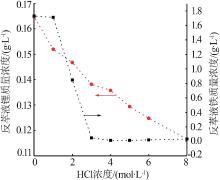
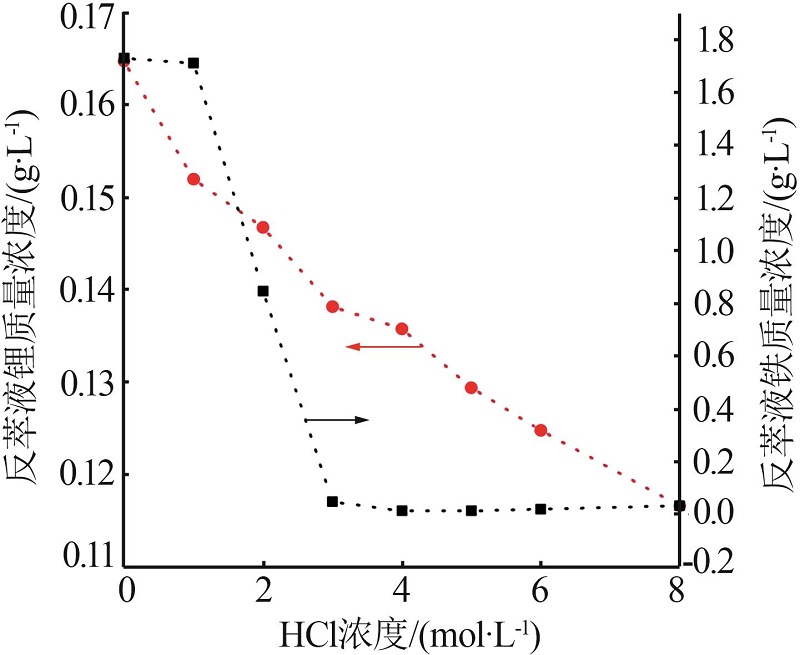
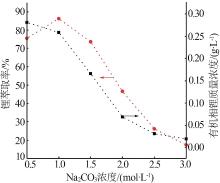
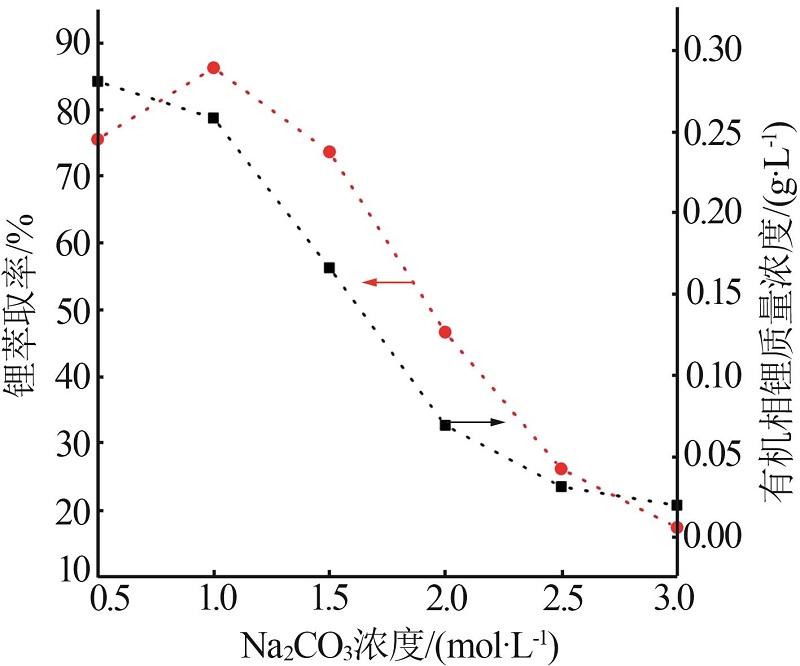
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (10): 63-69.doi: 10.19964/j.issn.1006-4990.2022-0713
• Research & Development • Previous Articles Next Articles
Study on extraction of lithium from low grade high clay leaching solution by solvent extraction
LI Huifang( ), WANG Xiao, BAI Youpeng, ZHANG Shixiang
), WANG Xiao, BAI Youpeng, ZHANG Shixiang
- School of Chemical Engineering,Qinghai University,Xining 810016,China
-
Received:2022-12-05Online:2023-10-10Published:2023-10-16
CLC Number:
Cite this article
LI Huifang, WANG Xiao, BAI Youpeng, ZHANG Shixiang. Study on extraction of lithium from low grade high clay leaching solution by solvent extraction[J]. Inorganic Chemicals Industry, 2023, 55(10): 63-69.
