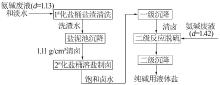
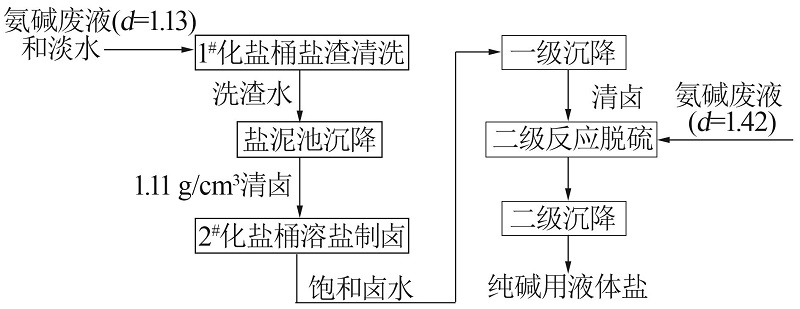
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (10): 46-52.doi: 10.19964/j.issn.1006-4990.2022-0183
• Development and utilization of salt lake resources • Previous Articles Next Articles
Study on pilot scale process of producing liquid salt from Jilantai salt lake tailings
SUN Zhicheng1( ),DU Wei1,WANG Runpu1,CHENG Penggao1,ZHANG Rong2,GAO Weixing2,WANG Zhanhe2,HU Kaibao2,TANG Na1(
),DU Wei1,WANG Runpu1,CHENG Penggao1,ZHANG Rong2,GAO Weixing2,WANG Zhanhe2,HU Kaibao2,TANG Na1( )
)
- 1. College of Chemical Engineering and Materials Science,Tianjin University of Science & Technology,Tianjin 300457,China
2. China Salt Inner Mongolia Chemical Co.,Ltd.
-
Received:2022-04-09Online:2022-10-10Published:2022-11-03 -
Contact:TANG Na E-mail:13132213521@163.com;tjtangna@tust.cn
CLC Number:
Cite this article
SUN Zhicheng,DU Wei,WANG Runpu,CHENG Penggao,ZHANG Rong,GAO Weixing,WANG Zhanhe,HU Kaibao,TANG Na. Study on pilot scale process of producing liquid salt from Jilantai salt lake tailings[J]. Inorganic Chemicals Industry, 2022, 54(10): 46-52.
share this article
Table 3
Composition of clarified brine after salt residuewashing under different proportions of ammonia alkaliwaste liquid and fresh water"
实验 编号 | V(氨碱废液)∶ V(淡水) | 液相离子质量浓度/(g?L-1) | 相对 密度 | ||||
|---|---|---|---|---|---|---|---|
| Cl- | Ca2+ | Mg2+ | SO42- | NaCl | |||
| 1 | 1∶3 | 113.30 | 10.21 | 0.12 | 1.32 | 158.01 | 1.11 |
| 2 | 1∶3 | 119.32 | 10.62 | 0.06 | 0.83 | 196.70 | 1.12 |
| 3 | 1∶3 | 118.45 | 8.80 | 0.17 | 1.08 | 170.08 | 1.11 |
| 4 | 1∶4 | 126.06 | 7.12 | 0.12 | 1.81 | 133.60 | 1.12 |
| 5 | 1∶4 | 105.41 | 7.80 | 0.10 | 3.77 | 173.77 | 1.12 |
| 6 | 1∶4 | 110.72 | 7.05 | 0.23 | 3.51 | 182.53 | 1.12 |
| 7 | 1∶7 | 123.45 | 3.84 | 0.09 | 1.52 | 193.74 | 1.13 |
| 8 | 1∶7 | 105.50 | 3.63 | 0.04 | 1.86 | 173.92 | 1.11 |
| 9 | 1∶7 | 89.98 | 4.06 | 0.07 | 1.81 | 138.32 | 1.10 |
| 10 | 1∶9 | 140.40 | 3.06 | 0.10 | 3.57 | 231.36 | 1.12 |
| 11 | 1∶9 | 110.56 | 3.67 | 0.09 | 1.66 | 188.09 | 1.11 |
| 12 | 1∶9 | 113.02 | 3.43 | 0.05 | 2.30 | 186.24 | 1.11 |
Table 5
