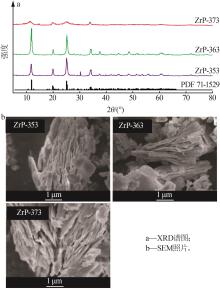
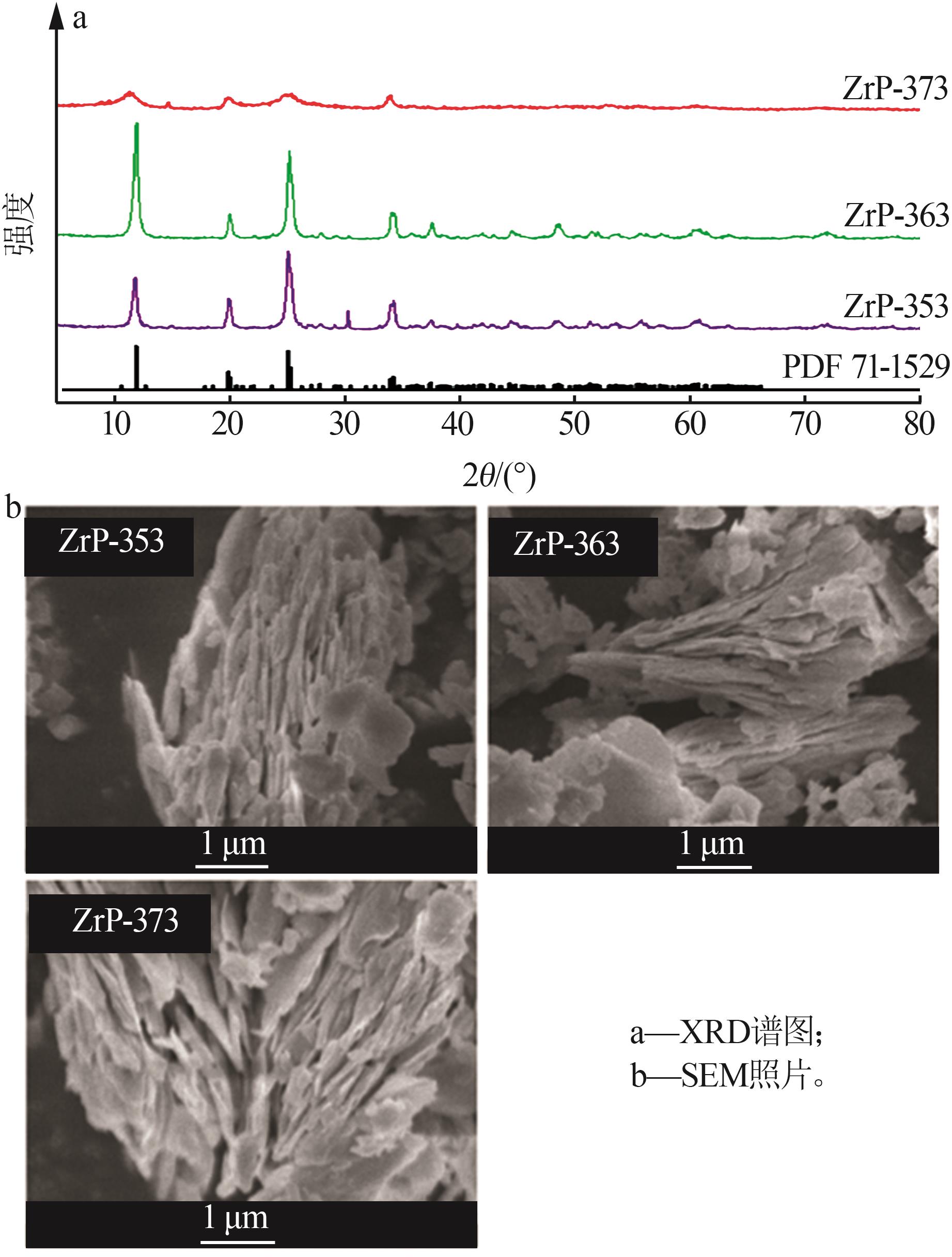
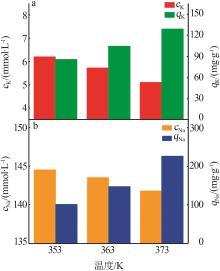
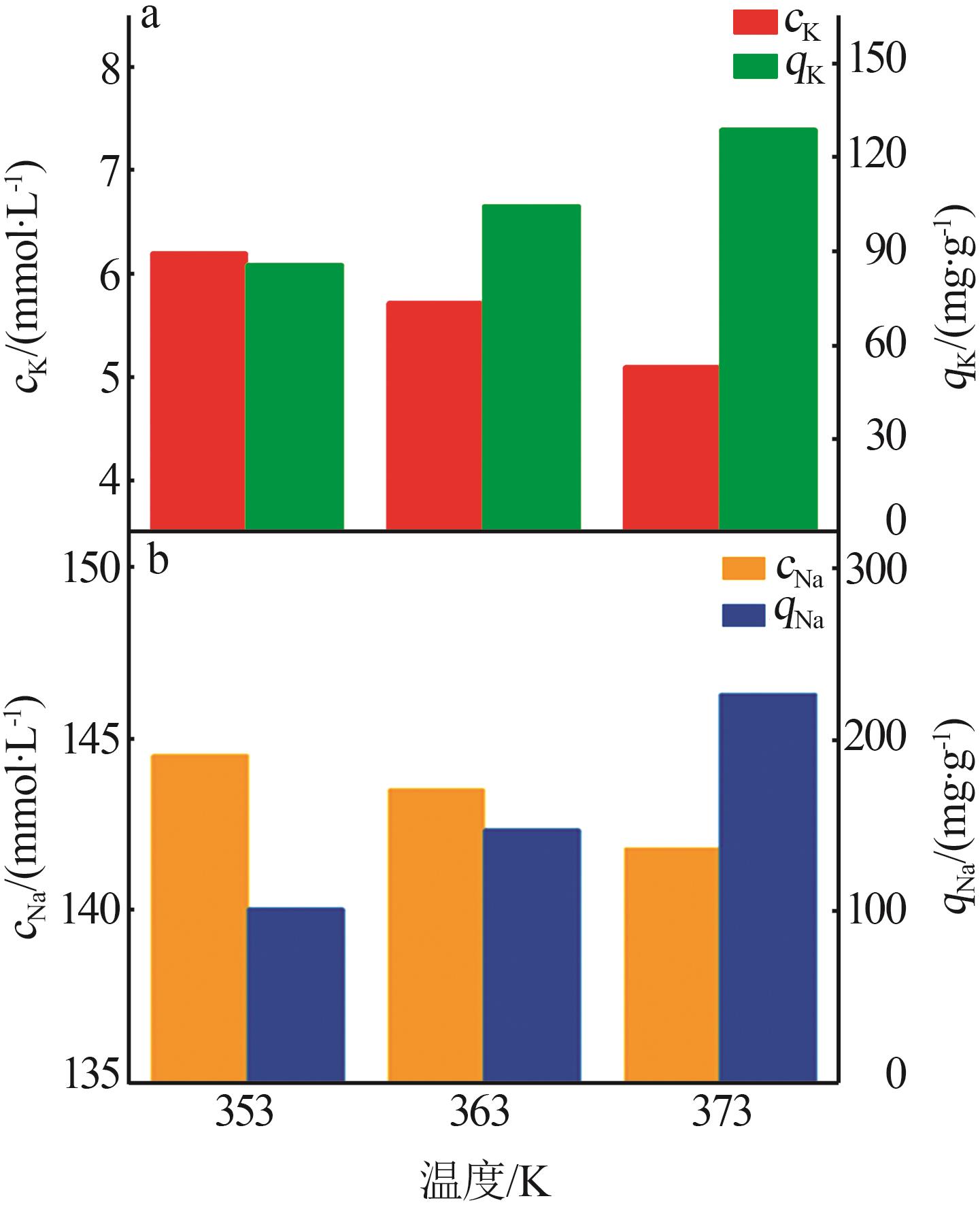
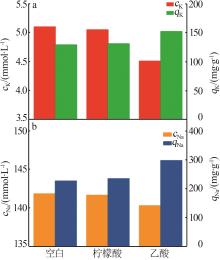
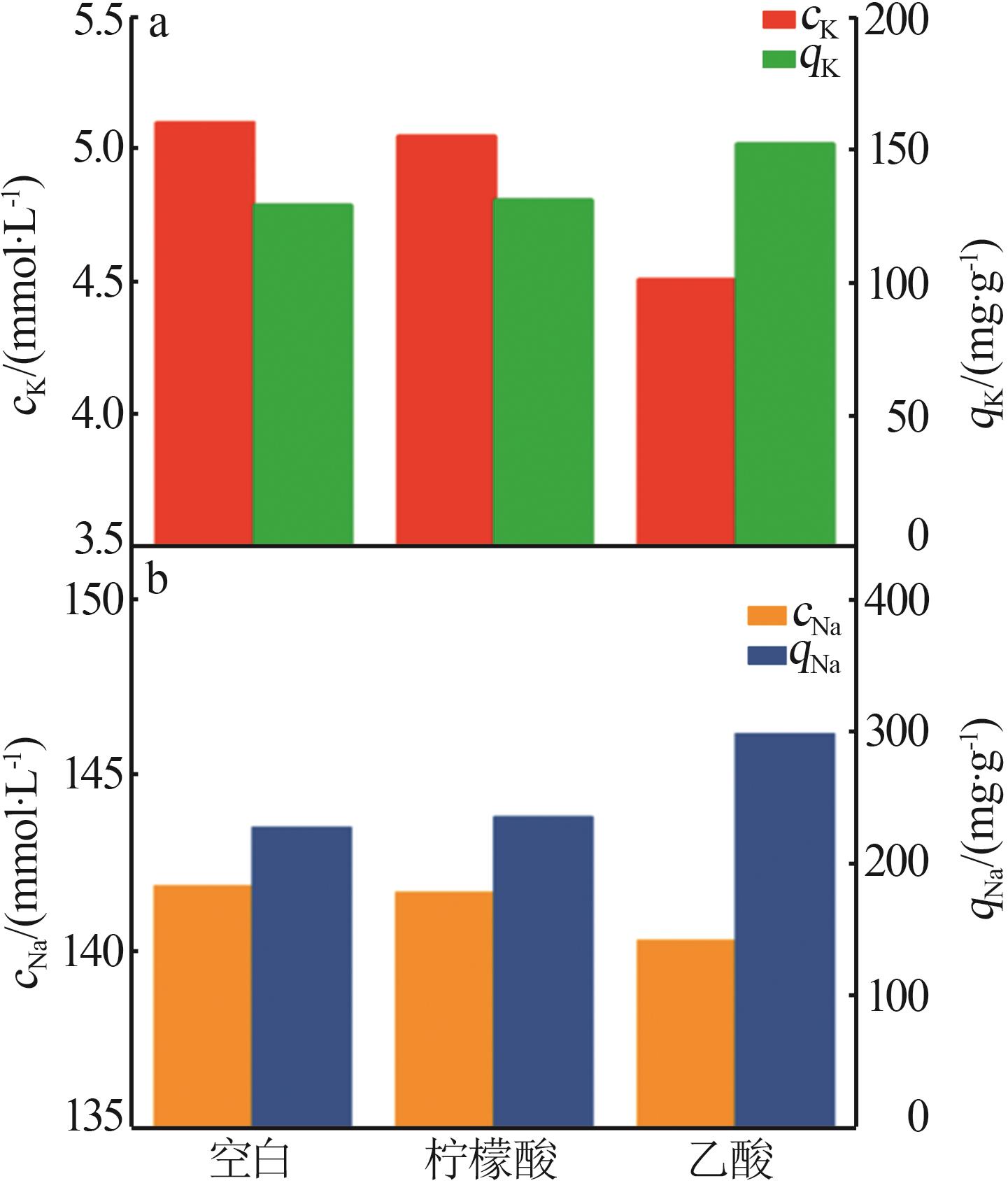
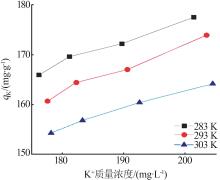
