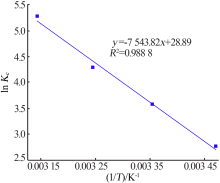
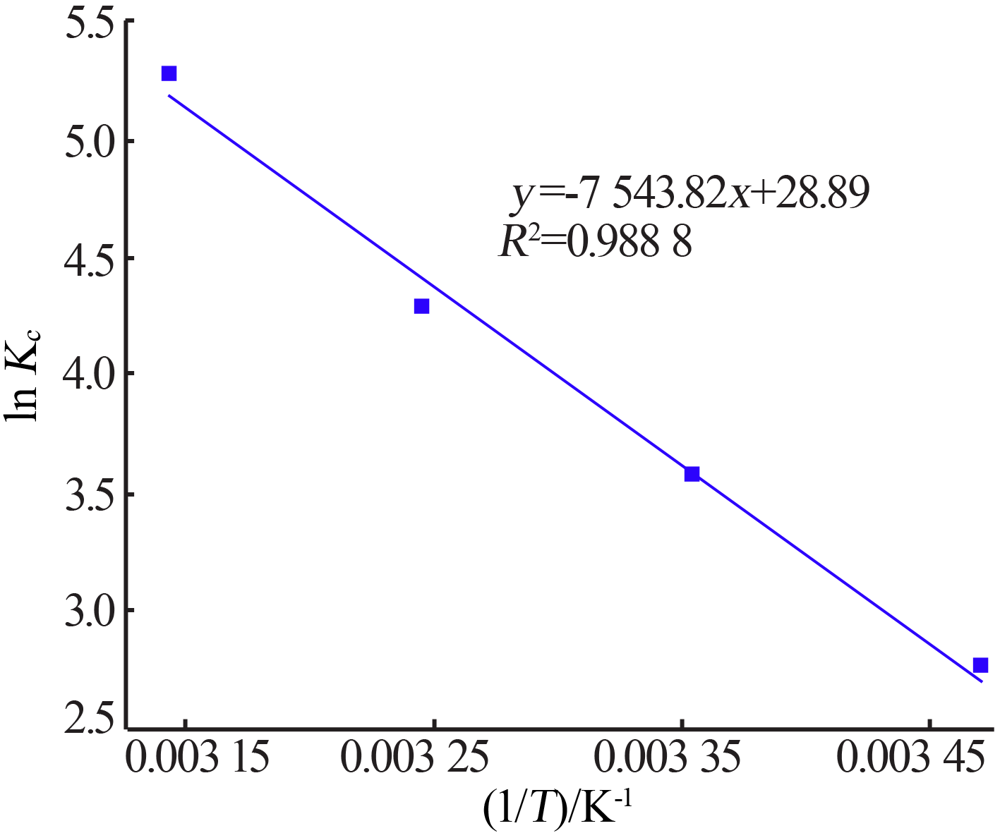
| [1] |
王志康, 黄路婷, 范娟娟, 等. α-Fe2O3活性炭的制备以及对亚甲基蓝去除影响[J]. 无机盐工业, 2019, 51(2):81-84,89.
|
| [2] |
陈俊平, 黄征青. 微波强化铸铁屑-活性炭-H2O2处理印染废水的研究[J]. 福建师范大学学报:自然科学版, 2018, 34(3):40-46.
|
| [3] |
王锐. 印染废水循环利用污染物富集规律与控制技术研究[D]. 西安: 西安建筑科技大学, 2020.
|
| [4] |
陈梦瑶, 王营茹, 曾伟, 等. 吸附-絮凝法处理亚甲基蓝染料废水的研究[J]. 工业安全与环保, 2017, 43(1):24-28.
|
| [5] |
许振钰, 魏笑, 金春姬, 等. 电化学氧化-臭氧氧化联合方法处理罗丹明B模拟染料废水[J]. 化工环保, 2020, 40(3):45-50.
|
| [6] |
王怡琴, 谢学辉, 郑秀林, 等. 激活剂促进微生物降解偶氮、蒽醌和三苯甲烷类染料研究进展[J]. 化工进展, 2019, 38(6):2968-2976.