Content of brine ions after making brineunder different ratio of ammonia-alkali wasteliquid to fresh water"
实验 编号 | V(氨碱废液)∶ V(淡水) | 液相离子质量浓度/(g?L-1) | ||||
|---|---|---|---|---|---|---|
| Cl- | Ca2+ | Mg2+ | SO42- | NaCl | ||
| 1 | 1∶3 | 187.57 | 5.61 | 0.19 | 1.91 | 309.20 |
| 2 | 1∶3 | 191.26 | 3.78 | 0.21 | 2.78 | 315.28 |
| 3 | 1∶3 | 192.34 | 5.51 | 0.56 | 1.22 | 317.07 |
| 4 | 1∶4 | 188.44 | 4.59 | 0.12 | 1.08 | 310.62 |
| 5 | 1∶4 | 190.17 | 2.69 | 0.16 | 3.38 | 308.97 |
| 6 | 1∶4 | 192.57 | 4.19 | 0.12 | 1.08 | 305.94 |
| 7 | 1∶7 | 190.39 | 2.04 | 0.21 | 4.31 | 313.74 |
| 8 | 1∶7 | 193.43 | 1.76 | 0.11 | 3.62 | 317.61 |
| 9 | 1∶7 | 190.17 | 1.74 | 0.12 | 3.77 | 312.42 |
| 10 | 1∶9 | 193.43 | 1.57 | 0.01 | 3.77 | 318.88 |
| 11 | 1∶9 | 191.26 | 1.25 | 0.11 | 3.70 | 315.29 |
| 12 | 1∶9 | 193.43 | 1.49 | 0.15 | 4.06 | 318.72 |
Table 6
Effect of different residence time on thecomposition of ions after making brine"
实验 编号 | 停留 时间/h | 液相离子质量浓度/(g?L-1) | ||||
|---|---|---|---|---|---|---|
| Cl- | Ca2+ | Mg2+ | SO42- | NaCl | ||
| 1 | 2.5 | 192.83 | 3.51 | 0.16 | 2.06 | 309.56 |
| 2 | 2.5 | 193.27 | 3.49 | 0.17 | 1.81 | 309.78 |
| 3 | 2.5 | 191.74 | 3.37 | 0.31 | 1.57 | 306.67 |
| 4 | 3.0 | 190.19 | 1.43 | 0.16 | 4.99 | 313.50 |
| 5 | 3.0 | 191.26 | 1.33 | 0.19 | 6.85 | 315.17 |
| 6 | 3.0 | 190.17 | 1.25 | 0.24 | 6.46 | 313.50 |
| 7 | 4.0 | 189.04 | 0.84 | 0.19 | 7.78 | 311.62 |
| 8 | 4.0 | 190.12 | 1.00 | 0.07 | 6.61 | 313.42 |
| 9 | 4.0 | 192.52 | 0.80 | 0.26 | 8.61 | 317.25 |
Table 7
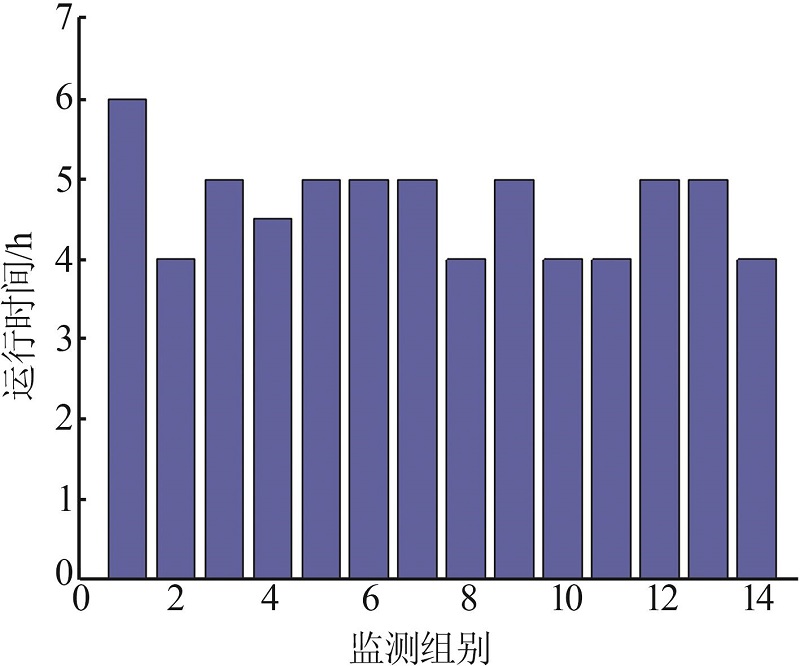
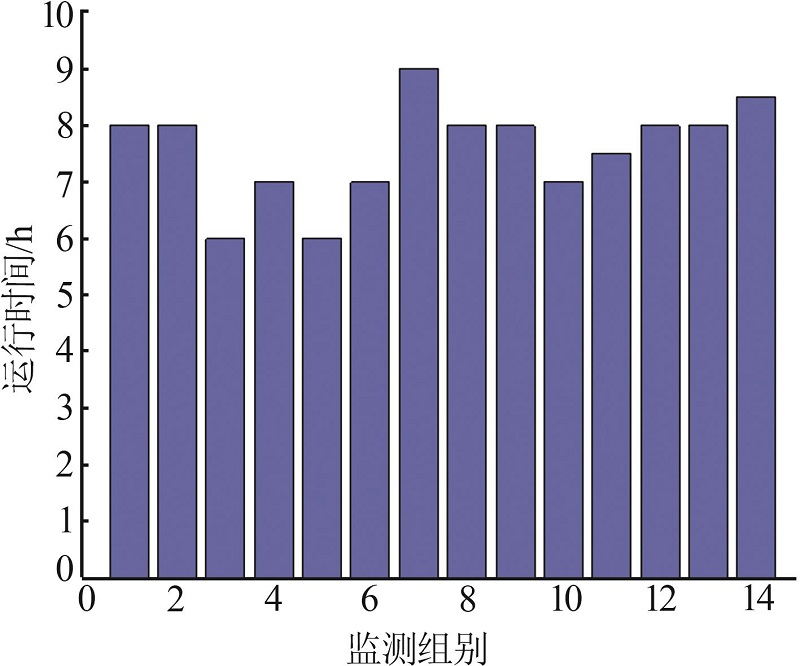
Monitoring data of primary settler for 26 h"
| 运行时间/h | 相对 密度 | 透光率/ % | 液相各离子质量浓度/(g·L-1) | ||||
|---|---|---|---|---|---|---|---|
| Ca2+ | SO42- | Mg2+ | Cl- | NaCl | |||
| 1 | 1.19 | 91.60 | 0.94 | 7.34 | 0.23 | 190.17 | 313.38 |
| 2 | 1.19 | 92.50 | 0.92 | 7.83 | 0.25 | 189.30 | 311.95 |
| 3 | 1.19 | 90.90 | 0.92 | 7.78 | 0.25 | 173.87 | 286.52 |
| 4 | 1.19 | 91.80 | 0.88 | 8.86 | 0.27 | 188.00 | 309.80 |
| 5 | 1.19 | 95.50 | 0.86 | 8.66 | 0.28 | 188.22 | 310.17 |
| 6 | 1.19 | 94.20 | 0.82 | 8.56 | 0.31 | 189.74 | 312.67 |