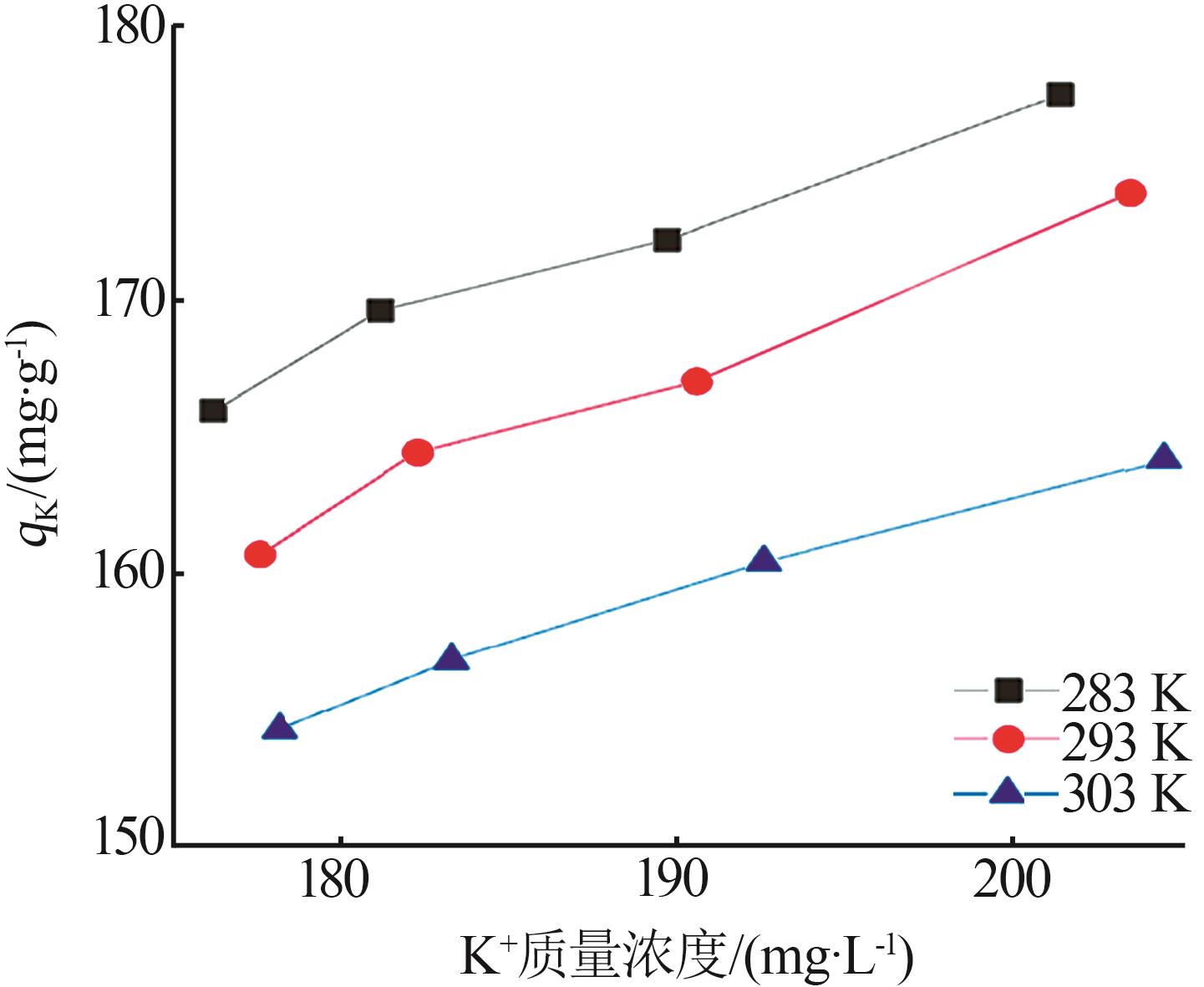
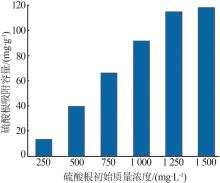
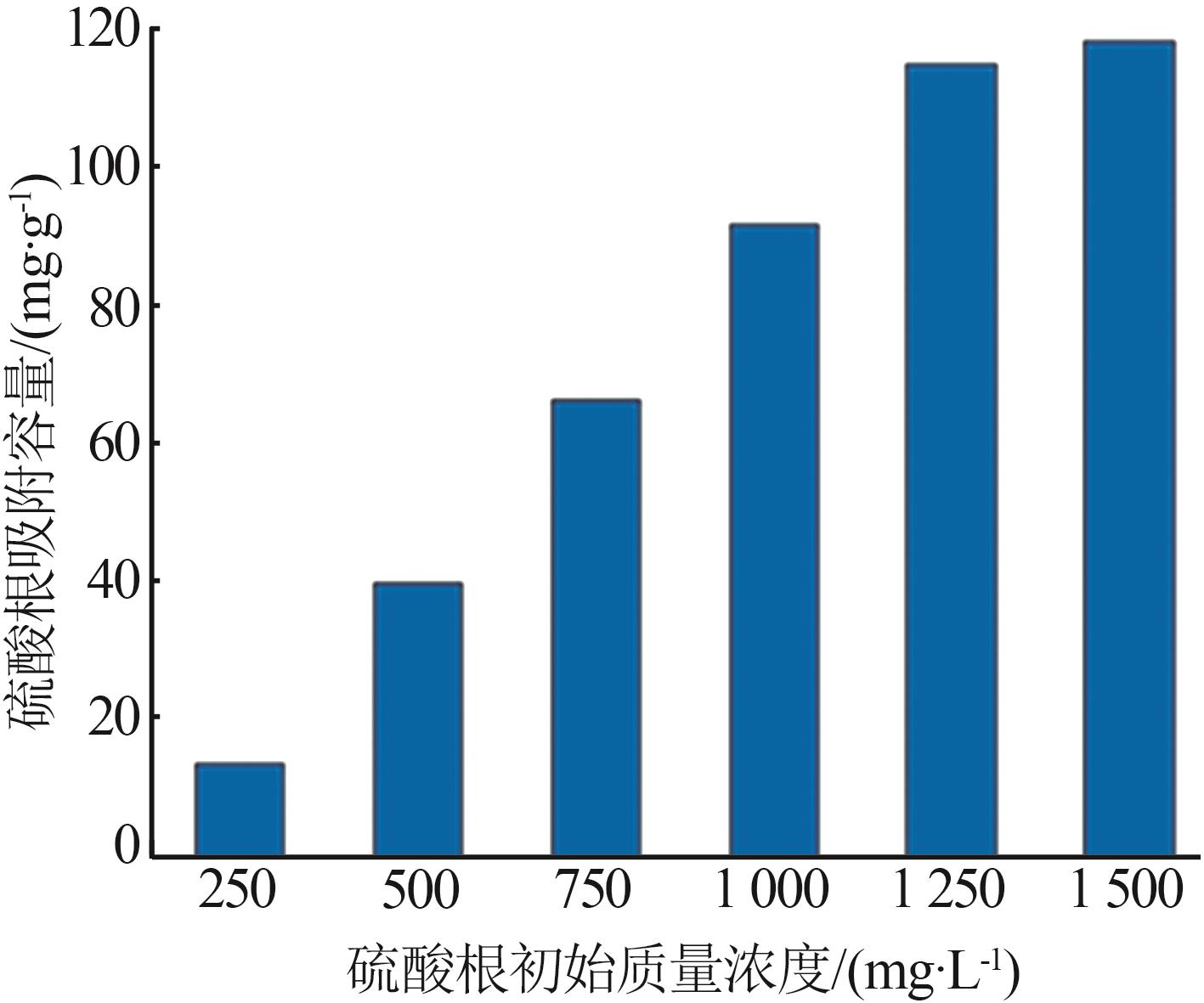
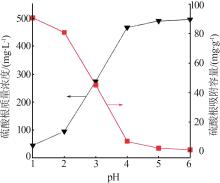
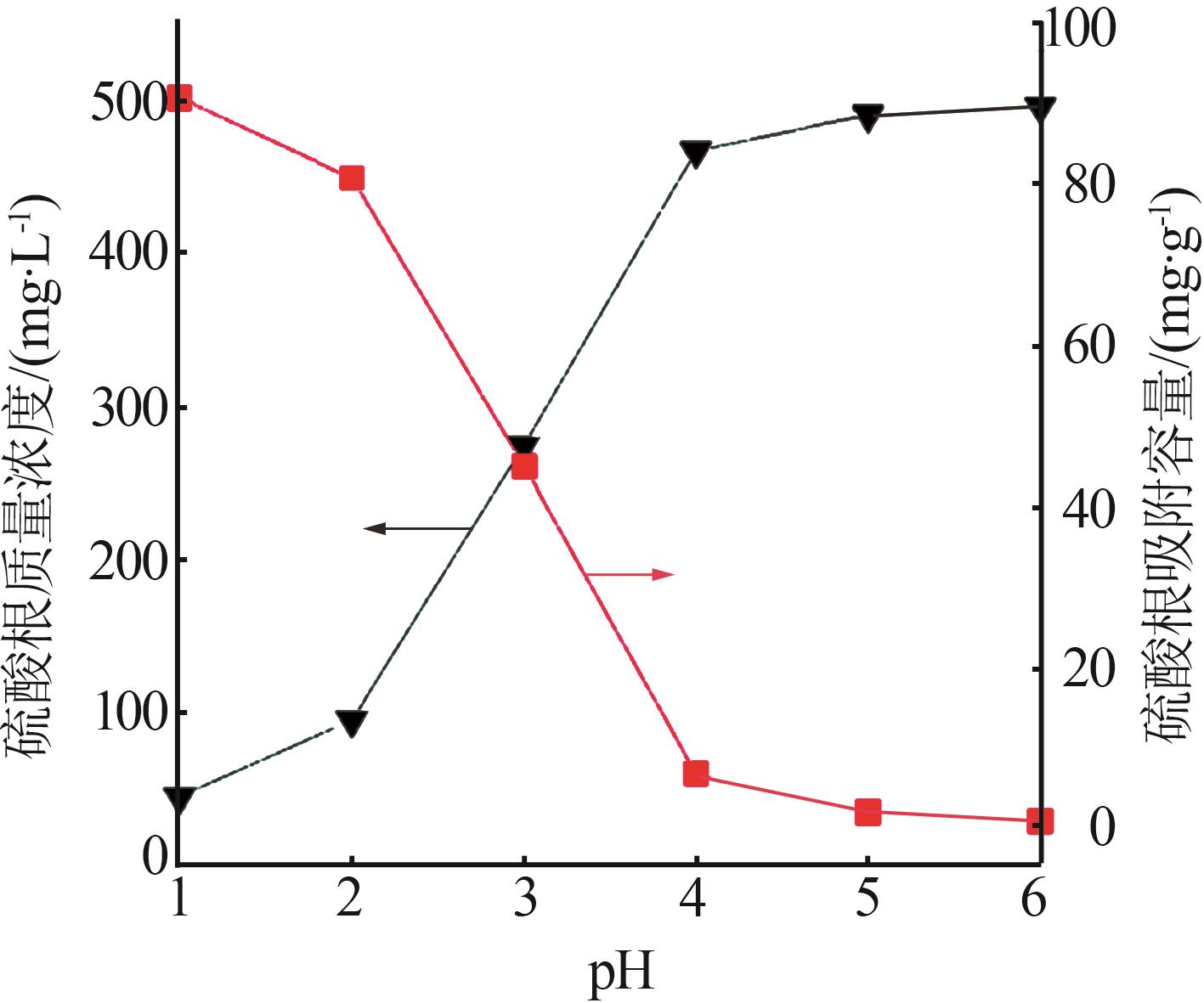
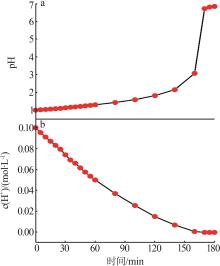
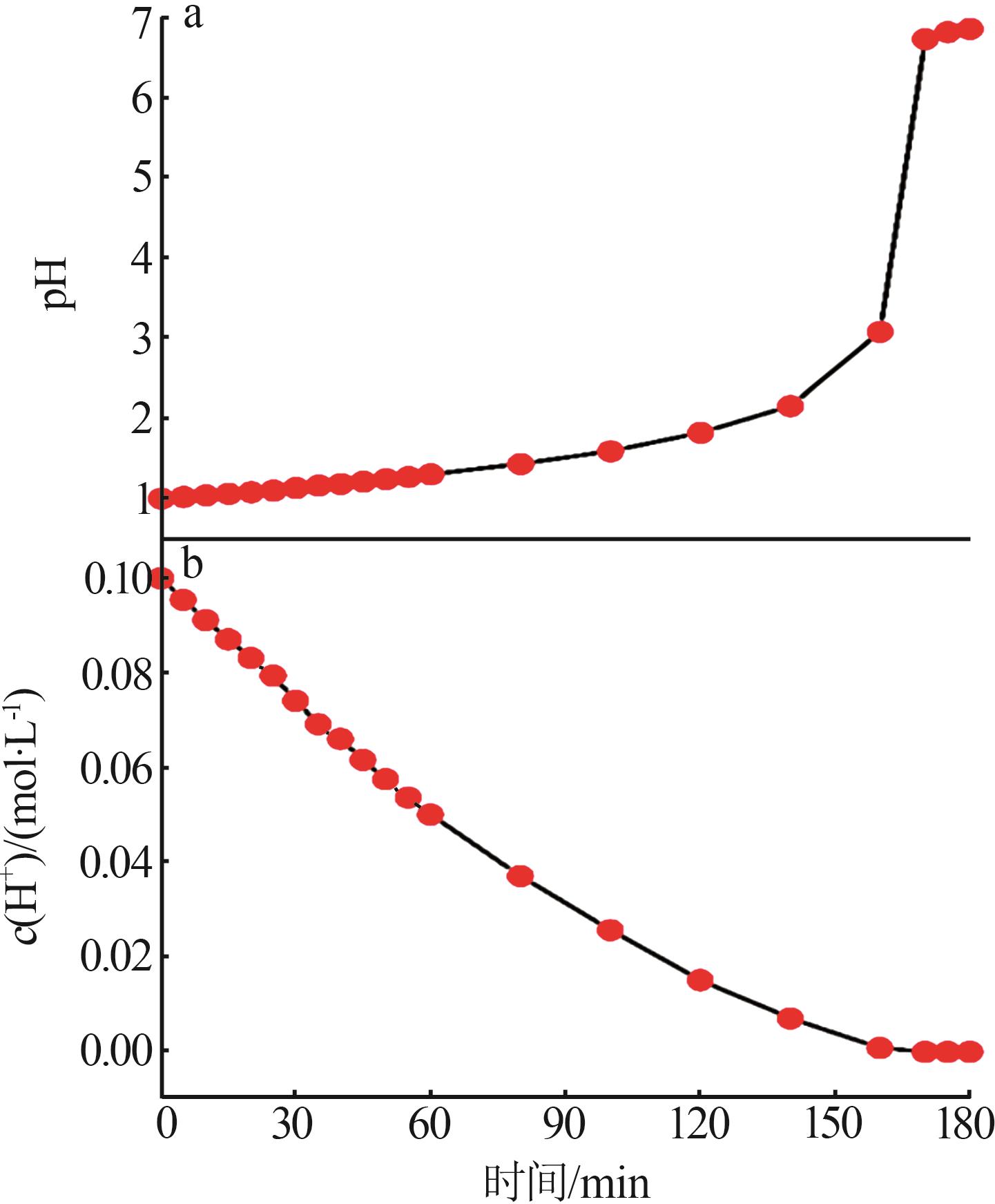
