Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (11): 60-65.doi: 10.19964/j.issn.1006-4990.2021-0019
• Research & Development • Previous Articles Next Articles
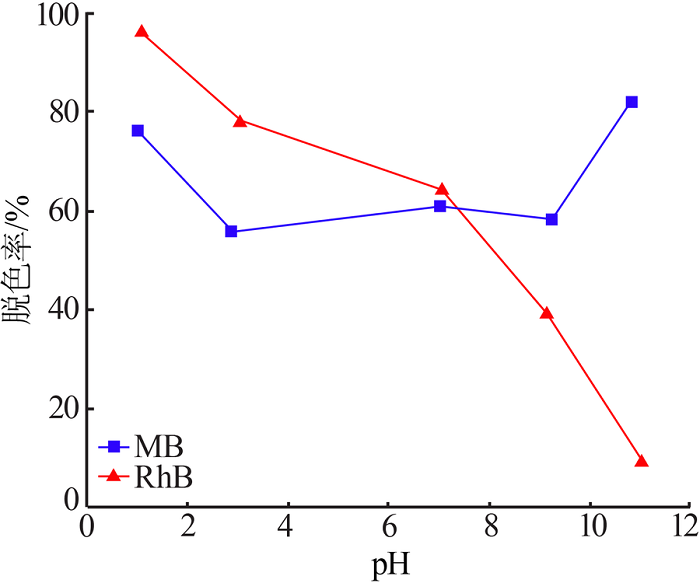
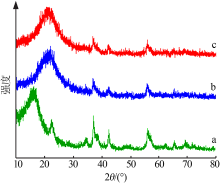
Study on hydrothermal synthesis of MnO2 and its decolorization performance on dyes
ZHU Danchen1,2( ),LI Mingjie1,CHEN Zhangxu1,2,LIN Qian1
),LI Mingjie1,CHEN Zhangxu1,2,LIN Qian1
- 1. College of Environmental and Biological Engineering,Putian University,Putian 351100,China
2. Fujian Key Laboratory of Ecology-toxicological Effects & Control for Emerging Contaminants
-
Received:2021-01-08Online:2021-11-10Published:2021-11-15
CLC Number:
Cite this article
ZHU Danchen,LI Mingjie,CHEN Zhangxu,LIN Qian. Study on hydrothermal synthesis of MnO2 and its decolorization performance on dyes[J]. Inorganic Chemicals Industry, 2021, 53(11): 60-65.
share this article
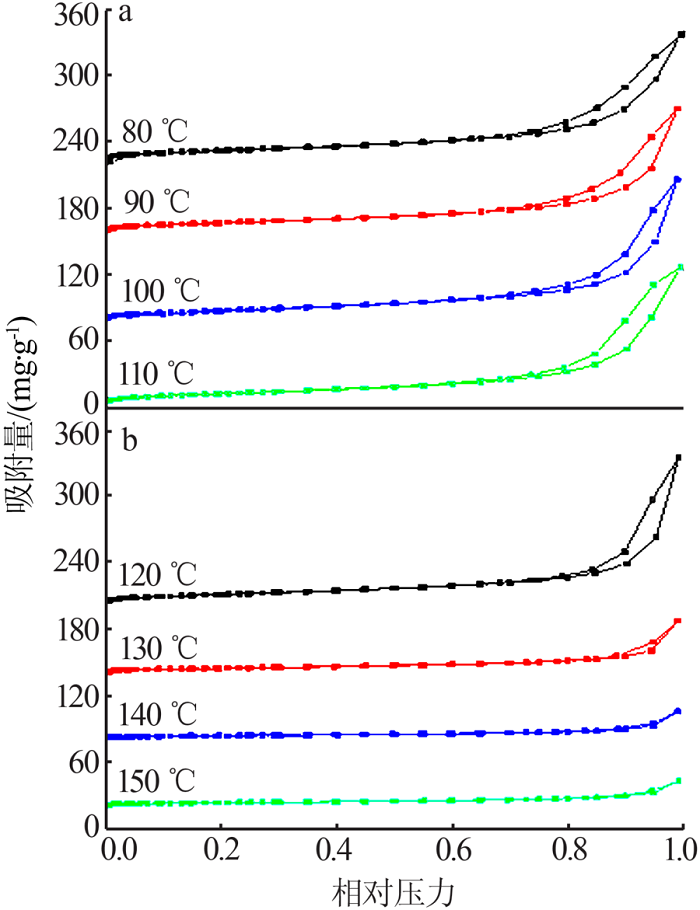
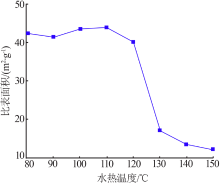
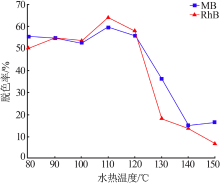
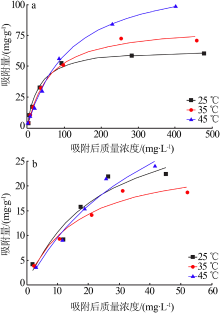
