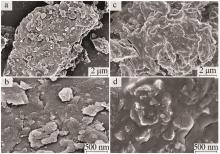
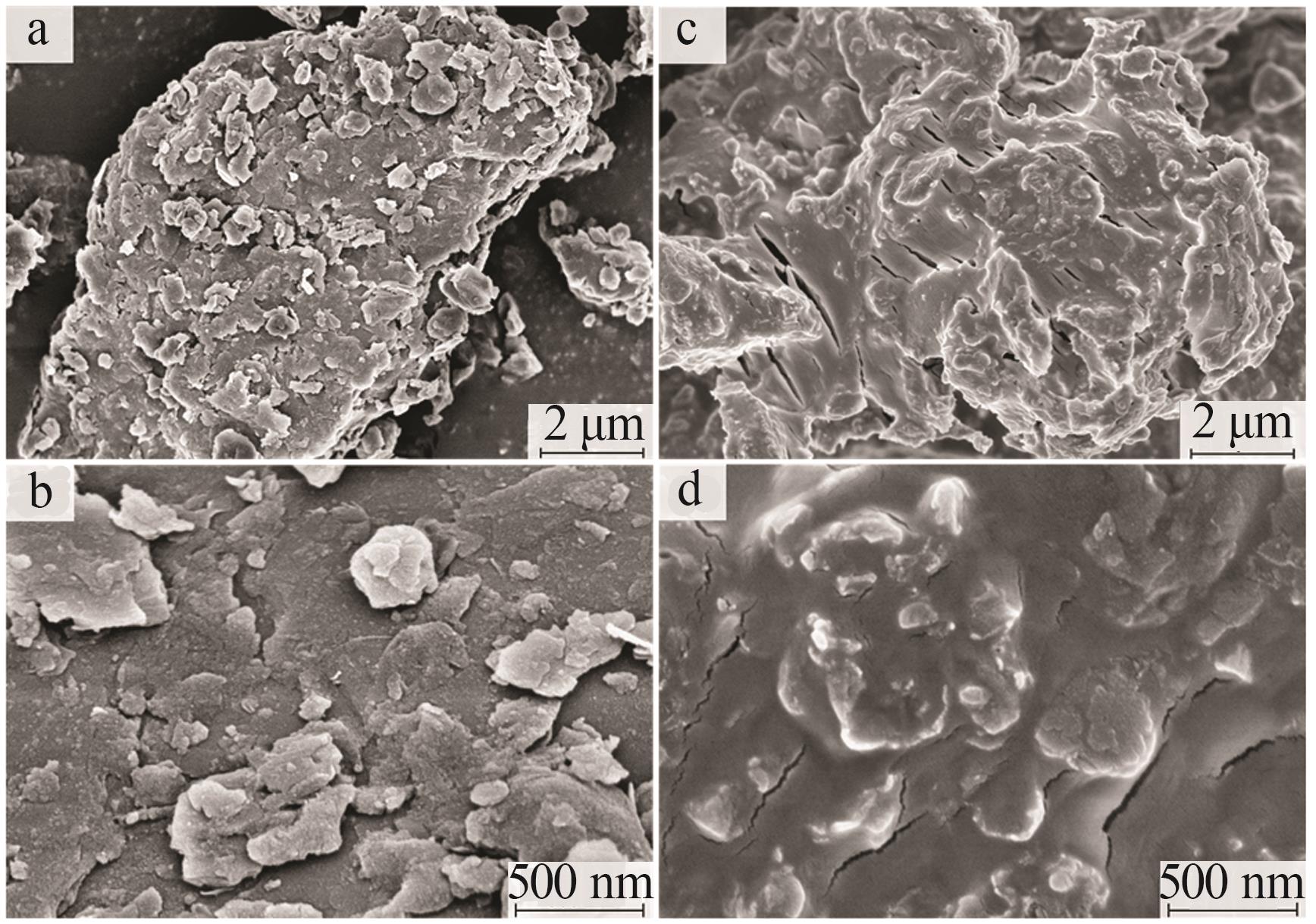
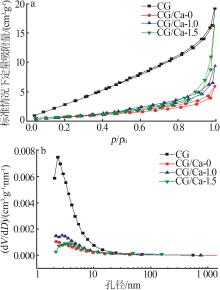
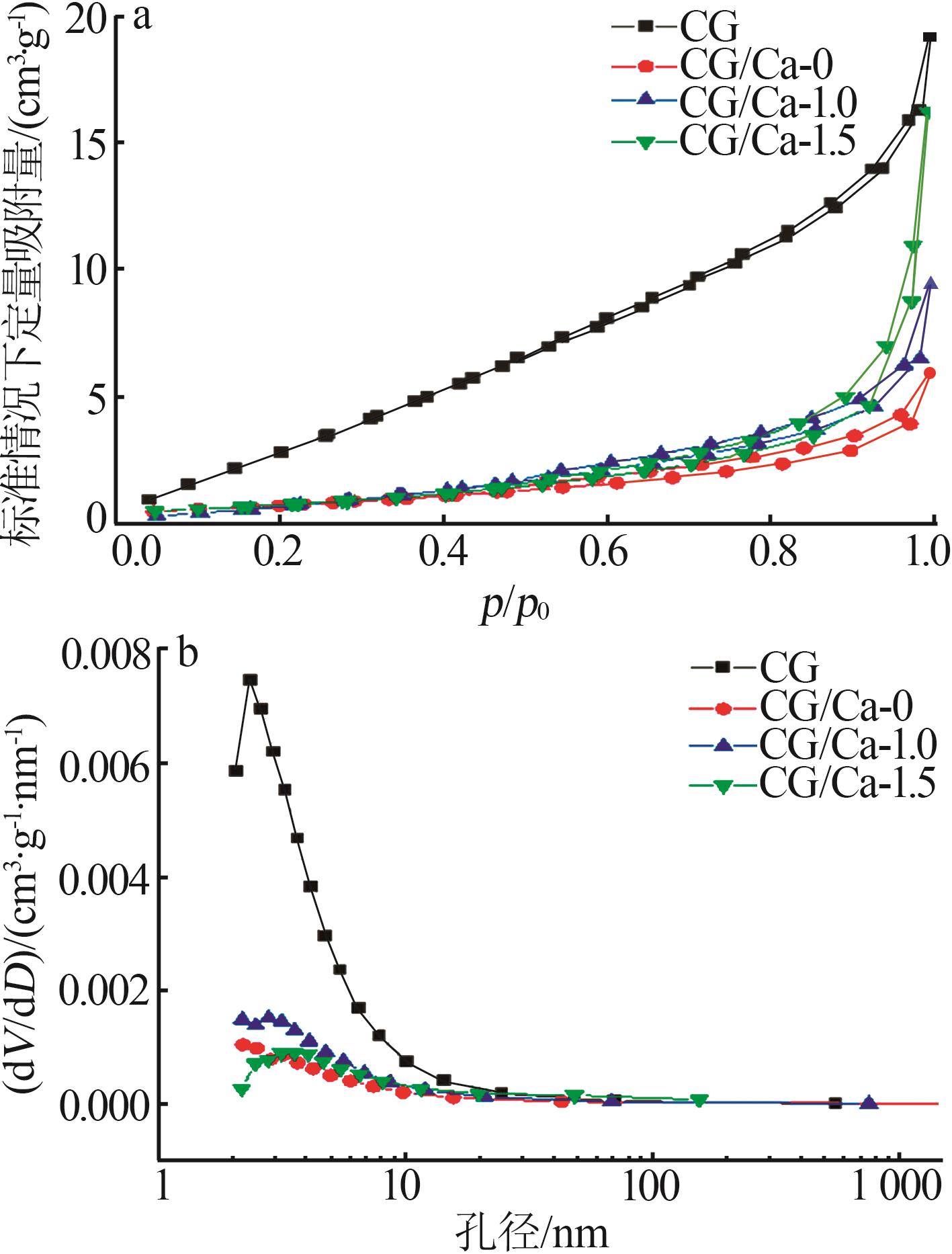
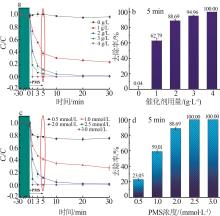
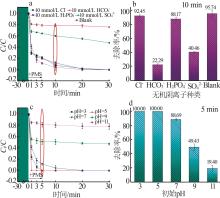
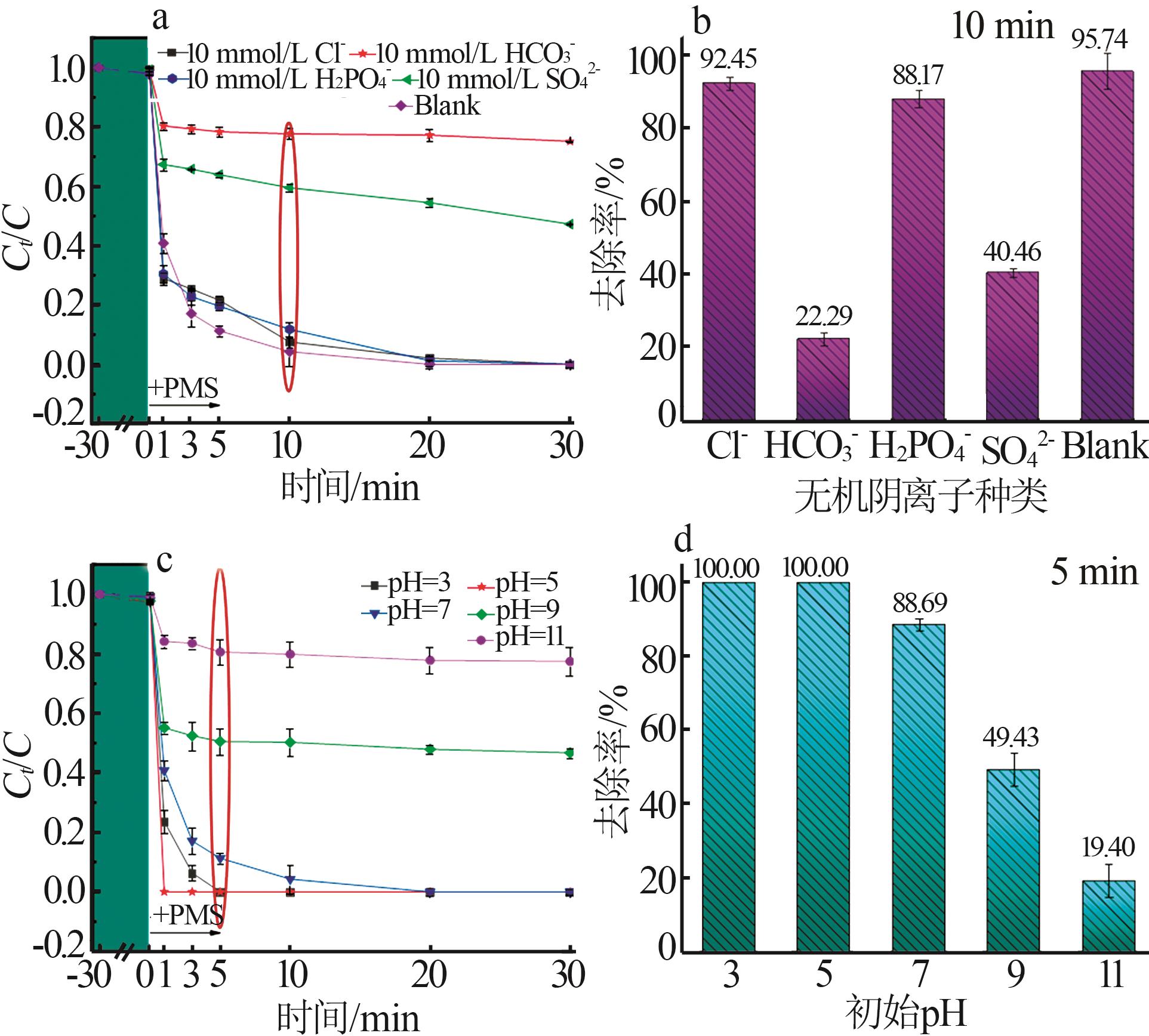
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (1): 103-112.doi: 10.19964/j.issn.1006-4990.2024-0193