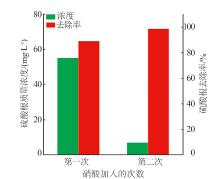
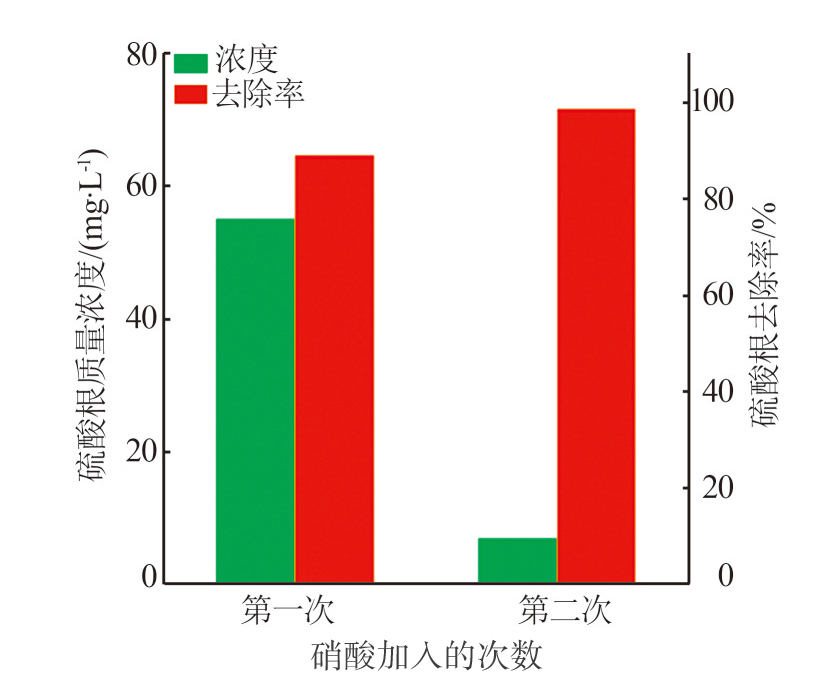
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (3): 116-124.doi: 10.19964/j.issn.1006-4990.2023-0305
• Environment·Health·Safety • Previous Articles Next Articles
Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance
