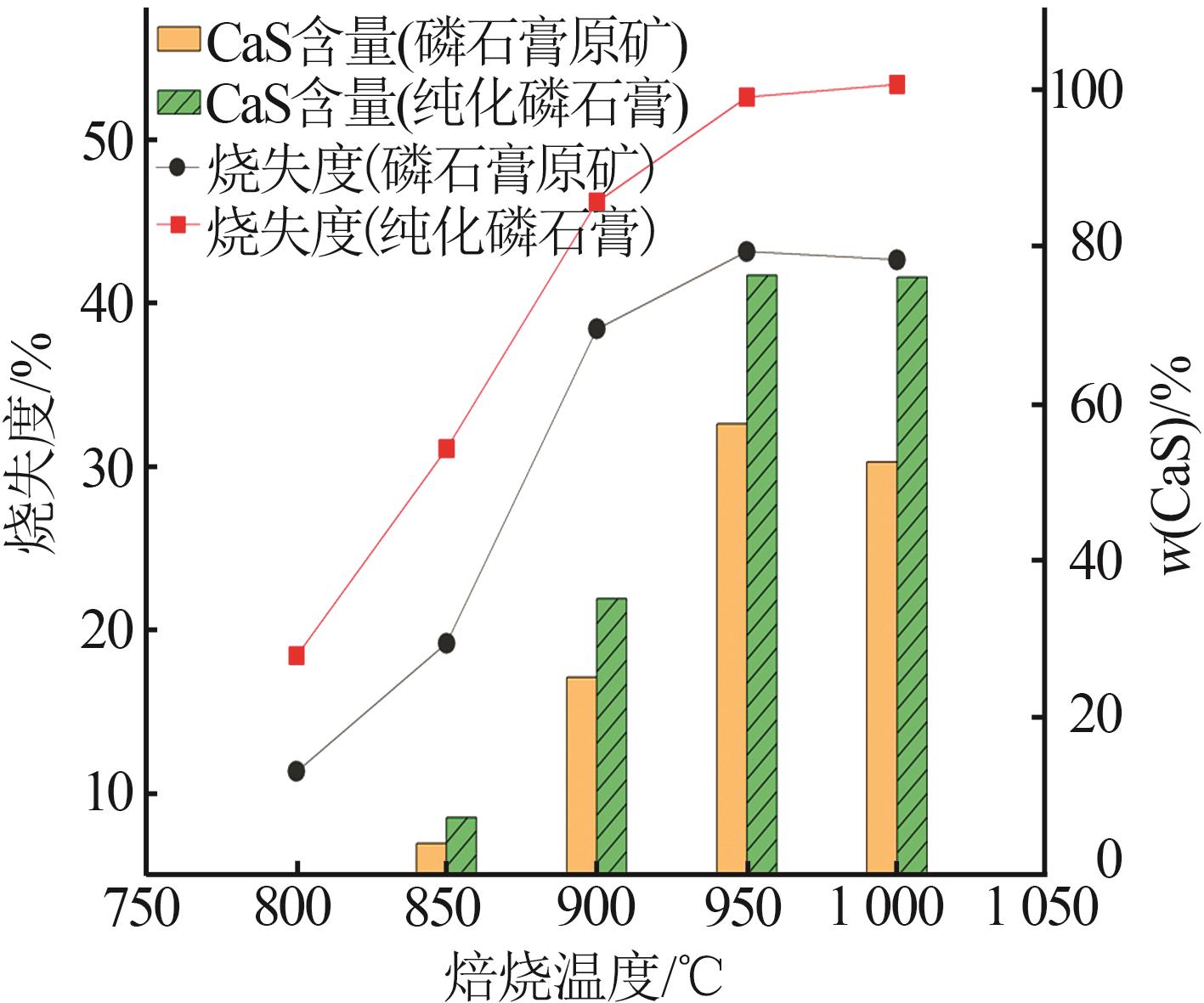
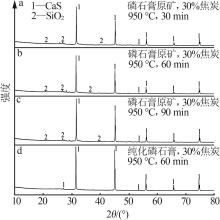
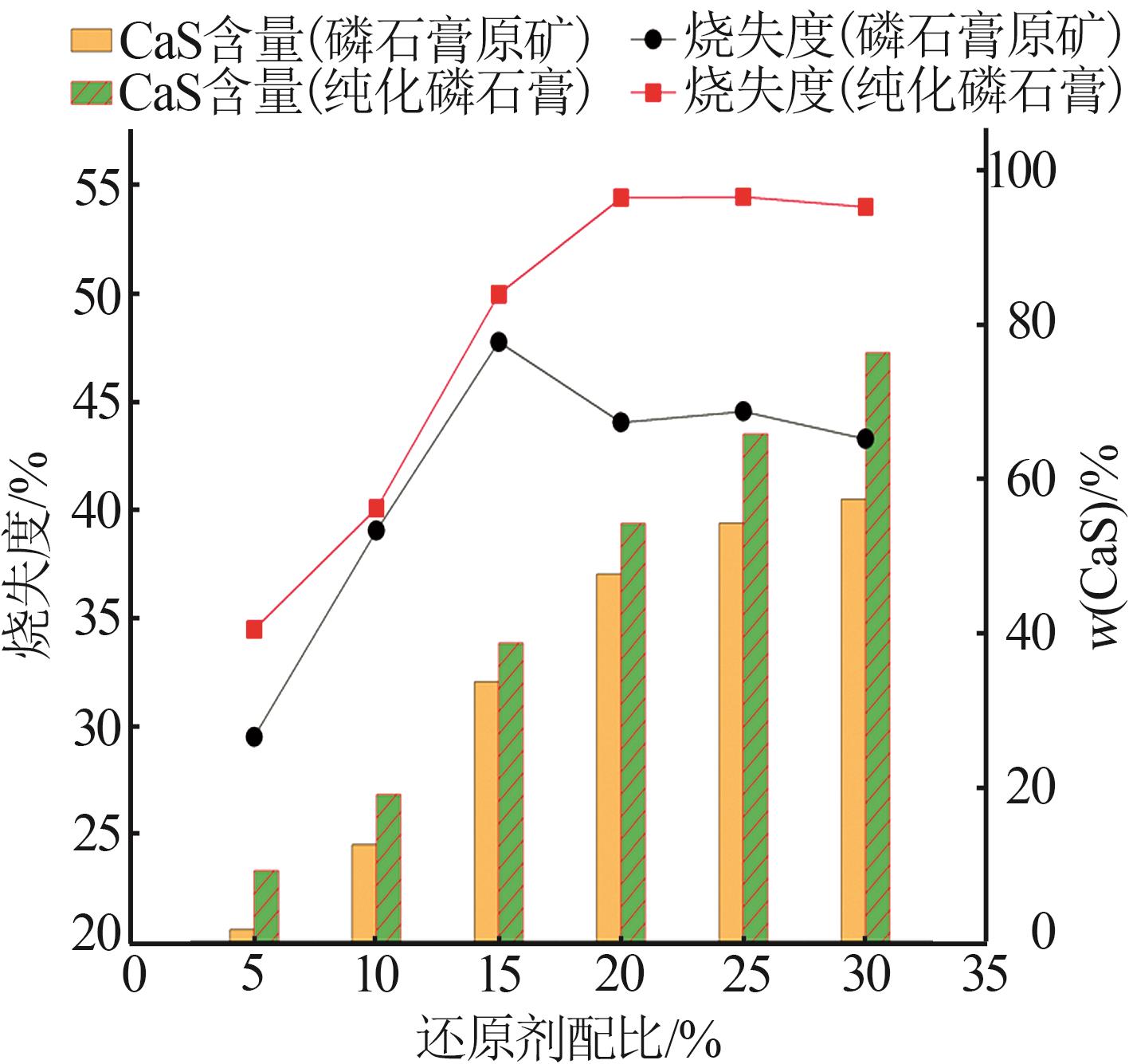
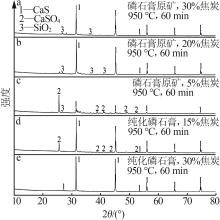
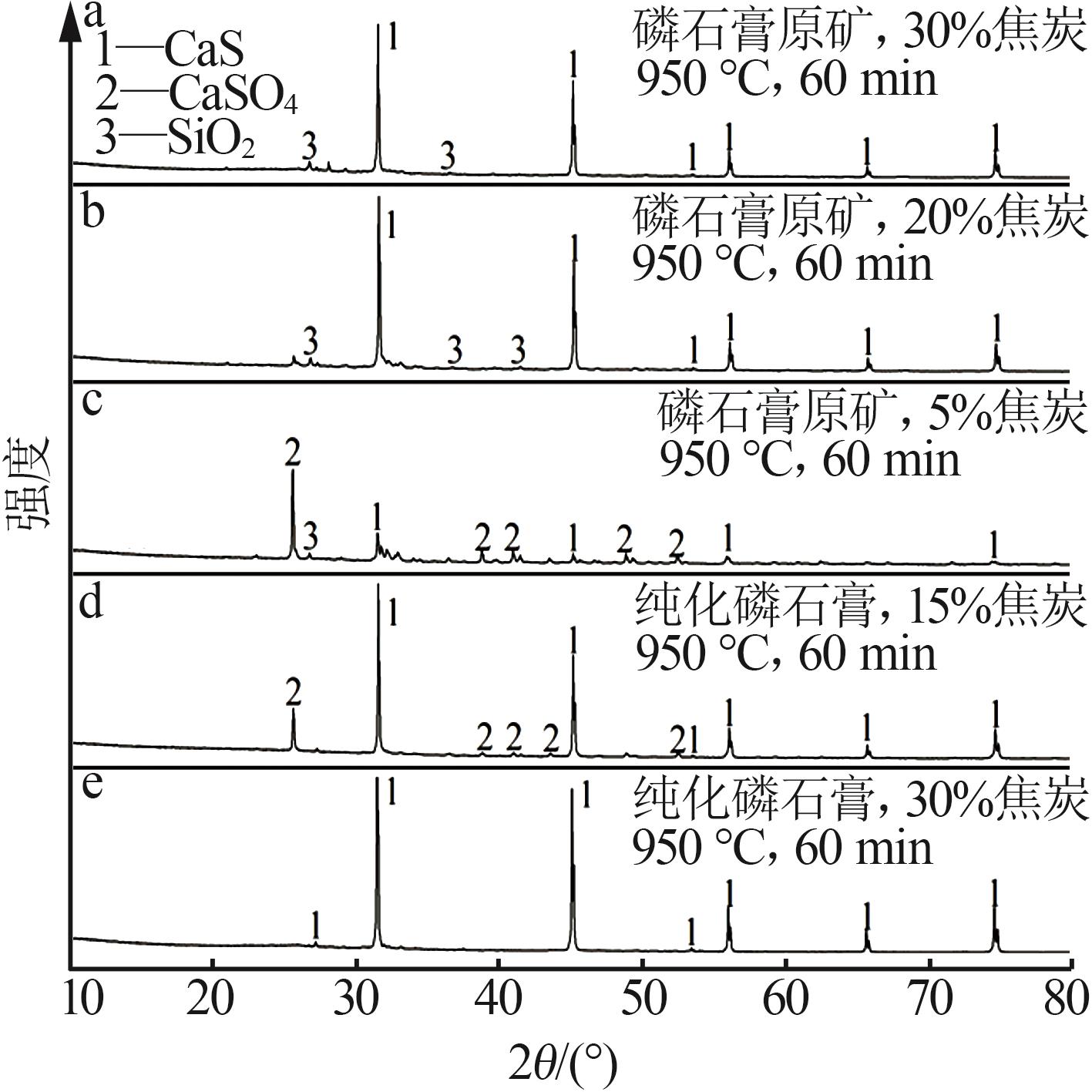
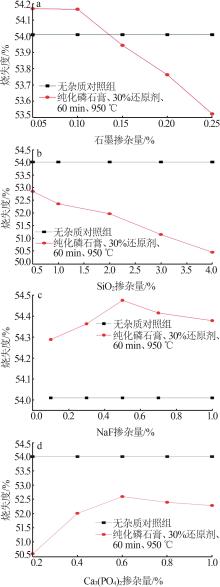