• Environment·Health·Safety • Previous Articles Next Articles
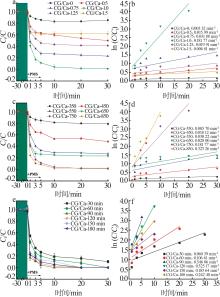
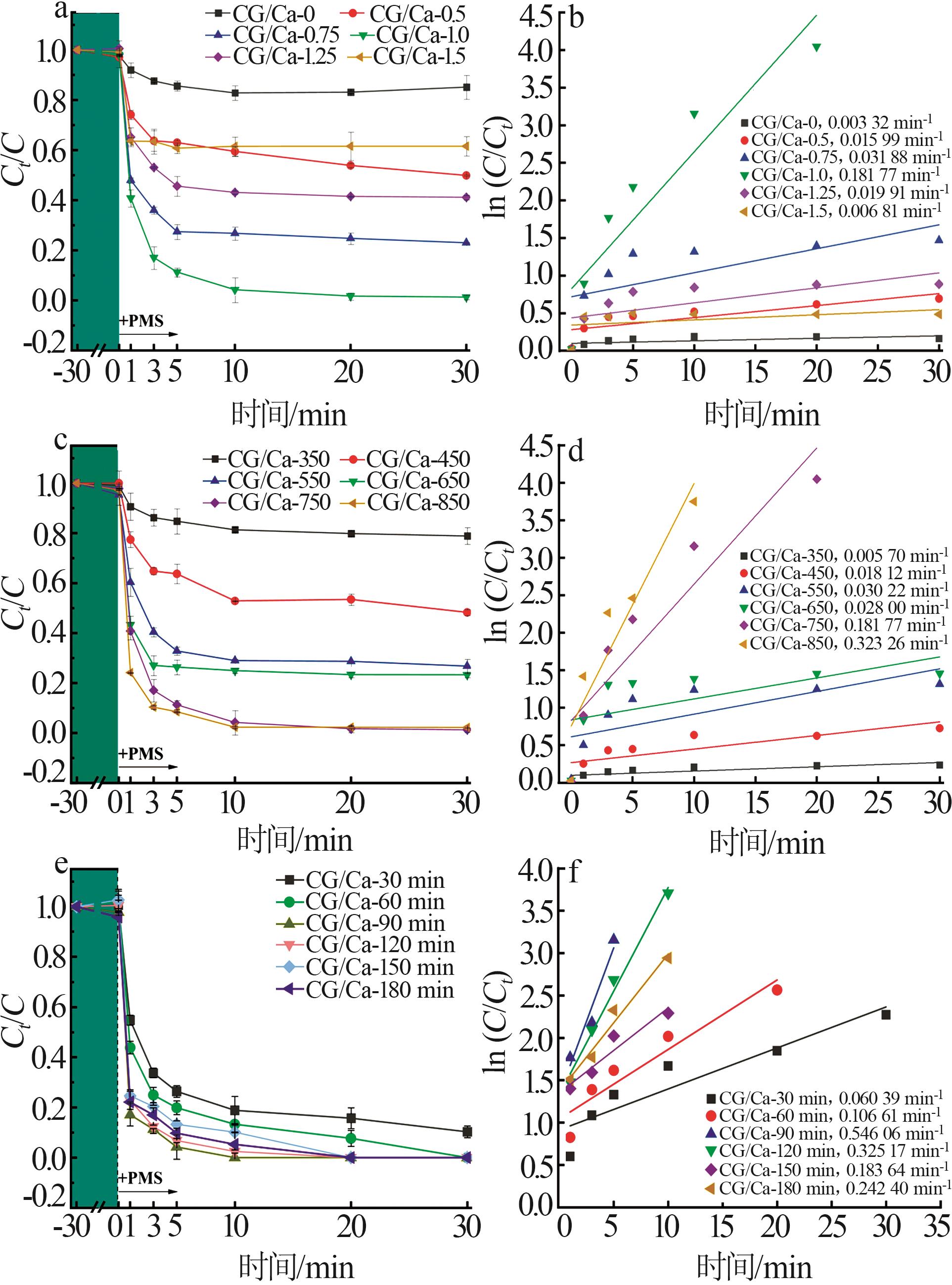
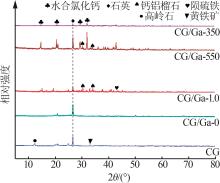
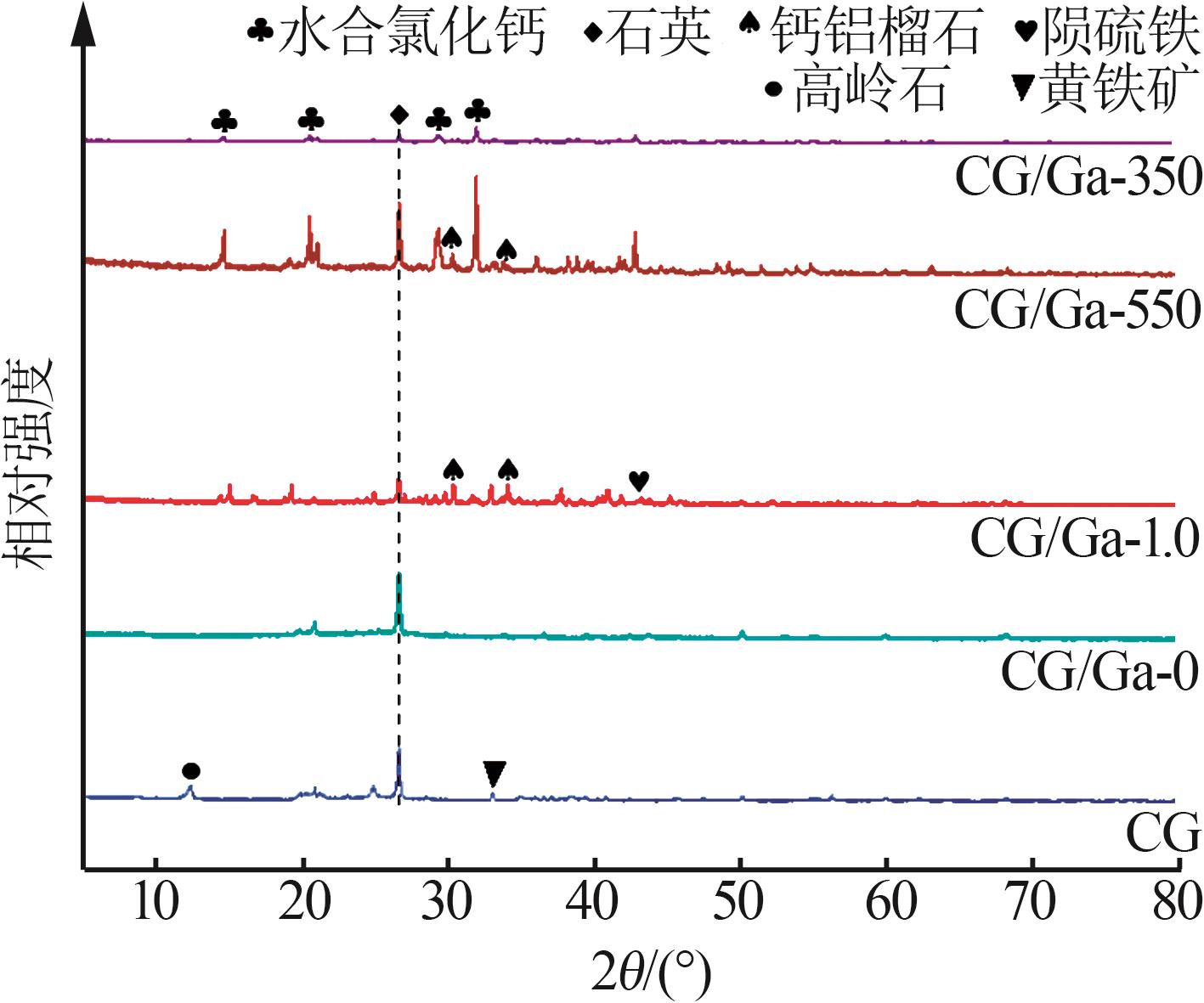
Study on performance of calcium chloride assisted thermal activation of coal gangue and its