|
| [7] |
边喜龙, 于景洋, 王宇清, 等. 电解-微生物燃料电池耦合系统处理染料废水性能[J]. 工业水处理, 2020, 40(10):59-62.
|
| [8] |
韦会鸽, 李岱原, 马俊辉, 等. 玉米芯基生物炭的制备及其吸附性能[J]. 兰州理工大学学报, 2020, 46(6):77-84.
|
| [9] |
谢亮, 王平, 李之锋, 等. Zn2SiO4/硅藻土复合材料制备及吸附亚甲基蓝性能的研究[J]. 硅酸盐通报, 2020, 39(3):974-979.
|
| [10] |
陈莹, 赵晓光. FeCl3改性柚子皮对亚甲基蓝的吸附性能[J]. 印染助剂, 2020, 37(2):42-47.
|
| [11] |
唐祝兴, 孙杨. 金属有机骨架材料Fe3O4@ZIF-67对亚甲基蓝的吸附[J]. 电镀与精饰, 2019, 41(2):23-27.
|
| [12] |
徐恩兵, 李坤权, 朱志强, 等. 双孔介孔碳的合成及其对亚甲基蓝的吸附[J]. 环境化学, 2015, 34(1):137-143.
|
| [13] |
王锐. 不同种类吸附剂对二级出水中磷及典型有机物吸附性能研究[D]. 西安: 西安理工大学, 2018.
|
| [14] |