XU Mengyao1( ), ZHANG Xin1, HE Kunpeng2, HE Jian2, JIANG Wei2(
), ZHANG Xin1, HE Kunpeng2, HE Jian2, JIANG Wei2( )
)
- 1.Sinopec Northwest Oilfield Branch,Urumchi 830011,China
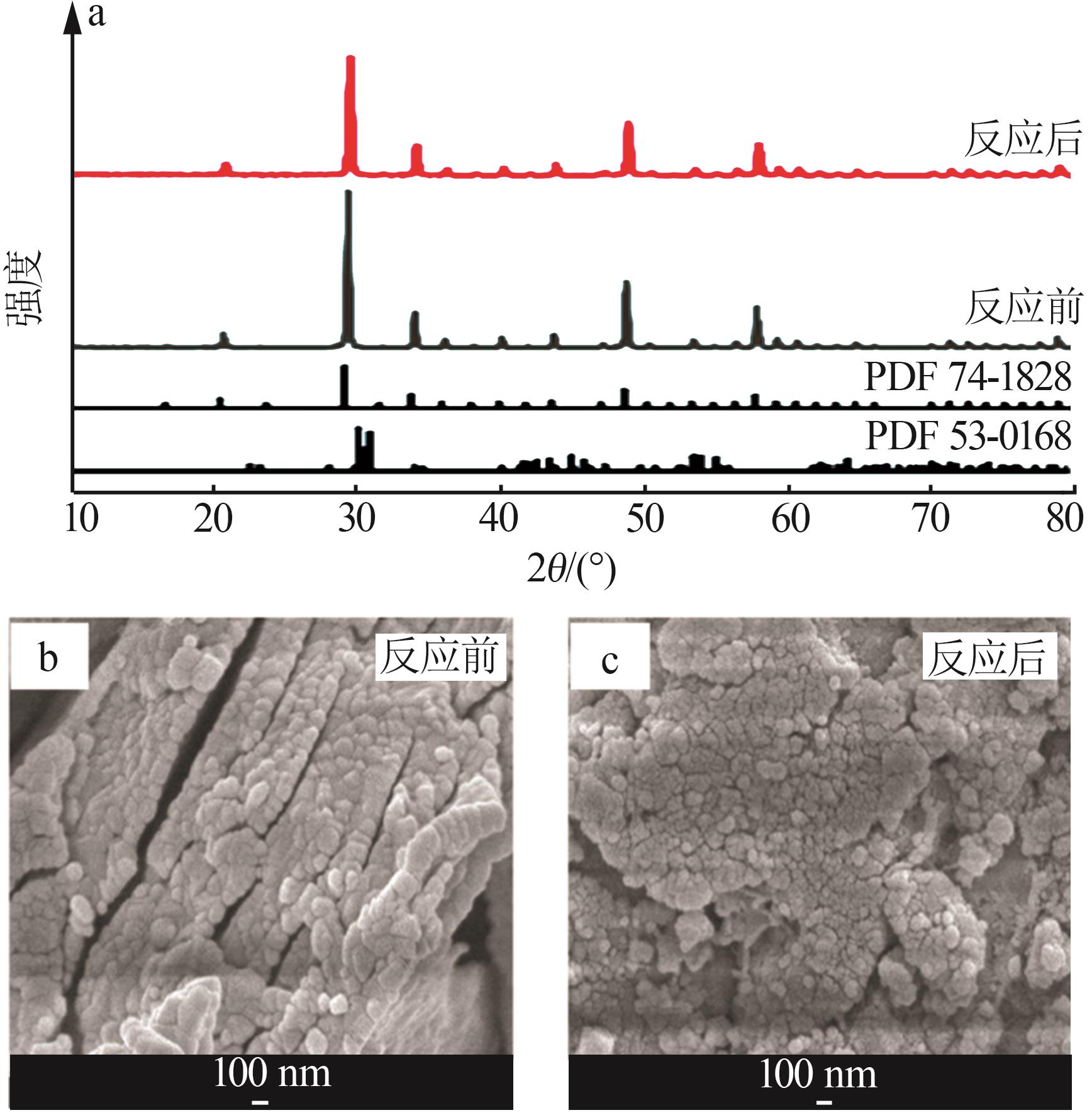
2.School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2023-06-02Online:2024-03-10Published:2024-03-14 -
Contact:JIANG Wei E-mail:xumengyao123.xbsj@sinopec.com;weijiang@scu.edu.cn
CLC Number:
Cite this article
XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance[J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124.