peroxymonosulfate activation toward benzo(a)pyrene degradation
HAN Wei1( ), SONG Yongming1, LIU Qi2, XU Jinling3, XU Rong3, LI Chunquan4, YIN Shuaijun4, SUN Zhiming4(
), SONG Yongming1, LIU Qi2, XU Jinling3, XU Rong3, LI Chunquan4, YIN Shuaijun4, SUN Zhiming4( )
)
- 1.Inner Mongolia Mengtai Buliangou Coal Industry Co.,Ltd.,Ordos 010399,China
2.Huadian Coal Industry Group Co.,Ltd.,Beijing 100035,China
3.Huadian Coal Industry Group Digital Intelligence Technology Co.,Ltd.,Beijing 102400,China
4.School of Chemical and Environmental Engineering,China University of Mining and Technology(Beijing),Beijing 100083,China
-
Received:2024-04-07Online:2025-01-10Published:2025-01-24 -
Contact:SUN Zhiming E-mail:weihanhuadian@163.com;zhimingsun@cumtb.edu.cn
CLC Number:
Cite this article
HAN Wei, SONG Yongming, LIU Qi, XU Jinling, XU Rong, LI Chunquan, YIN Shuaijun, SUN Zhiming. Study on performance of calcium chloride assisted thermal activation of coal gangue and its peroxymonosulfate activation toward benzo(a)pyrene degradation[J]. Inorganic Chemicals Industry, 2025, 57(1): 103-112.