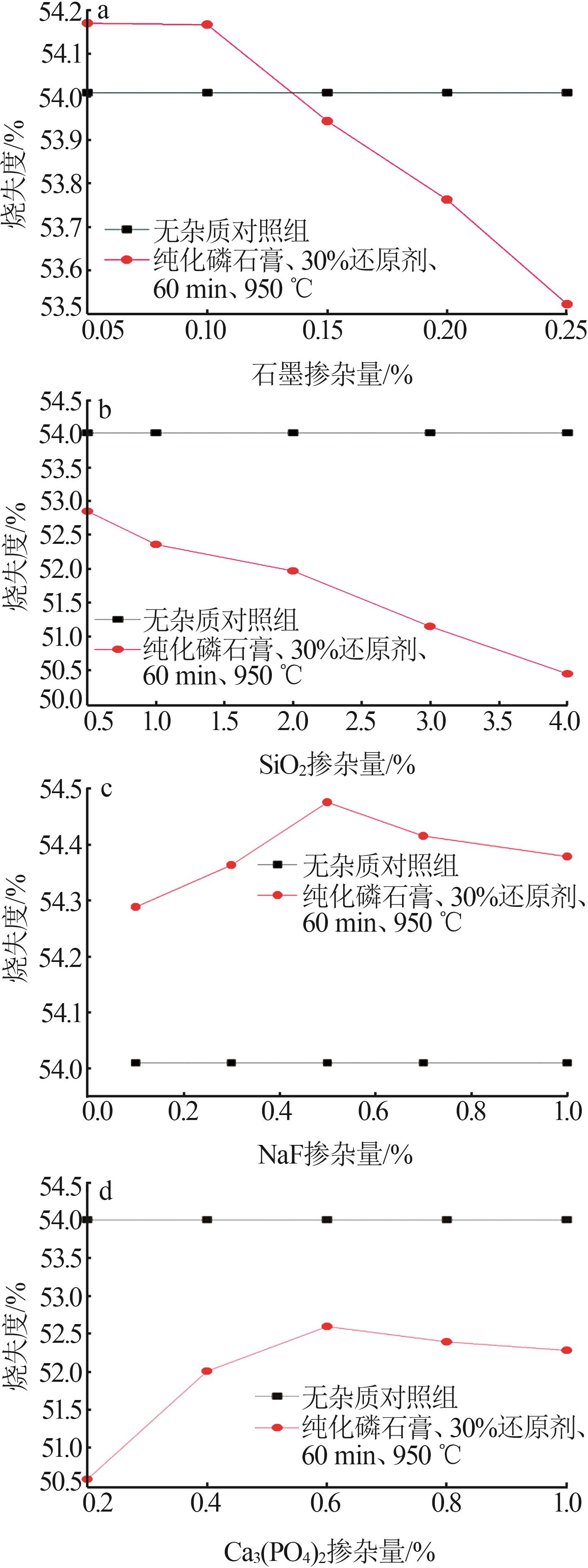
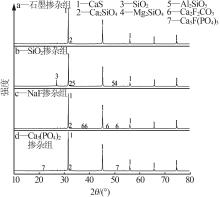
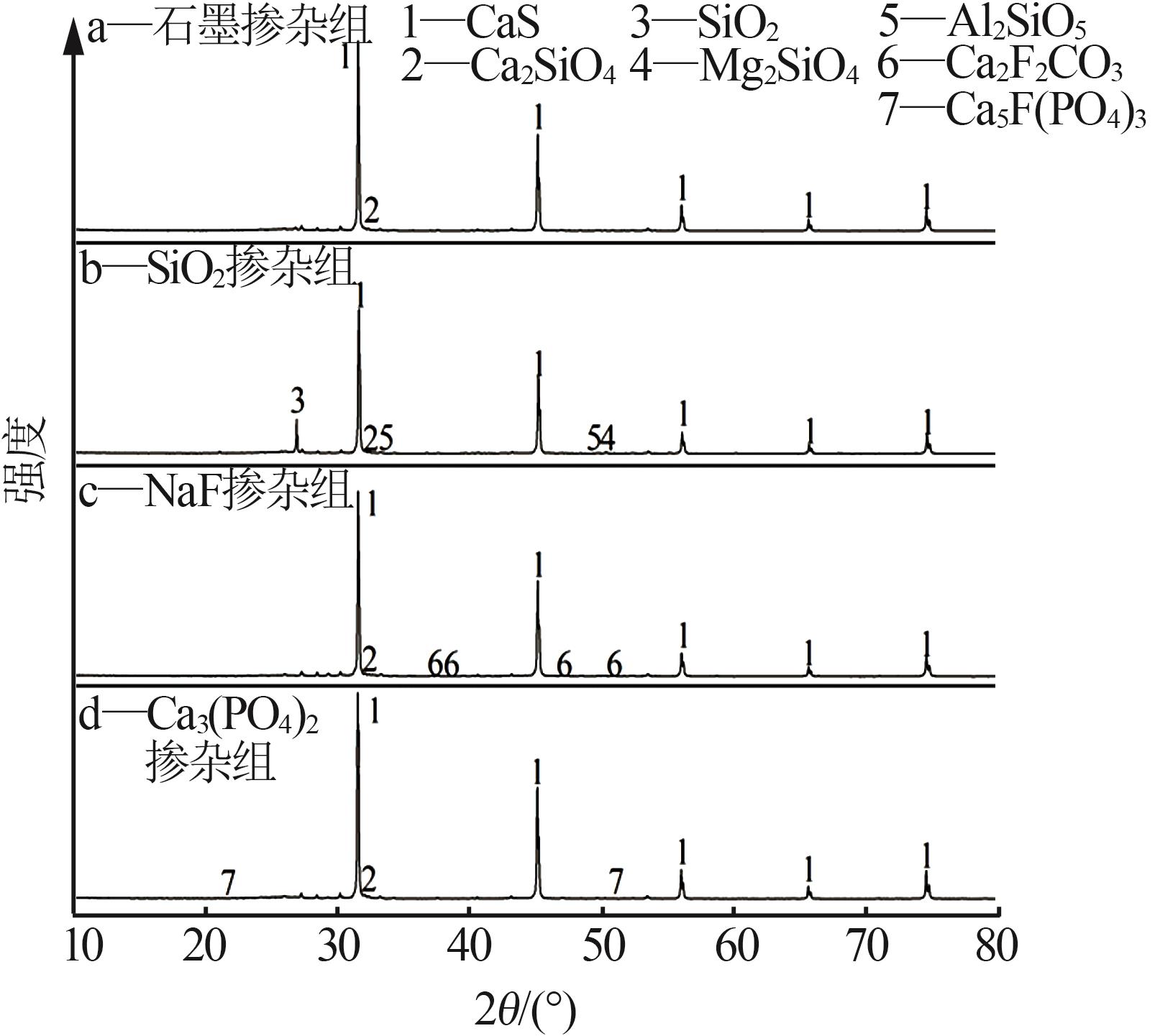
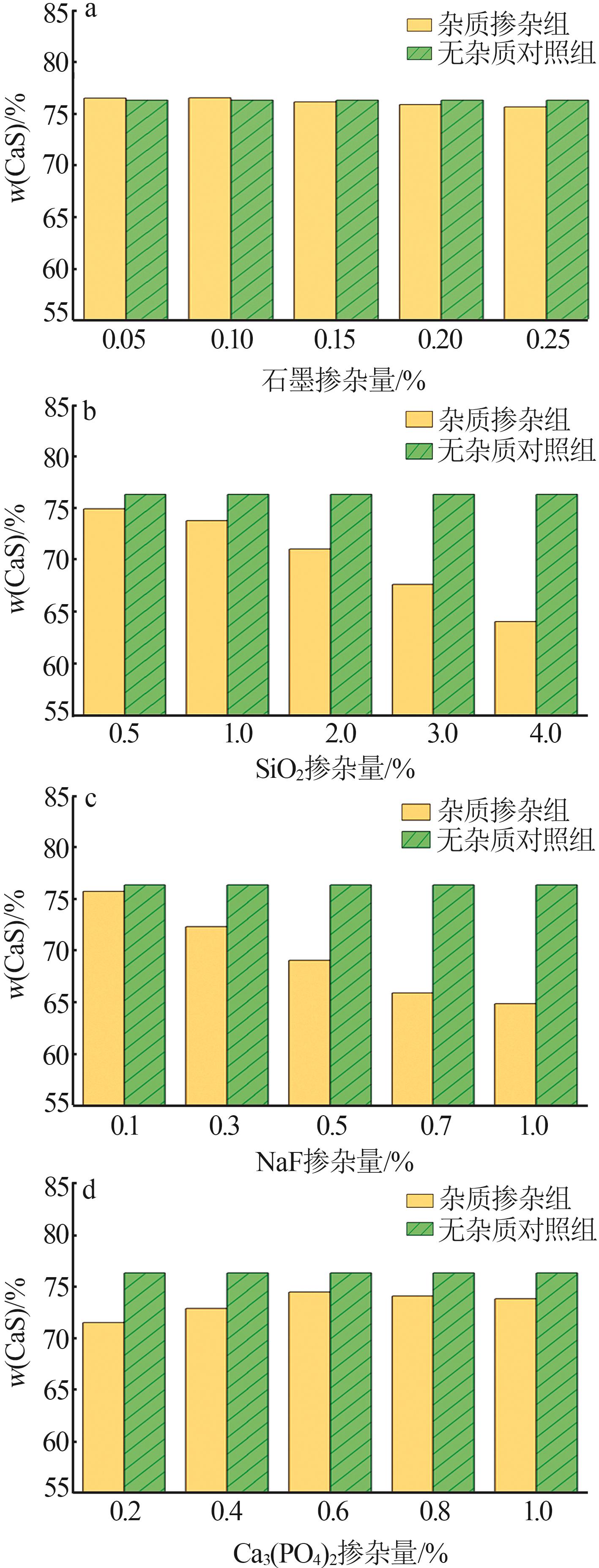
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (7): 88-95.doi: 10.19964/j.issn.1006-4990.2023-0525