share this article
| 1 | SWAIN B.Recovery and recycling of lithium:A review[J].Separation and Purification Technology,2017,172:388-403. |
| 2 | MESHRAM P, PANDEY B D, MANKHAND T R.Extraction of lithium from primary and secondary sources by pre-treatment,leac-hing and separation:A comprehensive review[J].Hydrometallurgy,2014,150:192-208. |
| 3 | ZHANG Xiufeng, TAN Xiumin, LI Chun,et al.Energy-efficient and simultaneous extraction of lithium,rubidium and cesium from lepidolite concentrate via sulfuric acid baking and water leaching[J].Hydrometallurgy,2019,185:244-249. |
| 4 | STERBA J, KRZEMIEŃ A, RIESGO FERNÁNDEZ P,et al.Lithium mining:Accelerating the transition to sustainable energy[J].Resources Policy,2019,62:416-426. |
| 5 | 乜贞,卜令忠,郑绵平.中国盐湖锂资源的产业化现状:以西台吉乃尔盐湖和扎布耶盐湖为例[J].地球学报,2010,31(1):95-101. |
| NIE Zhen, BU Lingzhong, ZHENG Mianping.Lithium resources industrialization of salt lakes in China:A case study of the Xitaijinaier salt lake and the Zabuye salt lake[J].Acta Geoscientica Sinica,2010,31(1):95-101. | |
| 6 | 贾旭宏,李丽娟,曾忠民,等.磷酸三丁酯萃取体系从盐湖卤水提取锂[J].无机盐工业,2011,43(8):29-32. |
| JIA Xuhong, LI Lijuan, ZENG Zhongmin,et al.Extracting lithium from salt lake brines by TBP extraction system[J].Inorganic Chemicals Industry,2011,43(8):29-32. | |
| 7 | MARTIN G, SCHNEIDER A, VOIGT W,et al.Lithium extraction from the mineral zinnwaldite:Part Ⅱ:Lithium carbonate recovery by direct carbonation of sintered zinnwaldite concentrate[J].Minerals Engineering,2017,110:75-81. |
| 8 | BARBOSA L I, VALENTE G, OROSCO R P,et al.Lithium extraction from β-spodumene through chlorination with chlorine gas[J].Minerals Engineering,2014,56:29-34. |
| 9 | VIECELI N, NOGUEIRA C A, PEREIRA M F C,et al.Effects of mechanical activation on lithium extraction from a lepidolite ore concentrate[J].Minerals Engineering,2017,102:1-14. |
| 10 | ZHANG Ye, HU Yuehua, WANG Li,et al.Systematic review of lithium extraction from salt-lake brines via precipitation approaches[J].Minerals Engineering,2019,139:105868. |
| 11 | HE Lihua, XU Wenhua, SONG Yunfeng,et al.Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation[J].Separation and Purification Technology,2017,187:214-220. |
| 12 | XIONG Jiachun, HE Lihua, ZHAO Zhongwei.Lithium extraction from high-sodium raw brine with Li0.3FePO4 electrode[J].Desalination,2022,535:115822. |
| 13 | LIU Dongfu, LI Zheng, HE Lihua,et al.Facet engineered Li3PO4 for lithium recovery from brines[J].Desalination,2021,514:115186. |
| 14 | LI Hongwei, WANG Ying, LI Tingyu,et al.Nanofiltration membrane with crown ether as exclusive Li+ transport channels achiev-ing efficient extraction of lithium from salt lake brine[J].Chemical Engineering Journal,2022,438:135658. |
| 15 | ZANTE G, BOLTOEVA M, MASMOUDI A,et al.Lithium extraction from complex aqueous solutions using supported ionic liquid membranes[J].Journal of Membrane Science,2019,580:62-76. |
| 16 | XIONG Yanhang, GE Tao, XU Liang,et al.A fundamental study on selective extraction of Li+ with dibenzo-14-crown-4 ether:Toward new technology development for lithium recovery from brines[J].Journal of Environmental Management,2022,310:114705. |
| 17 | SU Hui, TAN Boren, ZHANG Jian,et al.Modelling of lithium extraction with TBP/P507-FeCl3 system from salt-lake brine[J].Separation and Purification Technology,2022,282:120110. |
| 18 | LI Huifang, LI Lijuan, LI Wu.Lithium extraction from aqueous solution using different metal chloride as co-extraction reagent[J].Chemical Physics Letters,2020,754:137675. |
| 19 | LI Huifang, LI Lijuan, LI Wu,et al.The key factors and mechanism study on lithium extraction by TBP-FeCl3 extraction system[J].Chemical Physics Letters,2020,754:137740. |
| 20 | LI Huifang, LI Lijuan, JI Lianmin,et al.The extraction ability and mechanism in extraction lithium by several organic extractants[J].Chemical Physics Letters,2019,733:136668. |