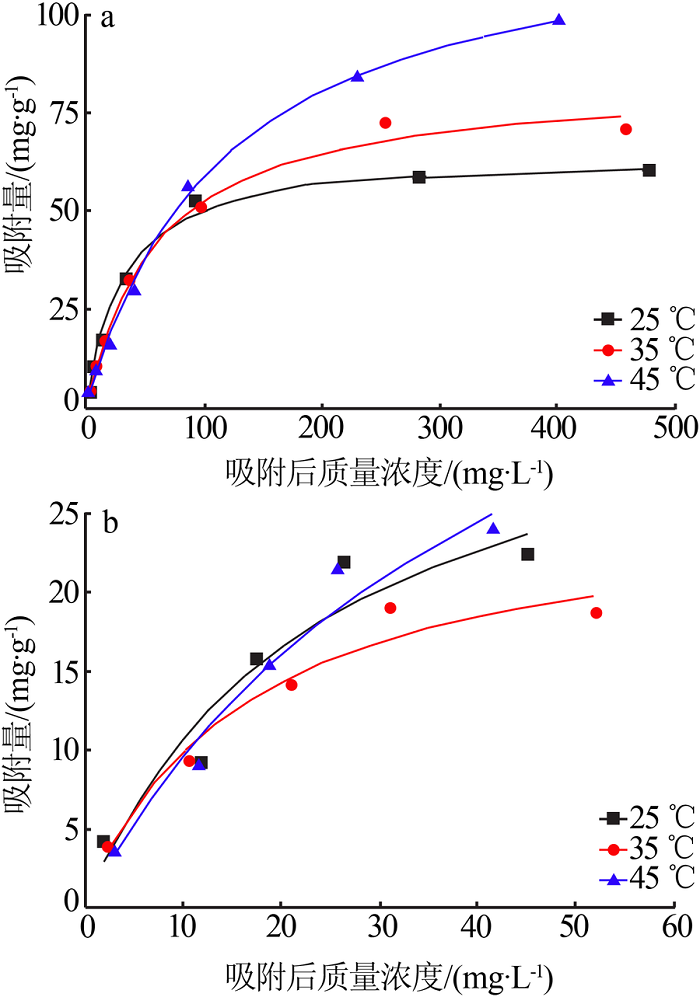
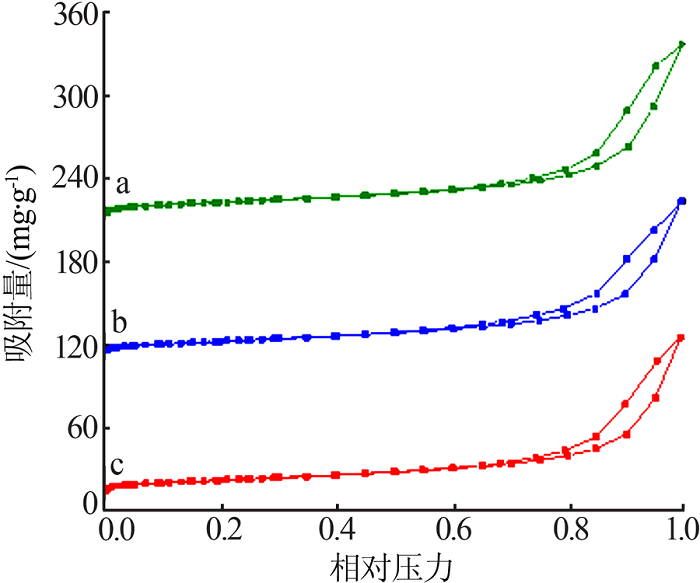
Table 1
Fitting parameters of the adsorption isotherm model(MB)"
| 温度/ ℃ | Langmuir等温模型 | Freundlich等温模型 | ||||
|---|---|---|---|---|---|---|
| KL/ (L·mg-1) | Qm/ (mg·g-1) | R2 | KF | 1/n | R2 | |
| 25 | 0.039 2 | 63.572 8 | 0.998 3 | 5.004 0 | 0.454 5 | 0.908 1 |
| 35 | 0.022 3 | 79.302 1 | 0.989 3 | 4.054 3 | 0.513 01 | 0.965 0 |
| 45 | 0.012 3 | 116.009 3 | 0.957 9 | 3.707 7 | 0.565 51 | 0.986 8 |
Table 2
Fitting parameters of the adsorption isotherm model(RhB)"
| 温度/ ℃ | Langmuir等温模型 | Freundlich等温模型 | ||||
|---|---|---|---|---|---|---|
| KL/ (L·mg-1) | Qm/ (mg·g-1) | R2 | KF | 1/n | R2 | |
| 25 | 0.050 4 | 32.894 7 | 0.822 2 | 2.500 7 | 0.605 2 | 0.943 9 |
| 35 | 0.072 4 | 24.330 9 | 0.959 2 | 2.566 6 | 0.541 7 | 0.964 7 |
| 45 | 0.022 5 | 51.020 4 | 0.722 5 | 1.510 9 | 0.767 8 | 0.972 3 |
| [1] |
HE Y, JIANG D B, CHEN J, et al. Synjournal of MnO2 nanosheets on montmorillonite for oxidative degradation and adsorption of methy-lene blue[J]. Journal of Colloid and Interface Science, 2018, 510:207-220.
doi: 10.1016/j.jcis.2017.09.066 |
| [2] |
SHAKOOR S, NASAR A. Removal of methylene blue dye from arti-fıcially contaminated water using citrus limetta peel waste as a very low cost adsorbent[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 66:154-163.
doi: 10.1016/j.jtice.2016.06.009 |
| [3] |
MA J P, CHEN L, WU Z, et al. Pyroelectric Pb(Zr0.52Ti0.48)O3 polariz-ed ceramic with strong pyro-driven catalysis for dye wastewater de-composition[J]. Ceramics International, 2019, 45(9):11934-11938.
doi: 10.1016/j.ceramint.2019.03.082 |
| [4] |
LIU Y N, QU R X, LI X Y, et al. A bifunctional β-MnO2 mesh for expeditious and ambient degradation of dyes in activation of perox-ymonosulfate(PMS) and simultaneous oil removal from water[J]. Journal of Colloid and Interface Science, 2020, 579:412-424.
doi: 10.1016/j.jcis.2020.06.073 |
| [5] |
AN Y Y, ZHENG H L, ZHENG X Y, et al. Use of a floating adsor-bent to remove dyes from water:A novel efficient surface separation method[J]. Journal of Hazardous Materials, 2019, 375:138-148.