• Environment·Health·Safety • Previous Articles Next Articles
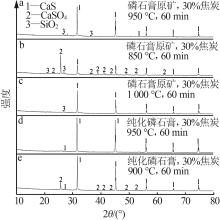
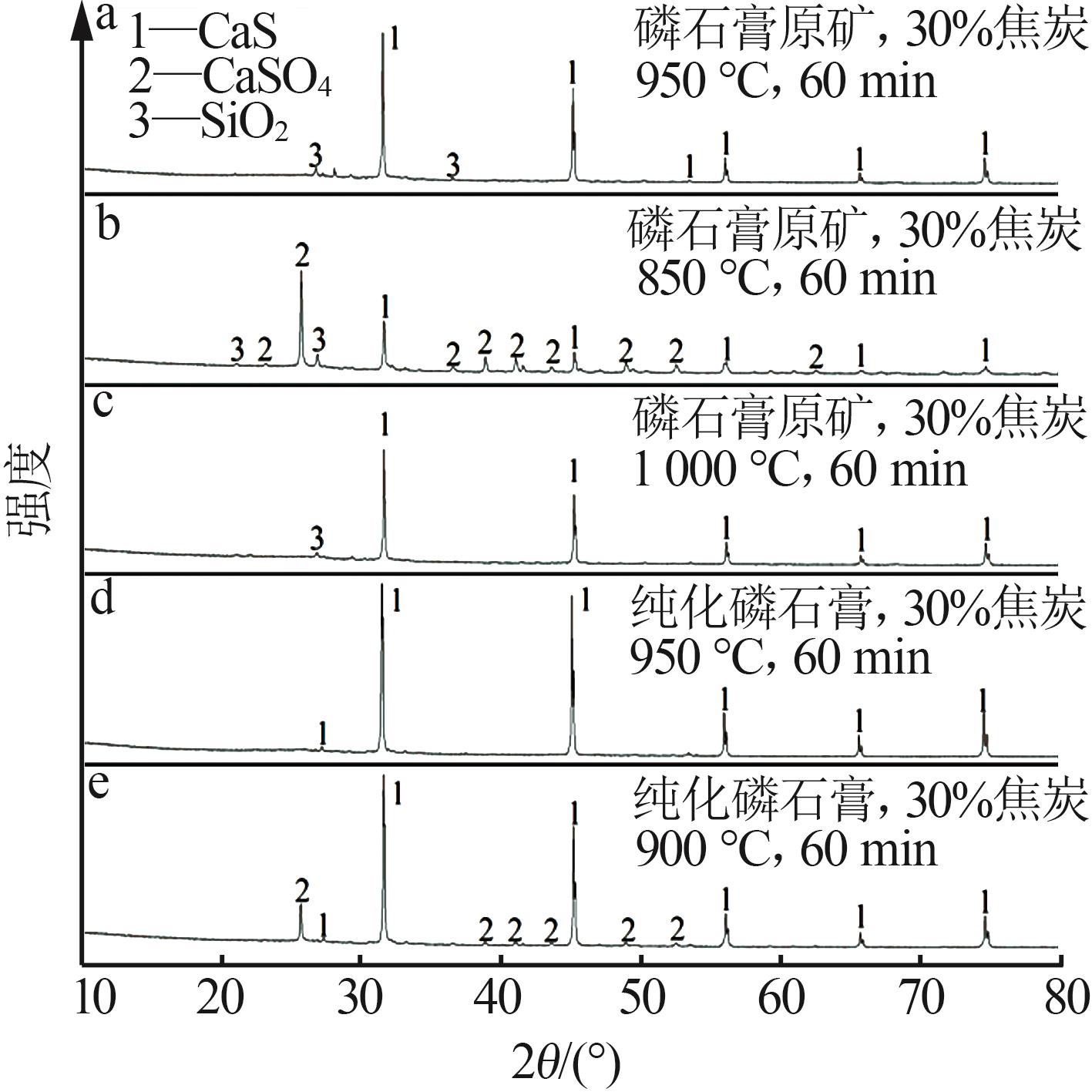
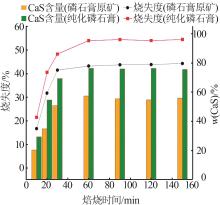
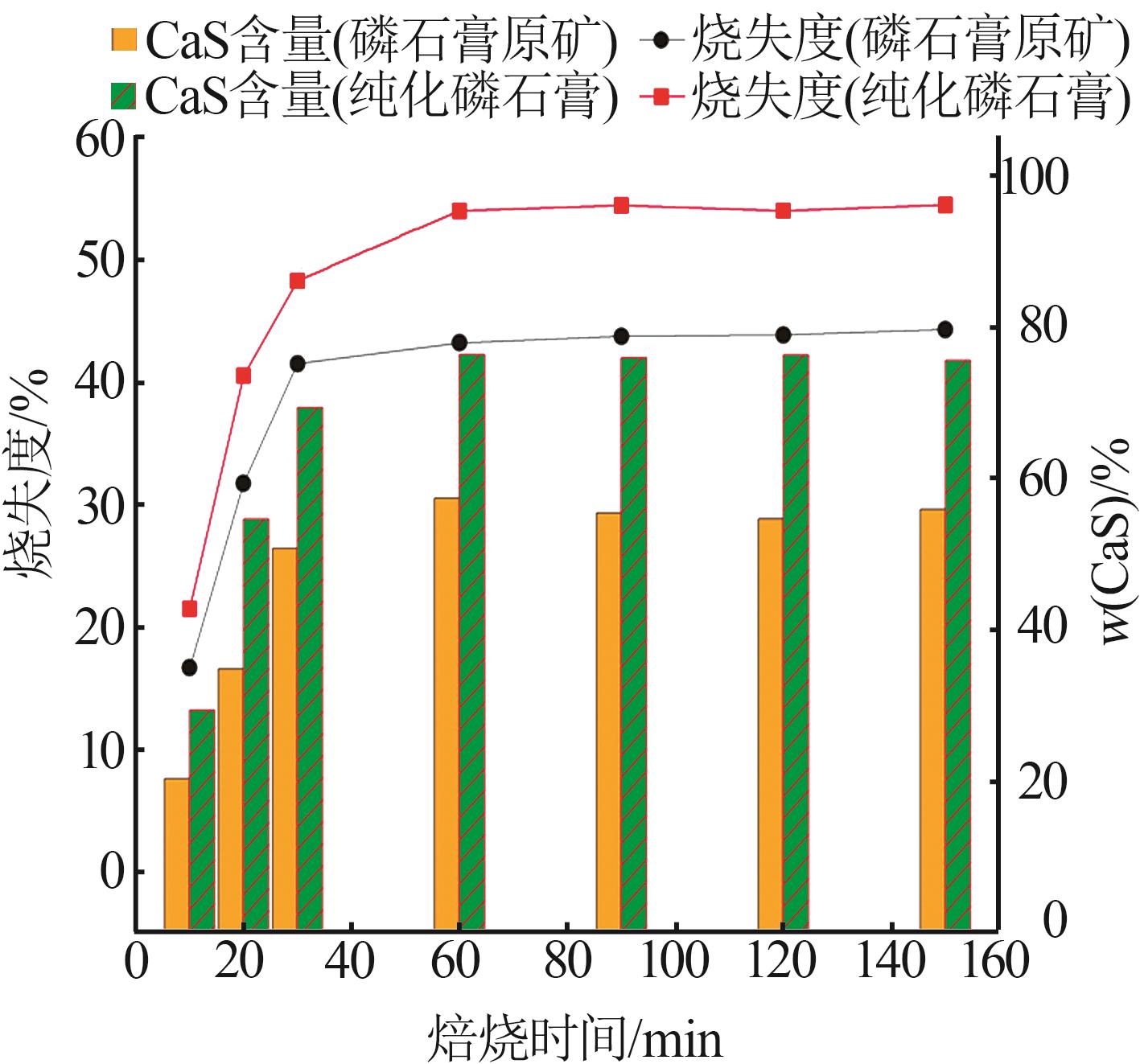
Research on effects of roasting process and typical impurities on reduction and decomposition process of phosphogypsum
HU Dian1( ), GUO Ze2, ZHANG Hanquan2, LU Manman2(
), GUO Ze2, ZHANG Hanquan2, LU Manman2( )
)
- 1.School of Optoelectronic Information and Energy Engineering,School of Mathematics and Physics,Wuhan;Institute of Technology,Wuhan 430205,China
2.School of Resources and Safety Engineering,Wuhan University of Engineering,Wuhan 430205,China
-
Received:2023-11-05Online:2024-07-10Published:2024-08-01 -
Contact:LU Manman E-mail:hthd701@163.com;lummwit@wit.edu.cn
CLC Number:
Cite this article
HU Dian, GUO Ze, ZHANG Hanquan, LU Manman. Research on effects of roasting process and typical impurities on reduction and decomposition process of phosphogypsum[J]. Inorganic Chemicals Industry, 2024, 56(7): 88-95.