share this article
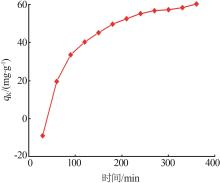
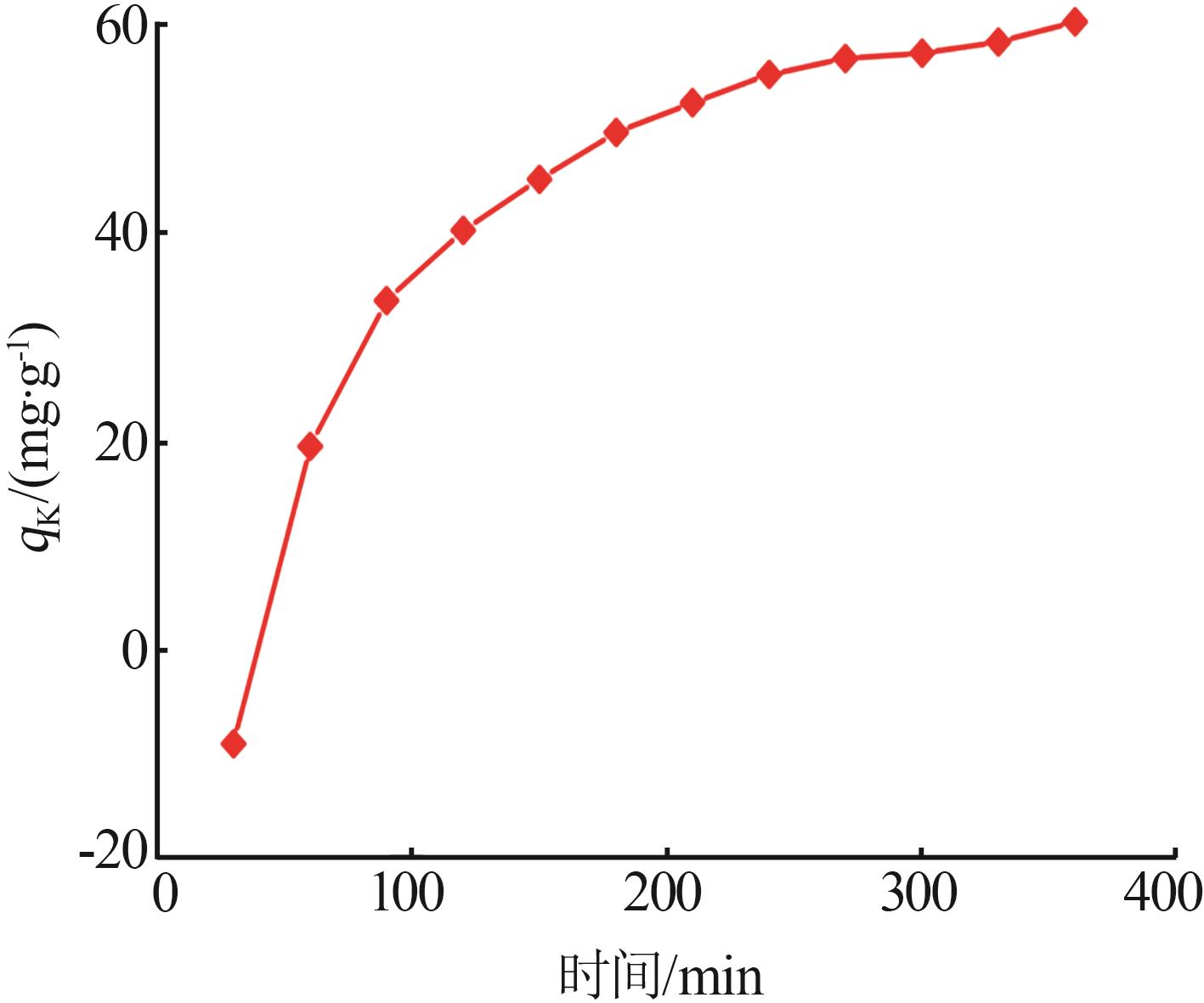
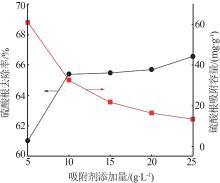
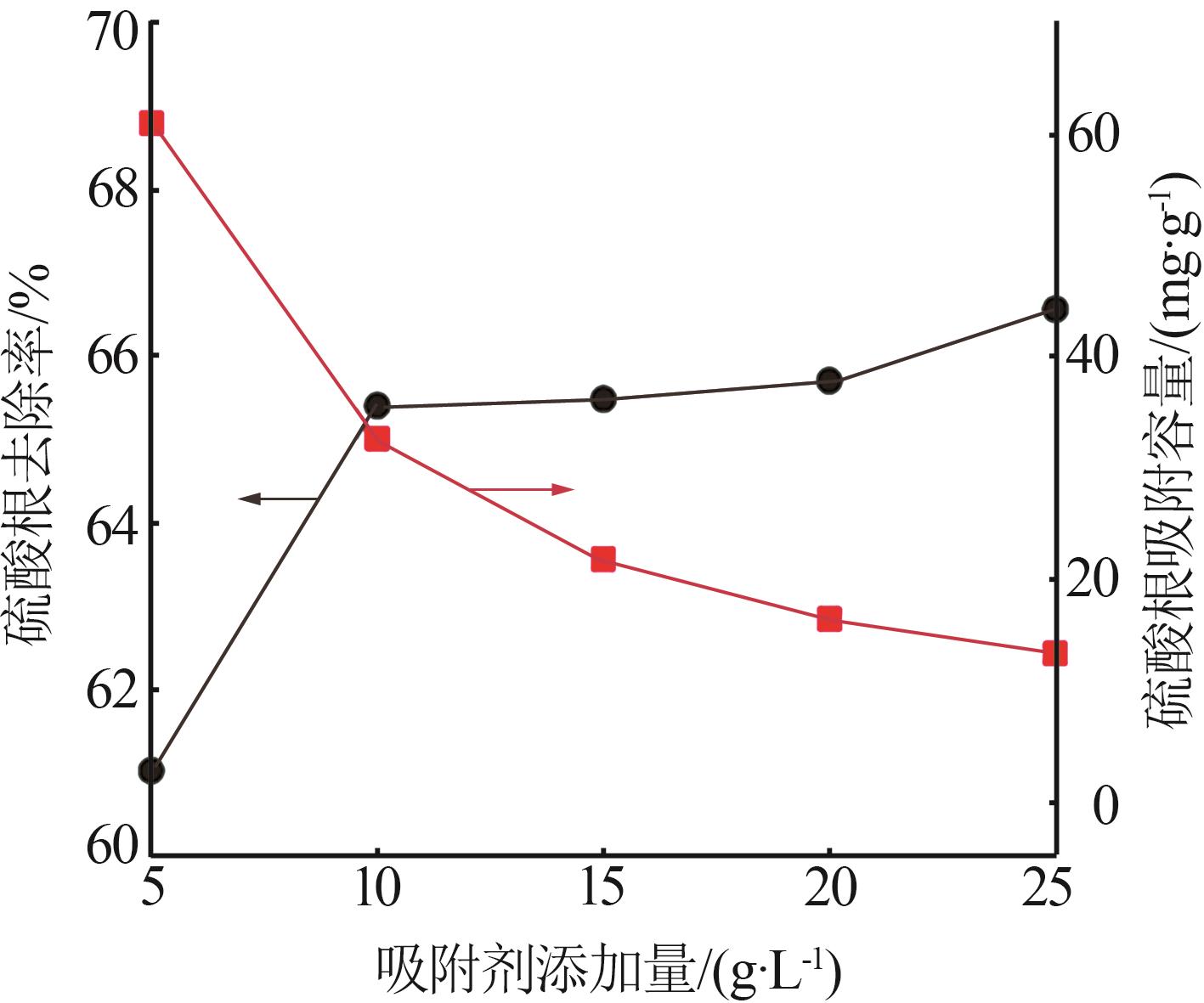
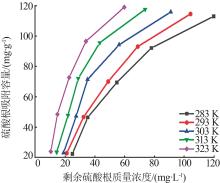
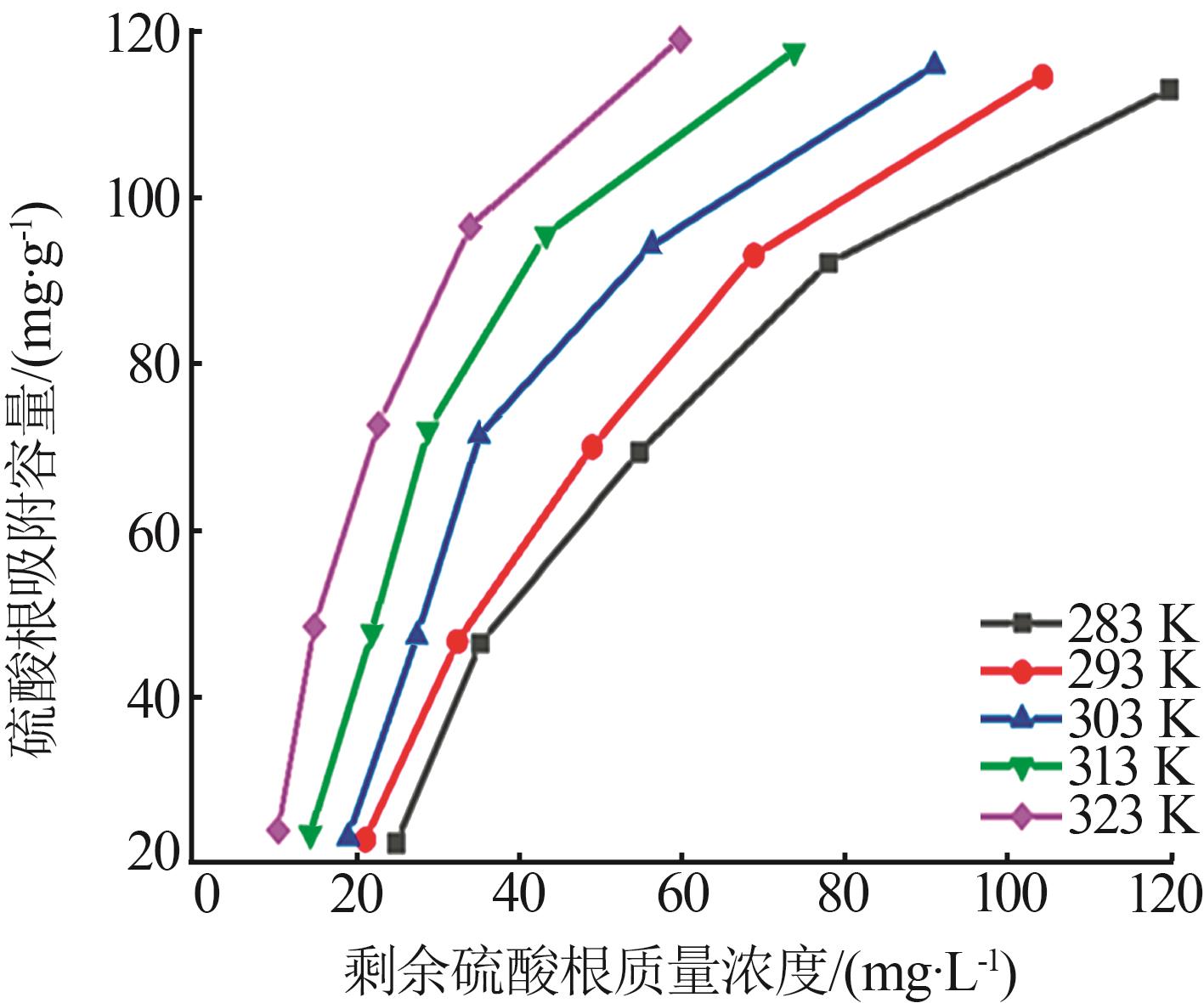
Table 5
Fitting parameters of adsorption isotherm models"
温度/ K | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
Qmax/ (mg·g-1) | KL/ (L·mg-1) | R2 | n | KF/(mg1-1/n · L1/n ·g-1) | R2 | ||
| 283 | 121.49 | 0.142 5 | 0.992 6 | 1.266 | 2.707 | 0.948 9 | |
| 293 | 126.91 | 0.173 6 | 0.998 2 | 1.235 | 2.786 | 0.947 1 | |
| 303 | 131.18 | 0.207 8 | 0.996 5 | 1.338 | 4.191 | 0.911 2 | |
| 313 | 133.46 | 0.220 9 | 0.995 2 | 1.367 | 5.348 | 0.914 3 | |
| 323 | 141.55 | 0.241 3 | 0.998 7 | 1.476 | 7.871 | 0.924 5 | |
| 1 | 林振汉.锆及其化合物的应用[J].世界有色金属,2011(7):68-69. |
| LIN Zhenhan.Application of zirconium and its compounds[J].World Nonferrous Metals,2011(7):68-69. | |
| 2 | 吴先锋.氧化锆磨削废料的回收工艺研究[D].青岛:山东科技大学,2014. |
| WU Xianfeng.Study on recovery technology of zirconia grinding waste[D].Qingdao:Shandong University of Science and Technology,2014. | |
| 3 | 喻杰.锆的核应用与我国锆材加工技术[J].机械制造,2009,47(5):48-50. |
| YU Jie.Nuclear application of zirconium and processing technology of zirconium in China[J].Machinery,2009,47(5):48-50. | |
| 4 | 陈鹏辉.钇稳定氧化锆(YSZ)透明陶瓷的制备与性能研究[D].镇江:江苏大学,2020. |
| CHEN Penghui.Fabrication and properties of yttria-stabilized ZrO2(YSZ)transparent ceramics[D].Zhenjiang:Jiangsu University,2020. | |
| 5 | 焦子豪.磷酸锆吸附剂的制备及其对锶离子的吸附性能和机理研究[D].南宁:广西大学,2021. |
| JIAO Zihao.Preparation of zirconium phosphate adsorbent and study on adsorption properties and mechanism of strontium[D].Nanning:Guangxi University,2021. | |
| 6 | 武越,周慧晴,史雍何,等.磷酸锆/介孔密胺树脂复合吸附材料的制备及性能研究[J].化工新型材料,2022,50(2):124-128. |
| WU Yue, ZHOU Huiqing, SHI Yonghe,et al.Study on preparation and property of Zr(HOP4)2 and mesoporous melamine resin as composite sorbent[J].New Chemical Materials,2022,50(2):124- 128. | |
| 7 | 刘志立.氧化钇纳米粉体的可控制备与表征[D].上海:上海第二工业大学,2021. |
| LIU Zhili.Controllable preparation and characterization of Y2O3 nanopowders[D].Shanghai:Shanghai Polytechnic University,2021. | |
| 8 | 于佳欣,白杨.2021年4季度稀土价格走势分析[J].稀土信息,2022(1):37-39. |