doi: 10.1016/j.jhazmat.2019.04.060 |
| [6] | 王建芳, 王君, 杨和平, 等. Fe3O4/δ-MnO2纳米片的制备及其对亚甲基蓝的吸附研究[J]. 无机盐工业, 2019, 51(10):93-96. |
| [7] | 胡月, 李盼, 吕宏凌, 等. 纳米MnO2/羧甲基纤维素复合膜制备及光催化降解罗丹明B性能研究[J]. 化工新型材料, 2019, 47(11):167-170,174. |
| [8] | 王春雨, 侯永江, 刘璇, 等. 不同晶型二氧化锰催化臭氧化降解亚甲基蓝废水[J]. 环境工程学报, 2017, 11(2):908-914. |
| [9] |
JIANG S B, YU T F, XIA R, et al. Realization of super high adsorp-tion capability of 2D δ-MnO2/GO through intra-particle diffusion[J]. Materials Chemistry and Physics, 2019, 232:374-381.
doi: 10.1016/j.matchemphys.2019.05.004 |
| [10] |
PARGOLETTI E, PIFFERI V, LUIGI F, et al. A detailed investiga-tion of MnO2 nanorods to be grown onto activated carbon.High efficiency towards aqueous methyl orange adsorption/degrada-tion[J]. Applied Surface Science, 2019, 472:118-126.
doi: 10.1016/j.apsusc.2018.03.170 |
| [11] |