Yang Q, Zhang F, Deng X, et al. Extraction of alumina from alu-mina rich coal gangue by a hydro-chemical process[J]. Royal So-ciety Open Science, 2020, 7(4).Doi: 10.1098/rsos.192132.
doi: 10.1098/rsos.192132
|
| [15] |
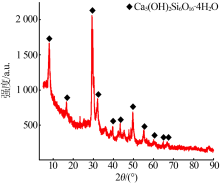
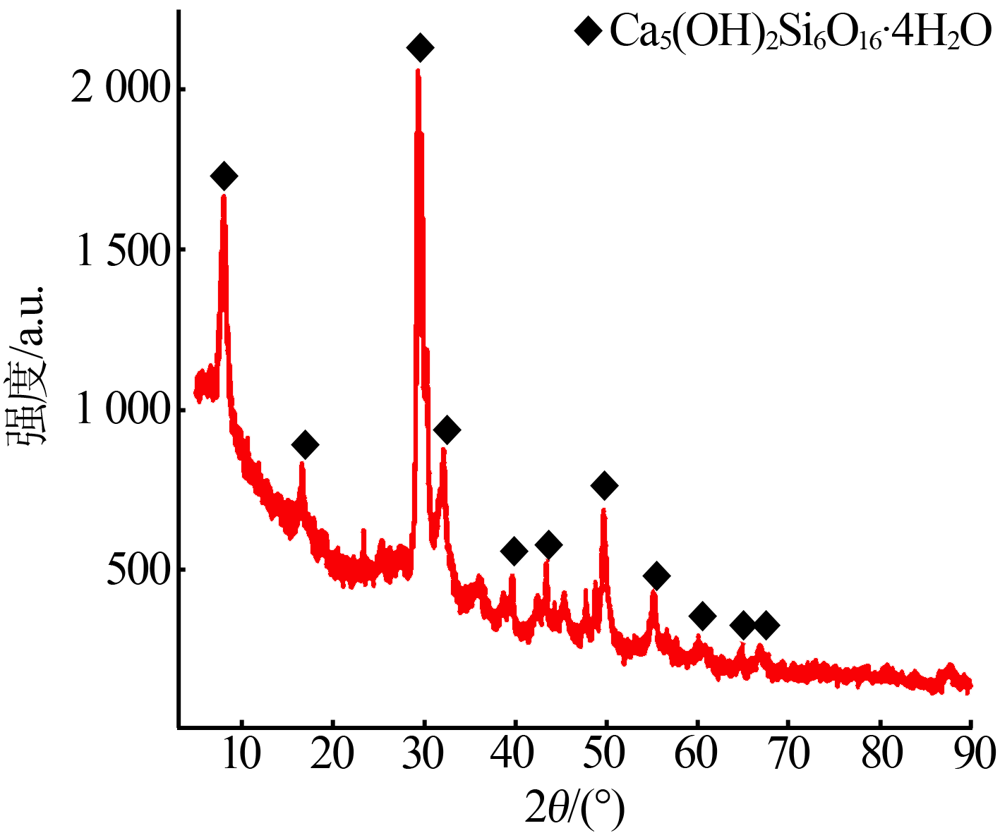
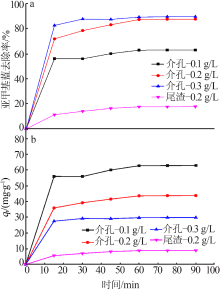
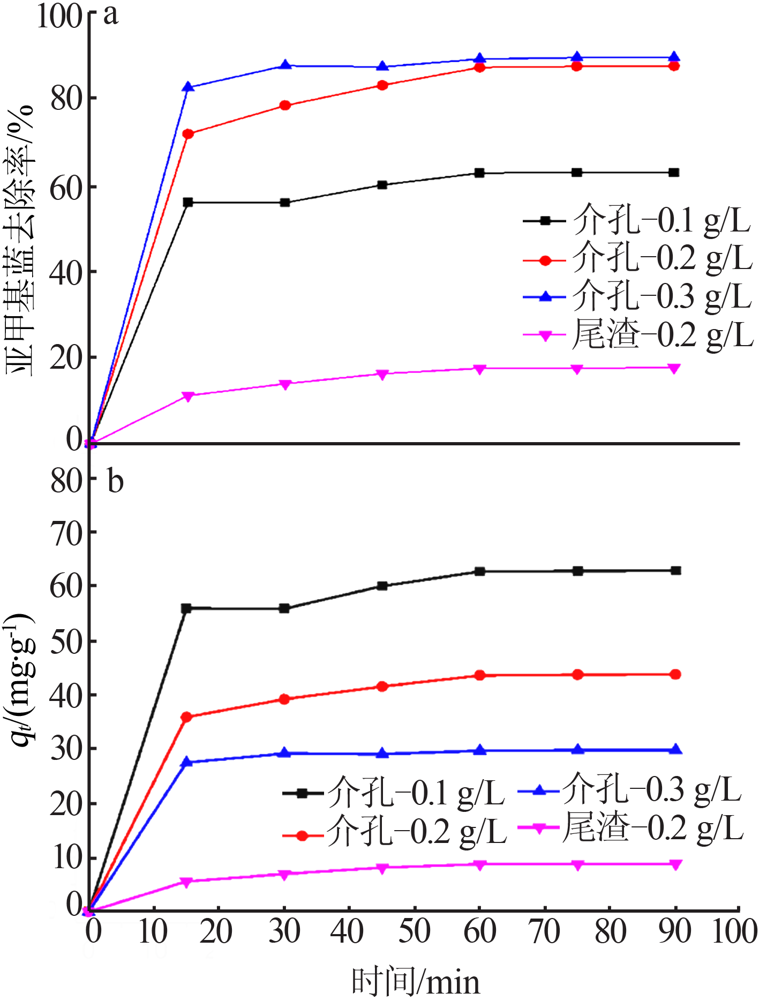
杨权成, 弓志明, 毛艳宇, 等. 煤矸石提铝尾渣水热法制备介孔硅酸钙研究[J]. 无机盐工业, 2020, 52(11):86-90.
|
| [16] |
常春, 刘天琪, 王瑀婷, 等. 水热法制备玉米叶基生物炭对亚甲基蓝的吸附性能研究[J]. 环境科学学报, 2017, 37(7):2680-2690.
|
 ),Zhang Kaiyong1,2,Guo De1,2,Shi Changsheng1,2,Ma Ruixin1,2,Tang Ligang1,2,Du Zhenyu1,2
),Zhang Kaiyong1,2,Guo De1,2,Shi Changsheng1,2,Ma Ruixin1,2,Tang Ligang1,2,Du Zhenyu1,2