| 7 | 1.19 | 92.70 | 0.88 | 8.22 | 0.27 | 188.87 | 311.24 |
| 8 | 1.19 | 88.70 | 1.02 | 7.58 | 0.19 | 191.91 | 316.25 |
| 9 | 1.19 | 91.00 | 1.02 | 7.58 | 0.19 | 191.26 | 315.29 |
| 10 | 1.19 | 93.00 | 0.96 | 8.07 | 0.31 | 189.30 | 312.07 |
| 11 | 1.19 | 92.00 | 1.08 | 9.05 | 0.21 | 191.26 | 315.29 |
| 12 | 1.19 | 92.40 | 1.12 | 6.36 | 0.15 | 190.17 | 313.50 |
| 13 | 1.19 | 93.60 | 1.22 | 7.49 | 0.12 | 187.08 | 308.40 |
| 14 | 1.19 | 84.20 | 0.92 | 7.34 | 0.36 | 189.25 | 311.98 |
| 15 | 1.19 | 93.60 | 0.92 | 9.30 | 0.31 | 189.25 | 311.98 |
| 16 | 1.19 | 75.90 | 0.84 | 7.83 | 0.30 | 189.04 | 311.62 |
| 17 | 1.19 | 81.10 | 0.90 | 8.47 | 0.22 | 188.17 | 310.19 |
| 18 | 1.19 | 84.00 | 0.92 | 8.81 | 0.19 | 189.04 | 311.62 |
| 19 | 1.19 | 86.20 | 0.82 | 8.81 | 0.25 | 185.99 | 306.60 |
| 20 | 1.19 | 87.30 | 0.80 | 9.05 | 0.26 | 189.25 | 311.98 |
| 21 | 1.19 | 90.00 | 0.90 | 6.56 | 0.20 | 187.95 | 309.83 |
| 22 | 1.19 | 92.00 | 0.92 | 9.05 | 0.25 | 190.34 | 313.78 |
| 23 | 1.19 | 79.70 | 0.96 | 9.98 | 0.21 | 188.17 | 310.20 |
| 24 | 1.19 | 82.00 | 0.84 | 9.30 | 0.25 | 189.91 | 313.07 |
| 25 | 1.19 | 84.40 | 0.78 | 9.79 | 0.33 | 171.43 | 315.57 |
| 26 | 1.19 | 86.10 | 1.00 | 8.66 | 0.28 | 190.34 | 313.66 |
Table 9
Effect of residence time in reactoron sulfate radical removal"
| 停留时间/min | n(硫酸根)∶ n(钙离子) | 液相各离子质量浓度/(g·L-1) | ||||
|---|---|---|---|---|---|---|
| Cl- | Ca2+ | Mg2+ | SO42- | NaCl | ||
| 10 | 1∶0.8 | 189.25 | 1.91 | 0.53 | 4.28 | 311.98 |
| 10 | 1∶0.75 | 190.34 | 1.54 | 0.49 | 5.01 | 313.78 |
| 10 | 1∶0.7 | 191.86 | 1.85 | 0.46 | 5.60 | 316.28 |
| 5 | 1∶0.8 | 190.34 | 1.93 | 0.49 | 4.92 | 313.78 |
| 5 | 1∶0.75 | 180.33 | 1.79 | 0.46 | 4.34 | 306.32 |
| 5 | 1∶0.7 | 183.65 | 1.69 | 0.42 | 5.18 | 310.20 |
Table 10
Reaction experimental data ofreactor staying for 5 minutes"
相对 密度 | 液相各离子质量浓度/(g·L-1) | n(硫酸根)∶ n(钙离子) | ||||
|---|---|---|---|---|---|---|
| Cl- | Ca2+ | Mg2+ | SO42- | NaCl | ||
| 1.19 | 185.82 | 1.55 | 0.48 | 5.26 | 307.07 | 1∶0.7 |
| 1.19 | 183.68 | 1.53 | 0.50 | 5.19 | 308.10 | 1∶0.7 |
| 1.19 | 185.74 | 1.60 | 0.44 | 5.20 | 307.12 | 1∶0.7 |
| 1.19 | 184.68 | 1.73 | 0.49 | 4.75 | 306.62 | 1∶0.75 |