YANG H, LI C, ZHANG J. Determining roles of in-situ measured surface potentials of phase controlled synthesized MnO2 nanostruc-tures for superficial adsorption[J]. Applied Surface Science, 2020, 513.Doi. org/10.1016/j.apsusc.2020.145752.
doi: org/10.1016/j.apsusc.2020.145752 |
| [12] | 孙宏, 张泽, 宋坤. 纳米层状二氧化锰去除水中罗丹明B的效能研究[J]. 印染助剂, 2016, 33(3):38-40. |
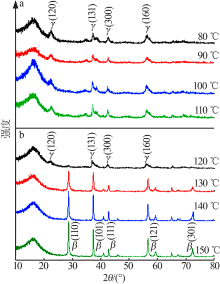
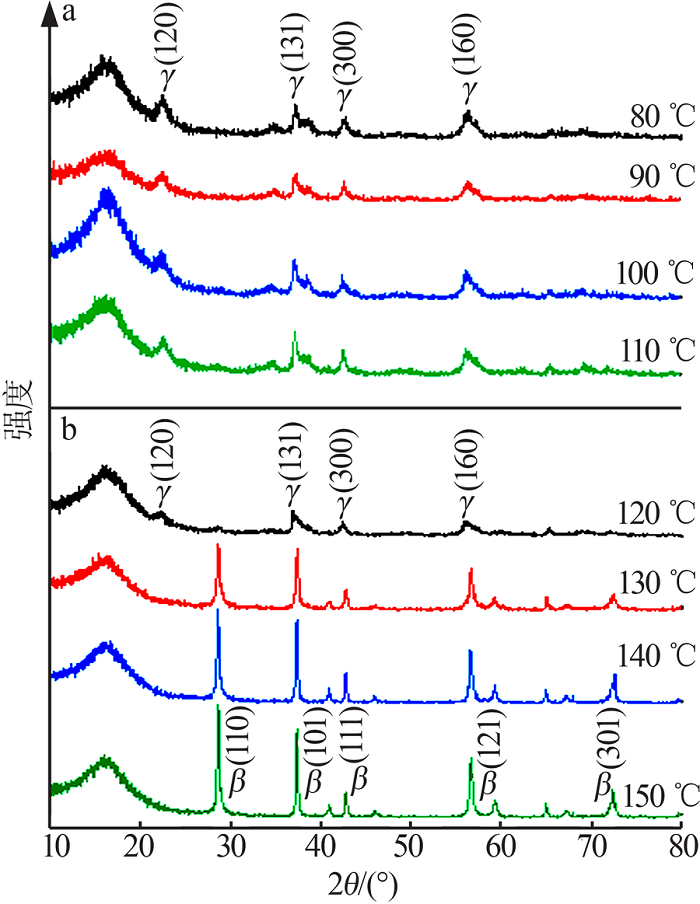
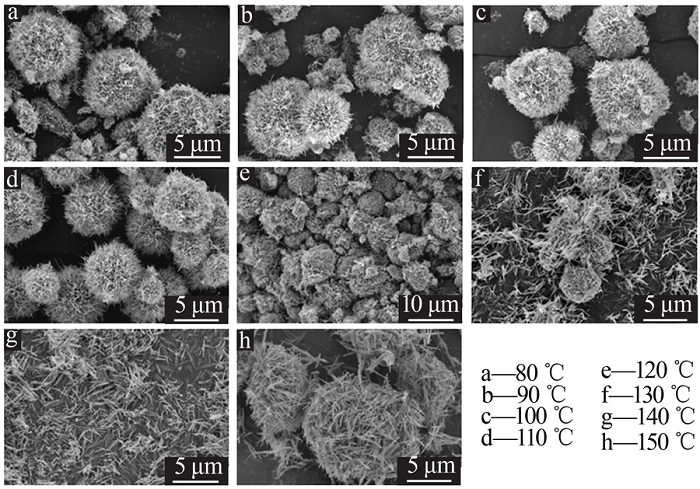
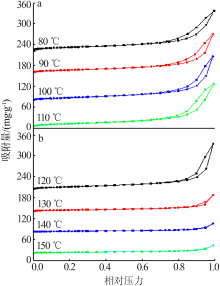
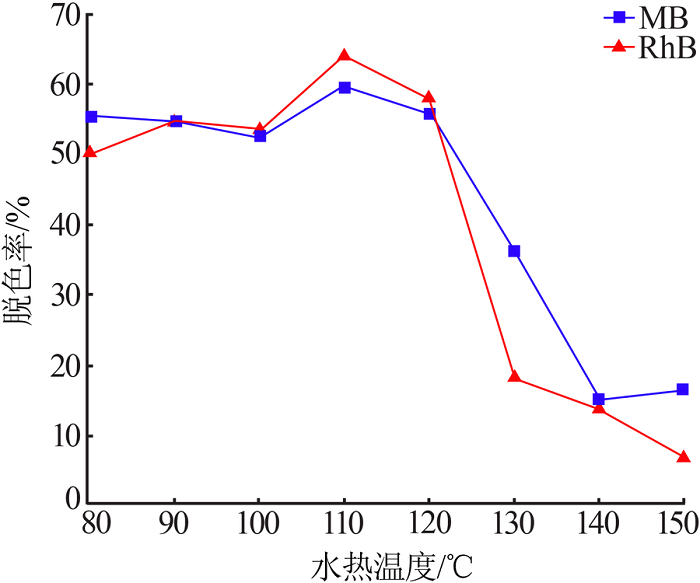
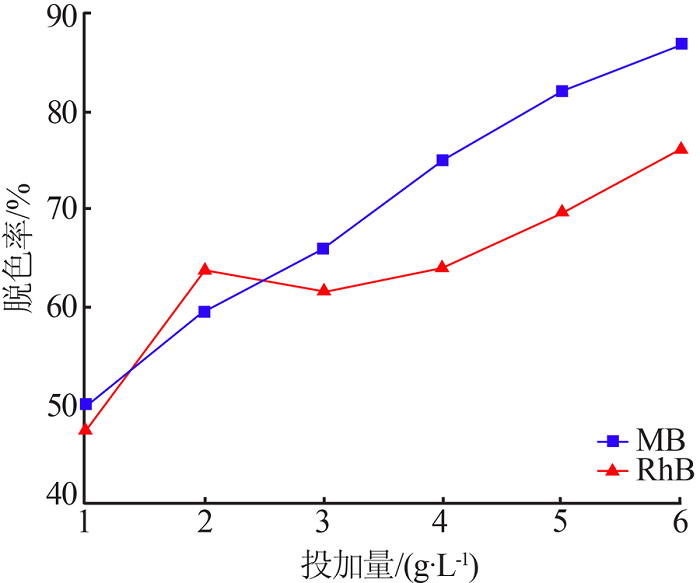
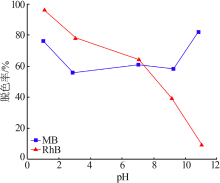
| [13] | 朱丹琛, 肖伽励, 杨丽丽, 等. 水热合成MnO2及其染料吸附性能的构效关系研究[J]. 化工进展, 2019, 38(8):3774-3781. |
| [14] | 蔡冬鸣, 李圭白. 天然粉末二氧化锰处理染料废水的试验研究[J]. 中国给水排水, 2007, 23(7):62-65. |
| [15] |
ZHAO B H, RAN R, XU X D, et al. Phase structures,morphologies,and NO catalytic oxidation activities of single-phase MnO2 catalysts[J]. Applied Catalysis A:General, 2016, 514:24-34.