| YU Jiaxin, BAI Yang.Analysis of rare earth price trend in the fo-urth quarter of 2021[J].Rare Earth Information,2022(1):37-39. | |
| 9 | 王彦.2021年美国钇统计分析[J].稀土信息,2022(2):24-25. |
| WANG Yan.Statistical analysis of yttrium in the United States in 2021[J].Rare Earth Information,2022(2):24-25. | |
| 10 | 钟竞德.超细粒度氧化钇生产的新工艺分析[J].科技创新与应用,2016(6):116. |
| ZHONG Jingde.Analysis of new technology for ultra-fine grained yttrium oxide production[J].Technology Innovation and Application,2016(6):116. | |
| 11 | CLEARFIELD A, STYNES J A.The preparation of crystalline zirconium phosphate and some observations on its ion exchange behaviour[J].Journal of Inorganic and Nuclear Chemistry,1964,26(1):117-129. |
| 12 | CHENG Yu, CHUAH G K.The synthesis and applications of α-zirconium phosphate[J].Chinese Chemical Letters,2020, 31(2):307-310. |
| 13 | 陆运涛.磷酸锆纳米载银抗菌剂及其抗菌中心静脉导管的体内外抗菌效果和安全性研究[D].上海:复旦大学,2010. |
| LU Yuntao.Study on the antibacterial effect and safety of silver-containing zirconium phosphate antibacterial agents of nanometer level and the made-up central venous catheter in vitro and in vivo[D].Shanghai:Fudan University,2010. | |
| 14 | 曾仁权,傅相锴.磷酸锆及其衍生物的离子交换性能[J].化学进展,2009,21(12):2536-2541. |
| ZENG Renquan, FU Xiangkai.Ion-exchange properties of zirconium phosphate and its derivatives[J].Progress in Chemistry,2009,21(12):2536-2541. | |
| 15 | 邱磊.蛋壳和桔皮对重金属的吸附性能及不同吸附工艺研究[D].天津:河北工业大学,2020. |
| QIU Lei.Studies on the adsorption properties of heavy metals by eggshell and orange peel and different adsorption processes[D].Tianjin:Hebei University of Technology,2020. |
| [1] | BAI Xingxing, LI Hanfei, TANG Yong, ZHANG Jun, ZHU Guangkai, LI Lishuo, TONG Zhangfa. Study on preparation of cellulose based hydrogel doped with nano-calcium carbonate and its adsorption properties of copper ions [J]. Inorganic Chemicals Industry, 2025, 57(2): 83-91. |
| [2] | WANG Ping, XU Rongsheng, SUN Dong, SHI Xiaohong, XU Wei, LI Mei. Study on preparation of nitrogen-doped biochar and its adsorption properties for methylene blue [J]. Inorganic Chemicals Industry, 2024, 56(9): 117-127. |
| [3] | SONG Zhaoxia, LIU Yongkang, GUO Yaokun, WANG Tengfei. Study on adsorption performance of wheat straw biochar on malachite green [J]. Inorganic Chemicals Industry, 2024, 56(9): 128-135. |