| 21 | ZHOU Zhiyong, FAN Jiahui, LIU Xueting,et al.Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent[J].Hydrometallurgy,2020,191:105244. |
| 22 | YU Xiaoping, FAN Xuebing, GUO Yafei,et al.Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate[J].Separation and Purification Technology,2019,211:790-798. |
| 23 | 时东,李丽娟,宋富根,等.N523-TBP混合萃取体系从盐湖卤水中萃取锂的机理研究[J].盐湖研究,2017,25(1):57- 63. |
| SHI Dong, LI Lijuan, SONG Fugen,et al.Mechanism study of extracting lithium from brine with N523-TBP mixed extraction system[J].Journal of Salt Lake Research,2017,25(1):57-63. |
| [1] | LI Chao, WANG Liping, GAO Guimei, ZHANG Yunfeng, HONG Yu, LIU Darui, XU Lijun, CUI Yongjie. Study on reaction mechanism of acid leaching lithium from circulating fluidized bed fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 101-107. |
| [2] | KONG Lingjie, LI Guangbi, XIE Jiahao, YANG Xinhui, BAI Xiaoqin. Research progress on lithium extraction technology from salt lake brine [J]. Inorganic Chemicals Industry, 2025, 57(1): 14-26. |
| [3] | ZHOU Wanji, LI Sixia. Study on extraction lithium from brine with high magnesium using ionic liquid/metal salt extraction system [J]. Inorganic Chemicals Industry, 2024, 56(9): 54-59. |
| [4] | FU Yu, ZHANG Boshuang, YANG Jianmao, LIU Jianyun. Research progress of lithium manganese oxide materials in electrochemical lithium extraction applications [J]. Inorganic Chemicals Industry, 2024, 56(12): 62-69. |
| [5] | CHEN Haixia, YAN Hong, SUN Yunlong, MA Guoqiang. Research progress of lithium resource extraction technology [J]. Inorganic Chemicals Industry, 2024, 56(1): 9-22. |
| [6] | FU Yu, DENG Mi, HUANG Donggen, WAN Jinbao. Research progress of lithium extraction technology from salt lake brine [J]. Inorganic Chemicals Industry, 2023, 55(9): 9-16. |
| [7] | ZHOU Qinglie, WANG Baoqi, ZHANG Zhiye, ZHANG Yinghu, WANG Jian, YANG Lin. Development of new process of removing metal cations from wet-process phosphoric acid by extraction [J]. Inorganic Chemicals Industry, 2023, 55(3): 84-91. |
| [8] | ZHU Ruisong, CAO Jing, LIU Taoran, LI Yingwen, GAO Fei, HU Xuesheng. Research progress of lithium extraction technology and industrialization of unconventional brines in global [J]. Inorganic Chemicals Industry, 2023, 55(11): 1-11. |
| [9] | HU Yue, MO Hengliang, LI Tianyu, PENG Wenjuan, SUN Guangdong, XIAO Hongkang, CHEN Yili, LI Suoding, TANG Yang. Study on spinning molding and performance of Ti-based lithium ion sieve powder and PVDF resin [J]. Inorganic Chemicals Industry, 2023, 55(11): 58-63. |
| [10] | JI Ying, ZHANG Ying, HOU Xuechao, ZHU Xiaofeng, JIANG Run, PENG Wenjuan, SUN Guangdong, LÜ Long. Application research of titanium adsorbent of carbonate-type salt lake in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 70-77. |
| [11] | LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao. Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92. |
| [12] | LIN Yuqing,ZHANG Yiren,QIU Yulong,ZHANG Jiayu,YU Jianguo. Progress and prospect of membrane technology in lithium extraction from salt lake brine [J]. Inorganic Chemicals Industry, 2023, 55(1): 33-45. |
| [13] | WANG Ping. Quantitative analysis of linkage between lithium supply and demand and lithium price [J]. Inorganic Chemicals Industry, 2022, 54(9): 1-13. |
| [14] | ZHAO Zhanyi,ZHONG Yongheng,LIU Jia,LI Xiaoyan,YONG Meijing. Research on global patent distribution and development countermeasures of lithium industry [J]. Inorganic Chemicals Industry, 2022, 54(7): 10-17. |
| [15] | ZHANG Liyuan,SHEN Ruqian,YANG Jinju,LI Yan,SHUI Yi,SU Min. Research progress on lithium ion sieves [J]. Inorganic Chemicals Industry, 2022, 54(5): 28-37. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||