| 1.19 | 182.69 | 1.75 | 0.45 | 4.80 | 305.23 | 1∶0.75 |
| 1.19 | 184.25 | 1.70 | 0.48 | 4.77 | 306.58 | 1∶0.75 |
| 1.19 | 190.45 | 2.05 | 0.48 | 4.55 | 309.02 | 1∶0.8 |
| 1.19 | 189.23 | 2.04 | 0.50 | 4.38 | 308.95 | 1∶0.8 |
| 1.19 | 190.12 | 2.03 | 0.49 | 4.53 | 309.27 | 1∶0.8 |
Table 12
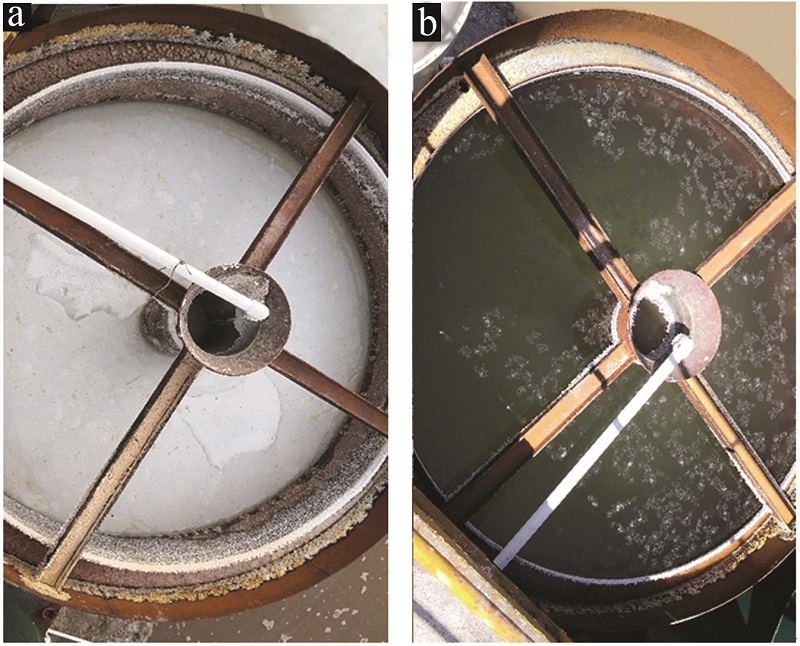
Monitoring experimental data of the secondaryprecipitator for continuous 12 h"
| 运行时间/h | 相对 密度 | 透光率/ % | 液相各离子质量浓度/(g·L-1) | ||||
|---|---|---|---|---|---|---|---|
| Ca2+ | SO42- | Mg2+ | Cl- | NaCl | |||
| 1 | 1.19 | 96.10 | 1.93 | 4.31 | 0.51 | 191.43 | 315.27 |
| 2 | 1.19 | 94.20 | 1.73 | 5.21 | 0.55 | 189.69 | 312.70 |
| 3 | 1.19 | 82.90 | 1.82 | 4.95 | 0.55 | 186.21 | 306.96 |
| 4 | 1.19 | 90.40 | 1.40 | 4.97 | 0.51 | 190.99 | 314.85 |
| 5 | 1.19 | 88.50 | 1.41 | 5.16 | 0.49 | 189.25 | 311.98 |
| 6 | 1.19 | 89.30 | 1.60 | 5.21 | 0.51 | 186.21 | 306.96 |
| 7 | 1.19 | 77.70 | 1.90 | 5.55 | 0.31 | 191.79 | 316.15 |
| 8 | 1.19 | 92.30 | 1.24 | 2.94 | 1.03 | 186.64 | 307.68 |
| 9 | 1.19 | 97.00 | 2.14 | 5.11 | 0.49 | 189.49 | 311.98 |
| 10 | 1.19 | 87.30 | 1.82 | 3.31 | 0.56 | 189.49 | 312.37 |
| 11 | 1.19 | 90.20 | 1.85 | 4.14 | 0.26 | 191.13 | 315.07 |
| 12 | 1.19 | 92.50 | 1.79 | 4.19 | 0.32 | 190.59 | 314.17 |
| [1] | 李育童, 咏梅.吉兰泰盐湖采盐技术变迁[J].内蒙古师范大学学报:自然科学版, 2021, 50(3): 261-267. |
| LI Yutong, YONG Mei.Transition of mining technology in Jilantai salt lake[J].Journal of Inner Mongolia Normal University:Natural Science Edition, 2021, 50(3): 261-267. | |
| [2] | 史星星, 胡开宝, 王占和, 等. 吉兰泰盐湖卤水冻硝及冻硝母液蒸发析盐规律研究[J].无机盐工业, 2020, 52(6): 54-58. |
| SHI Xingxing, HU Kaibao, WANG Zhanhe, et al.Study on salt crystallization law of brine frozen mirabilite and frozen mirabilite mother liquor evaporation in Jilantai salt lake[J].Inorganic Che-Industry micals, 2020, 52(6): 54-58. | |
| [3] | 胡开宝, 崔金贵, 张荣, 等. 吉兰泰盐湖资源开采与创新发展[J].盐科学与化工, 2018(3): 40-43. |
| HU Kaibao, CUI Jingui, ZHANG Rong, et al.Resources exploitation and innovation development of Jilantai salt lake[J].Journal of Salt Science and Chemical Industry, 2018(3): 40-43. | |
| [4] | 程芳琴, 成怀刚, 崔香梅.中国盐湖资源的开发历程及现状[J].无机盐工业, 2011, 43(7): 1-4, 12. |