| [4] | XIONG Chengrong, CHEN Yan, TANG Miao, ZHANG Fengze, ZHOU Tianxiang, OUYANG Ruifeng, DONG Gang, SHI Wei, ZENG Tao, CHEN Yunxia, SU Xiaoli. Research progress on preparation of 2D vermiculite nanosheets and their environmental adsorption of pollutants [J]. Inorganic Chemicals Industry, 2024, 56(8): 19-26. |
| [5] | ZHANG Bangcheng, WANG Li. Preparation and adsorption properties of waste polyester⁃based activated carbon activated by ZnCl2 [J]. Inorganic Chemicals Industry, 2024, 56(7): 126-134. |
| [6] | ZHANG Lijin, LÜ Qing, CHEN Xiaolang, LI Qingxin, SHI Hongyu, QIN Jun. Preparation of Ca-based LDO composite material and its adsorption performance for phosphate [J]. Inorganic Chemicals Industry, 2024, 56(7): 37-45. |
| [7] | ZHAO Chuang, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation technology of polycyclic aromatic hydrocarbons by adsorbent [J]. Inorganic Chemicals Industry, 2024, 56(7): 61-68. |
| [8] | LIU Kailong, ZHU Kongyi, GUO Chunlei, MA Xiaobiao, WANG Yujian, SHENG Qiang, LI Xiang, WANG Yinbin, JIN Fengying. Effects of process condition on performance of diesel aromatic to light aromatic [J]. Inorganic Chemicals Industry, 2024, 56(6): 139-146. |
| [9] | ZHU Zenghu, WANG Min, PENG Zhengjun, JIA Guofeng, LI Yan. Study on adsorption of potassium ions in lithium chloride solution [J]. Inorganic Chemicals Industry, 2024, 56(6): 61-66. |
| [10] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [11] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [12] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [13] | SHI Hongyu, LÜ Qing, ZHANG Lijin, LI Qingxin, QIN Jun. Study on site energy of phosphate adsorption by layered double hydrotalcites with mesoporous morphologies [J]. Inorganic Chemicals Industry, 2024, 56(10): 38-46. |
| [14] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [15] | YAN Zhen, QIU Zhaofu, JIN Xibiao, WANG Yuan, LIU Chang, YANG Ji. Study on 4A molecular sieve loaded with Ce and γ-Fe2O3 for removal of Sb(Ⅲ) and Sb(Ⅴ) in water [J]. Inorganic Chemicals Industry, 2024, 56(1): 81-89. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||