share this article
| 1 | 李群峰,沈江平.不同气氛下掺加活性炭对磷石膏分解特性的影响[J].中国新技术新产品,2022(14):64-66. |
| LI Qunfeng, SHEN Jiangping.Effect of activated carbon on decomposition characteristics of phosphogypsum in different atmospher⁃es[J].New Technology & New Products of China,2022(14):64-66. | |
| 2 | 贺雷,朱干宇,郑光明,等.湿法磷酸体系磷石膏结晶过程与机理研究[J].无机盐工业,2022,54(7):110-116. |
| HE Lei, ZHU Ganyu, ZHENG Guangming,et al.Study on crystallization process and mechanism of phosphogypsum in wet process phosphoric acid system[J].Inorganic Chemicals Industry,2022,54(7):110-116. | |
| 3 | 刘琦,敖先权,陈前林,等.磷石膏高温还原分解体系研究进展[J].磷肥与复肥,2021,36(6):25-29. |
| LIU Qi, AO Xianquan, CHEN Qianlin,et al.Progression in high⁃temperature reduction and decomposition system of PG[J].Phosphate & Compound Fertilizer,2021,36(6):25-29. | |
| 4 | 郑绍聪,宁平,汪帆,等.磷石膏热分解制备二氧化硫和氧化钙研究[J].无机盐工业,2013,45(9):45-47. |
| ZHENG Shaocong, NING Ping, WANG Fan,et al.Study on preparation of sulfur dioxide and lime by thermal decomposition of phosphogypsum[J].Inorganic Chemicals Industry,2013,45(9):45- 47. | |
| 5 | 白海丹.我国磷石膏综合利用形势及对策建议[J].磷肥与复肥,2020,35(12):1-3. |
| BAI Haidan.Situation and countermeasures of comprehensive utilization of phosphogypsum in China[J].Phosphate & Compound Fertilizer,2020,35(12):1-3. | |
| 6 | 吴泳霖,张伟,奠波,等.磷石膏热分解研究现状[J].硅酸盐通报,2022,41(9):3129-3137. |
| WU Yonglin, ZHANG Wei, DIAN Bo,et al.Research status of phosphogypsum pyrolysis[J].Bulletin of the Chinese Ceramic Society,2022,41(9):3129-3137. | |
| 7 | 郑绍聪,宁平,成飞翔,等.磷石膏分解制备硫化钙的试验分 析[J].土木建筑与环境工程,2013,35(S2):160-163. |
| ZHENG Shaocong, NING Ping, CHENG Feixiang,et al.Experimental analysis on preparation of calcium sulfide by decomposition of phosphogypsum[J].Journal of Civil and Environmental Engineering,2013,35(S2):160-163. | |
| 8 | 董泽,翟延波,任志威,等.磷石膏建材资源化利用研究进 展[J].无机盐工业,2022,54(4):5-9. |
| DONG Ze, ZHAI Yanbo, REN Zhiwei,et al.Research progress on phosphogypsum utilization in building materials[J].Inorganic Che⁃Industry micals,2022,54(4):5-9. | |
| 9 | 资泽城.磷石膏还原分解过程中杂质的影响及其迁移变化研究[D].昆明:昆明理工大学,2014. |
| ZI Zecheng.Study on the influence of impurities and their migration during the reduction and decomposition of phosphogyps⁃um[D].Kunming:Kunming University of Science and Technology,2014. | |
| 10 | 李恒,郭旭东,钟晋,等.磷石膏杂质及净化研究现状[J].磷肥与复肥,2022,37(5):22-26. |
| LI Heng, GUO Xudong, ZHONG Jin,et al.Research status of phosphogypsum impurities and purification[J].Phosphate & Fertilizer Compound,2022,37(5):22-26. | |
| 11 | 宋小霞,唐绍林,季强,等.磷石膏杂质的处理方法研究[J].中国环保产业,2018(10):68-70. |
| SONG Xiaoxia, TANG Shaolin, JI Qiang,et al.Research progress on treatment of phosphorous gypsum impurity[J].China Environmental Protection Industry,2018(10):68-70. | |
| 12 | 刘林程,左海滨,许志强.工业石膏的资源化利用途径与展望[J].无机盐工业,2021,53(10):1-9. |
| LIU Lincheng, ZUO Haibin, XU Zhiqiang.Resource utilization approach of industrial gypsum and its prospect[J].Inorganic Chemicals Industry,2021,53(10):1-9. | |
| 13 | LAASRI F, GARCIA A C, LATIFI M,et al.Reaction mechanism of thermal decomposition of phosphogypsum[J].Waste Management,2023,171:482-490. |
| 14 | MA Liping, DU Yalei, NIU Xuekui,et al.Thermal and kinetic analysis of the process of thermochemical decomposition of phosphogypsum with CO and additives[J].Industrial & Engineering Chemistry Research,2012,51:6680-6685. |
| 15 | 王艳语,谷守玉,侯翠红,等.磷石膏配料碳热熔融还原硫逸出及熔渣物相分析[J].无机盐工业,2024,56(2):86-94. |
| WANG Yanyu, GU Shouyu, HOU Cuihong,et al.Sulfur escape and slag physical phase analysis by carbon thermal reduction melting based on phosphogypsum ingredients[J].Inorganic Che⁃Industry micals,2024,56(2):86-94. | |
| 16 | 谢容生,刘树根,宁平,等.CaF2作用下磷石膏还原分解过程的动力学特性[J].硅酸盐通报,2016,35(8):2604-2610. |
| XIE Rongsheng, LIU Shugen, NING Ping,et al.Kinetic characteristics of phosphogypsum reductive decomposition with mineralization agent CaF2 [J].Bulletin of the Chinese Ceramic Society,2016,35(8):2604-2610. | |