doi: 10.1016/j.apcata.2016.01.005 |
| [16] |
ZHAO Z W, GENG C, YANG C, et al. A novel flake-ball-like ma-gnetic Fe3O4/γ-MnO2 meso-porous nano-composite:Adsorption of fluorinion and effect of water chemistry[J]. Chemosphere, 2018, 209:173-181.
doi: 10.1016/j.chemosphere.2018.06.104 |
| [17] |
DUAN Y P, PANG H F, ZHANG Y H, et al. Morphology-controlled synjournal and microwave absorption properties of β-MnO2 micro-ncube with rectangular pyramid[J]. Materials Characterization, 2016, 112:206-212.
doi: 10.1016/j.matchar.2015.12.024 |
| [18] |
ZHANG Y G, CHEN L Y, ZHENG Z, et al. A redox-hydrothermal route to β-MnO2 hollow octahedra[J]. Solid State Sciences, 2009, 11(7):1265-1269.
doi: 10.1016/j.solidstatesciences.2009.03.018 |
| [19] | 陈丽雅, 程高, 刘冠良, 等. 三维海胆状二氧化锰微球对电催化氧还原反应的晶型效应[J]. 无机化学学报, 2020, 36(3):458-466. |
| [20] | 靳福娅, 余林, 蓝邦, 等. 水热法制备二氧化锰及在过氧化氢传感器中的应[J]. 化工进展, 2017, 36(9):3380-3387. |
| [21] |
SHARMA G, DIONYSIOU D D, SHARMA S, et al. Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of Rhodamine B[J]. Catalysis Today, 2019, 335:437-451.
doi: 10.1016/j.cattod.2019.03.063 |
| [22] |
WANG Y R, ZHANG X F, HE X, et al. In situ synjournal of MnO2 coated cellulose nanofibers hybrid for effective removal of methy-lene blue[J]. Carbohydrate Polymers, 2014, 110:302-308.
doi: 10.1016/j.carbpol.2014.04.008 |
| [23] | SHAYESTEH H, ASHRAFI A, RAHBAR-KELISHAMI A. Evalua-tion of Fe3O4@MnO2 core-shell magnetic nanoparticles as an adsor-bent for decolorization of methylene blue dye in contaminated wa-ter:Synjournal and characterization,kinetic,equilibrium,and ther-modynamic studies[J]. Journal of Molecular Structure, 2017, 1149:199-205. |
| [24] | 赵颖, 王仁国, 曾武, 等. 纳米二氧化锰的制备及其对亚甲基蓝的吸附研究[J]. 水处理技术, 2012, 38(1):55-58. |
| [25] |
YAMAGUCHI S, MINBUTA S, MATSUI K. Rhodamine B adsorp-tion on anodic porous alumina in sodium dodecyl sulfate solutio-ns[J]. Colloids and Surfaces A, 2018, 555:209-216.
doi: 10.1016/j.colsurfa.2018.06.079 |
| [26] | RACHNAA K, AGRWALB A, SINGH N B. Preparation and charac-terization of zinc ferrite-polyaniline nanocomposite for removal of rhodamine B dye from aqueous solution[J]. Environmental Nano-technology,Monitoring & Management, 2018(9):154-163. |
| [27] |
ZHAO Y J, ZHU L, LI W H, et al. Insights into enhanced adsorptive removal of Rhodamine B by different chemically modifıed garlic peels:Comparison,kinetics,isotherms,thermodynamics and me-chanism[J]. Journal of Molecular Liquids, 2019, 293.Doi: org/10.1016/j.molliq.2019.111516.