| CHENG Fangqin, CHENG Huaigang, CUI Xiangmei.Development history and present status of salt lake resources in China[J].Inorganic Chemicals Industry, 2011, 43(7): 1-4, 12. | |
| [5] | 刘海宁, 叶秀深, 张慧芳, 等. 盐湖稀有元素吸附分离提取研究[J].盐湖研究, 2019, 27(3): 11-20. |
| LIU Haining, YE Xiushen, ZHANG Huifang, et al.Separation and extraction of rare elements in salt lake brine[J].Journal of Salt Lake Research, 2019, 27(3): 11-20. | |
| [6] | 胡开宝.吉兰泰盐湖资源开采与创新发展[J].中国盐业, 2020(1): 51-54. |
| HU Kaibao.Exploitation and innovative development of Jilantai salt lake resources[J].China Salt Industry, 2020(1): 51-54. | |
| [7] | 王润璞, 杜威, 胡开宝, 等. 氨碱废液溶采二层盐制备优级液体盐工艺研究[J].无机盐工业, 2021, 53(8): 83-86. |
| WANG Runpu, DU Wei, HU Kaibao, et al.Study on the process of preparing liquid salt of guaranteed reagent by dissolving two-layer salt in ammonia-alkali wastewater[J].Inorganic Chemicals Industry, 2021, 53(8): 83-86. | |
| [8] | 高卫星, 赵春刚, 张玮, 等. 吉兰太盐湖贫瘠矿床资源新工艺的实践与应用[J].盐科学与化工, 2017, 46(7): 42-44. |
| GAO Weixing, ZHAO Chungang, ZHANG Wei, et al.Practice and application of new technology and resource exploration in Jilantai salt lake deposit ore[J].Journal of Salt Science and Chemical Industry, 2017, 46(7): 42-44. | |
| [9] | 宋信信, 熊泽华, 邓强, 等. 利用氨碱废液生产液体盐的新型工艺[J].盐科学与化工, 2020, 49(11): 8-10. |
| SONG Xinxin, XIONG Zehua, DENG Qiang, et al.A new technology of producing liquid salt using waste ammonia liquid from ammonia soda[J].Journal of Salt Science and Chemical Industry, 2020, 49(11): 8-10. | |
| [10] | 崔玉虎.硫酸钠型盐矿卤水脱硫的研究[J].苏盐科技, 2012(4): 1-3. |
| CUI Yuhu.Study on desulphurization of brine of sodium sulfate type salt mine[J].Jiangsu Salt Science & Technology, 2012(4): 1-3. | |
| [11] | ZHAO Chuanliang, ZHOU Junyuan, YAN Yi, et al.Application of coagulation/flocculation in oily wastewater treatment:A revi-ew[J].Science of the Total Environment, 2021,765.Doi:10.1016/j.scitotenv.2020.142795. |
| [12] | TIKHONOV A Y, MYASNIKOV S K, KULOV N N.Kinetics of nucleation and growth of calcium carbonate and calcium sulphate crystals from aqueous solutions[J].Theoretical Foundations of Chemical Engineering, 2020, 54(4): 529-538. |