| 17 | 张磊,李蒙,向文国,等.磷石膏循环流化床煅烧分解工艺可行性分析[J].无机盐工业,2023,55(6):85-91. |
| ZHANG Lei, LI Meng, XIANG Wenguo,et al.Feasibility analysis of calcination and decomposition process of phosphogypsum in circulating fluidized bed[J].Inorganic Chemicals Industry,2023,55(6):85-91. | |
| 18 | 朱凯,解桂林,陈昭睿,等.磷石膏在一氧化碳气氛下的还原反应特性[J].硅酸盐学报,2013,41(11):1540-1545. |
| ZHU Kai, XIE Guilin, CHEN Zhaorui,et al.Reaction characteristics of phosphogypsum under carbon monoxide atmosphere[J].Journal of the Chinese Ceramic Society,2013,41(11):1540-1545. | |
| 19 | 徐悦,林强,李建锡,等.磷石膏炭热分解的热动力学研究[J].硅酸盐通报,2017,36(3):839-845. |
| XU Yue, LIN Qiang, LI Jianxi,et al.Thermal and kinetic study on decomposition of phosphogypsum by carbon[J].Bulletin of the Chinese Ceramic Society,2017,36(3):839-845. |
| [1] | LI Yongxiang, LIU Chenxi, LI Yundong, MA Hang, MEI Lianping, DANG Hui, SUN Zhi, WAN Banglong. Research progress of preparation technology of black phosphorus [J]. Inorganic Chemicals Industry, 2025, 57(3): 18-29. |
| [2] | LI Chao, WANG Liping, GAO Guimei, ZHANG Yunfeng, HONG Yu, LIU Darui, XU Lijun, CUI Yongjie. Study on reaction mechanism of acid leaching lithium from circulating fluidized bed fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 101-107. |
| [3] | LENG Manxi, ZHU Yu, YAN Jikang, XIE Ke, ZOU Yongjie. Study on crystallization characteristics and mineral flotation performance of industrial waste phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(3): 108-115. |
| [4] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [5] | TAN Shanyi, WEN Huizi, HE Shuyu, ZHANG Liwen, CHEN Shaohua, XI Benjun. Study on leaching behavior and kinetics of phosphorus from phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(2): 105-112. |
| [6] | TANG Kaijing, LIU Chuanbei, LI Yingding, JIANG Yong, WU Junnan, ZHANG Tao. Research on preparation and mechanism of superhydrophobic phosphogypsum products [J]. Inorganic Chemicals Industry, 2025, 57(1): 97-102. |
| [7] | CHEN Xue, OUYANG Quansheng, SHAO Jiaojing. Recent research progress of lithium-sulfur batteries based on solid-solid reaction mechanism [J]. Inorganic Chemicals Industry, 2024, 56(9): 12-23. |
| [8] | FANG Fan, YAO Benlin, XIAO Yiqun, JIA Yanhong, CHEN Hui, LI Bin, HE Hui. Research progress on dissolution behavior and mechanism of uranium dioxide in nitric acid [J]. Inorganic Chemicals Industry, 2024, 56(9): 34-43. |
| [9] | TANG Xuemei, WANG Meibo, XU Li, ZHANG Xujie, TAI Shijun, YI Xianmei, LIU Hongjuan, PAN Linyi. Study on whitening of phosphogypsum by low⁃temperature calcination [J]. Inorganic Chemicals Industry, 2024, 56(8): 110-115. |
| [10] | LI Zhao, YIN Youyou, LIU Chenhui, WANG Fang, GAO Jiyun. Study on preparation of two⁃dimensional titanium carbide/zinc oxide nanoparticles and their ethanol gas sensitive properties [J]. Inorganic Chemicals Industry, 2024, 56(8): 33-39. |
| [11] | SUN Lan, CHEN Shiying, YANG Liuxu, NIU Yiming, ZHAO Aonan. Basic study on fixing soluble phosphorus in phosphogypsum with magnesium slag [J]. Inorganic Chemicals Industry, 2024, 56(8): 92-98. |
| [12] | HU Cheng, LIU Meng, XIANG Weiheng, CHEN Ping, WANG Neng, LU Guanju, ZHOU Jinlan. Preparation of α-hemihydrous gypsum from CaCl2 and MgCl2 and their composite solution [J]. Inorganic Chemicals Industry, 2024, 56(7): 112-117. |
| [13] | YE Fen, CHENG Hao, XIANG Yuan, LIU Song, SHI Wei. Preparation of ceramic aggregate from electrolytic manganese slag and its application in concrete [J]. Inorganic Chemicals Industry, 2024, 56(6): 127-132. |
| [14] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [15] | TU Yanping, BAI Dengxian, CHENG Shukai, XIE Junjie, HUANG Zhiliang, CHEN Guofu. Effect of high temperature modification of mineral powder and quicklime on properties of phosphogypsum cement based materials [J]. Inorganic Chemicals Industry, 2024, 56(6): 94-101. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||