doi: org/10.1016/j.molliq.2019.111516 |
| [28] | 陈丽群, 张红杰, 朱荣耀, 等. 木质素/Fenton污泥基磁性活性炭对亚甲基蓝和苯酚吸附特性的研究[J]. 中国造纸, 2020, 39(5):23-28. |
| [29] | 段贤扬, 何梦奇, 徐继红, 等. CTS-G-PAA/ATP水凝胶制备及对染料的吸附性能[J]. 功能材料, 2020, 51(2):2203-2208. |
| [1] | LI Zihan, ZHANG Jiaqi, LI Shizhuo, LI Xinyu, LIU Shaozhuo, WANG Yihao, HAO Yucui, LIU Jian, LI Yanhua. Study on synthesis and catalytic mechanism of CdS/g-C3N4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2025, 57(3): 124-132. |
| [2] | LIU Guangming. Study on photocatalytic and mechanical properties of C3N5/NH2-MIL-125(Ti) modified concrete mortar [J]. Inorganic Chemicals Industry, 2025, 57(1): 120-128. |
| [3] | ZHANG Feigang, LIU Zhongli. Study on application of CuO/g-C3N4 composites in organic dye degradation and supercapacitors [J]. Inorganic Chemicals Industry, 2025, 57(1): 129-136. |
| [4] | WANG Ping, XU Rongsheng, SUN Dong, SHI Xiaohong, XU Wei, LI Mei. Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(9): 117-127. |
| [5] | ZHANG Bangcheng, WANG Li. Preparation and adsorption properties of waste polyester⁃based activated carbon activated by ZnCl2 [J]. Inorganic Chemicals Industry, 2024, 56(7): 126-134. |
| [6] | WANG Yawen, WANG Fangfang, GENG Siyu, JU Jia, CHEN Lei, CHEN Changdong. Study on preparation and photocatalytic performance of SrTiO3-SrWO4 [J]. Inorganic Chemicals Industry, 2024, 56(7): 143-149. |
| [7] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [8] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [9] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [10] | LIU Fujie, HE Qian, SU Long, JIANG Caiyun. Adsorption properties of methylene blue by surface functionalized magnetic biochar with sodium alginate [J]. Inorganic Chemicals Industry, 2024, 56(2): 65-73. |
| [11] | WANG Ruirui, ZHU Chaoliang, MU Bing, MA Wanxia, FAN Jie, XU Guowang, SHI Yifei, DENG Xiaochuan, QING Binju. Preparation of cubic manganese carbonate by hydrothermal method and its application in extraction of lithium [J]. Inorganic Chemicals Industry, 2024, 56(12): 94-103. |
| [12] | CUI Xiangdong, LIU Sile. Study on photoelectric performance analysis of g-C3N5 nanorods and removal of Cr(Ⅵ) and methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(10): 159-168. |
| [13] | ZHANG Lijie, LI Degang, HAN Wenyuan, XU Huijun, ZHANG Weimin, YU Chen. Preparation of phosphorus-doped carbon quantum dots and activation of peroxymonosulfate for degradation of methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(1): 126-133. |
| [14] | SUN Haijie, CHENG Yuan, TIAN Yuan, LIU Hongyan, CHEN Zhihao. Preparation of BiOI/g-C3N4 catalyst and its photocatalytic degradation performance of Rhodamine B [J]. Inorganic Chemicals Industry, 2023, 55(8): 36-44. |
| [15] | CHEN Zhangxu, ZHU Danchen, FU Minglian. Study on preparation of g-C3N4/TiO2 composites and application for rhodamine B removal [J]. Inorganic Chemicals Industry, 2023, 55(7): 130-136. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||