| [13] | 王少青.吉兰泰盐湖卤水除杂及副产超细氢氧化镁工艺[D].呼和浩特:内蒙古工业大学, 2007. |
| WANG Shaoqing.Removal of impurities from brine of Jilantai salt lake and preparation process of ultrafine magnesium hydroxide powder as a byproduct[D].Hohhot:Inner Mongolia University of Tehchnology, 2007. | |
| [14] | 朱明丽, 李俊杰, 曹正伟, 等. 纯碱行业卤水中硫酸根的脱除[J].无机盐工业, 2016, 48(11): 57-61. |
| ZHU Mingli, LI Junjie, CAO Zhengwei, et al.Removal of sulfate radical from brine in soda industry[J].Inorganic Chemicals Industry, 2016, 48(11): 57-61. | |
| [15] | 颜亚盟, 任青考.不同温度下硫酸钙在盐水中的沉降速率实验研究[J].盐科学与化工, 2018, 47(6): 24-27. |
| YAN Yameng, REN Qingkao.Study on settlement rate of calcium sulfate in brine at different temperature[J].Journal of Salt Science and Chemical Industry, 2018, 47(6): 24-27. | |
| [16] | FELDMANN T, DEMOPOULOS G P.Influence of impurities on crystallization kinetics of calcium sulfate dihydrate and hemihydrate in strong HCl-CaCl2 solutions[J].Industrial & Engineering Chemistry Research, 2013, 52(19): 6540-6549. |
| [1] | WANG Baoming, WANG Xinglong, YANG Ying, ZHAO Bo, HUA Quanxian, LIU Yong, LIU Pengfei, SHEN Bo, DING Junxiang, TANG Jianwei. Current status and research progress of comprehensive utilization of phosphorus tailings [J]. Inorganic Chemicals Industry, 2024, 56(10): 1-11. |
| [2] | DENG Hua, HOU Shuomin, LI Zhongjun, XU Gang, CHI Ru′an, XI Benjun. Current situation and prospect of comprehensive utilization of phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(1): 1-8. |
| [3] | LIU Guanfa, ZENG Chaocong, WANG Zekai, ZENG Xiangrong, HUANG Wanfu. Study on preparation of high strength sintered ceramsite from tungsten smelting slag and its property [J]. Inorganic Chemicals Industry, 2023, 55(8): 132-139. |
| [4] | JIANG Ziwen,QUAN Xuejun,LI Gang,LU Cunfang,GUO Yijun,CHEN Hao,ZHOU Yankuo,CHENG Zhiliang. Research progress of resource utilization of chromium slag [J]. Inorganic Chemicals Industry, 2023, 55(2): 26-35. |
| [5] | YANG Liyan,MA Xin,MEI Ruifeng,YANG Dashan,LIANG Jialin,LI Chunquan,SUN Zhiming. Study on preparation and performance of ceramsite from sediment in Yellow River desilting basin [J]. Inorganic Chemicals Industry, 2022, 54(5): 109-115. |
| [6] | CUI Rongzheng,BAI Haidan,Gao Yongfeng,XIU Xuefeng. Current situation of comprehensive utilization of phosphogypsum and its development trend of 14th Five-Year Plan [J]. Inorganic Chemicals Industry, 2022, 54(4): 1-4. |
| [7] | Wang Runpu,Du Wei,Hu Kaibao,Wang Zhanhe,Gao Weixing,Tang Na. Study on the process of preparing liquid salt of guaranteed reagent by dissolving two-layer salt in ammonia-alkali wastewater [J]. Inorganic Chemicals Industry, 2021, 53(8): 83-86. |
| [8] | Liu Lincheng,Zuo Haibin,Xu Zhiqiang. Resource utilization approach of industrial gypsum and its prospect [J]. Inorganic Chemicals Industry, 2021, 53(10): 1-9. |
| [9] | Shi Xingxing,Hu Kaibao,Wang Zhanhe,Pan Jingzhong,Cui Jingui,Luo Ruiyin,Du Wei,Tang Na. Study on salt crystallization law of brine frozen mirabilite and frozen mirabilite mother liquor evaporation in Jilantai Salt Lake [J]. Inorganic Chemicals Industry, 2020, 52(6): 54-58. |
| [10] | Zhang Xiangcheng,Meng Yongbiao. Brief analysis on present situation of comprehensive utilization of fly ash in China [J]. Inorganic Chemicals Industry, 2020, 52(2): 1-5. |
| [11] | Liu Yuelong,Wang Linlin,Liu Gousheng. Extraction of rubidium and cerium salts from lithium tail liquid of medium and low grade lithium clay by ammonium sulfate process [J]. Inorganic Chemicals Industry, 2020, 52(11): 60-63. |
| [12] | Fu Yuhang1,2,3,Yan Rongbei1,2,3,Peng Chuanfeng1,2,3. Study on comprehensive utilization of residues in salt making area [J]. Inorganic Chemicals Industry, 2019, 51(7): 55-57. |
| [13] | GU Han-Nian, CUI Shan-Shan, WANG Ning, ZHAO Cheng-Dong. Distribution features and leaching effect of alkali in red mud [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 45-. |
| [14] | WAN Ya-Meng, WANG Bao-Qing, WANG Dan, REN Bao-Zeng. Research progress of alumina recovery technology from coal fly ash [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(11): 7-. |
| [15] | XIA Han-Bo, LI Bao-Shan. Liquid absorption and comprehensive utilization of NO in flue gas [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 54-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||