share this article
| 1 | 杨越.我国煤矸石堆存现状及其大宗量综合利用途径[J].中国资源综合利用,2014,32(6):18-22. |
| YANG Yue.Coal gangue stacked and its comprehensive utilizati-on[J].China Resources Comprehensive Utilization,2014,32(6):18-22. | |
| 2 | LI Dan, WU Daishe, XU Feigao,et al.Literature overview of Chinese research in the field of better coal utilization[J].Journal of Cleaner Production,2018,185:959-980. |
| 3 | 杨长俊.关于煤矸石回填工艺技术的研究和思考[J].矿业安全与环保,2022,49(1):104-108. |
| YANG Changjun.Research and thinking about coal gangue backfilling technology[J].Mining Safety & Environmental Protection,2022,49(1):104-108. | |
| 4 | 关杰,李英顺.煤矸石综合利用现状及前景[J].环境与可持续发展,2008,33(1):34-36. |
| GUAN Jie, LI Yingshun.Current situation and prospects of comprehensive utilization of coal gangue[J].Environment and Sustainable Development,2008,33(1):34-36. | |
| 5 | 周楠,姚依南,宋卫剑,等.煤矿矸石处理技术现状与展望[J].采矿与安全工程学报,2020,37(1):136-146. |
| ZHOU Nan, YAO Yinan, SONG Weijian,et al.Present situation and prospect of coal gangue treatment technology[J].Journal of Mining & Safety Engineering,2020,37(1):136-146. | |
| 6 | LI Linhao, LONG Guangcheng, BAI Chaoneng,et al.Utilization of coal gangue aggregate for railway roadbed construction in practi- ce[J].Sustainability,2020,12(11):4583. |
| 7 | LI Jiayan, WANG Jinman.Comprehensive utilization and environmental risks of coal gangue:A review[J].Journal of Cleaner Production,2019,239:117946. |
| 8 | 孙晓刚,马征宇,赵家琪,等.黄金尾砂和煤矸石协同制备发泡陶瓷及其性能研究[J].金属矿山,2021(8):214-220. |
| SUN Xiaogang, MA Zhengyu, ZHAO Jiaqi,et al.Preparation and properties of foamed ceramics from gold tailings and coal gan-gue[J].Metal Mine,2021(8):214-220. | |
| 9 | 范雯阳,李侠,周珊,等.利用煤矸石合成沸石及其矿物成分的影响[J].非金属矿,2016,39(1):75-77. |
| FAN Wenyang, LI Xia, ZHOU Shan,et al.Synthesis of zeolite using coal gangue and the effect of mineral composition[J].Non-Metallic Mines,2016,39(1):75-77. | |
| 10 | 孙勇,张祥,吕树光.基于过硫酸盐的多环芳烃污染场地原位修复技术研究进展[J].环境污染与防治,2023,45(1):105-112,121. |
| SUN Yong, ZHANG Xiang, Shuguang LÜ.Research progress of in situ remediation technology based on persulfate for polycyclic aromatic hydrocarbons contaminated site[J].Environmental Pollution & Control,2023,45(1):105-112,121. | |
| 11 | 张季,刘伟,王慎.锰氧化物在芘污染土壤修复中的应用研究[J].中国锰业,2022,40(3):27-32. |
| ZHANG Ji, LIU Wei, WANG Shen.An application of manganese oxides in remediation of pyrene contaminated soil[J].China′s manganese industry,2022,40(3):27-32. | |
| 12 | 施维林,罗王捷.有机物污染土壤修复技术研究与应用进展[J].苏州科技大学学报(自然科学版),2022,39(2):1-8. |
| SHI Weilin, LUO Wangjie.A review of remediation technology of organic contaminated soil[J].Journal of Suzhou University of Science and Technology(Natural Science Edition),2022,39(2):1-8. | |
| 13 | 夏强,廖小刚,李纲.化学浴沉积法制备氢氧化镍及其催化过一硫酸盐降解亚甲基蓝[J].无机盐工业,2021,53(10):119-124. |
| XIA Qiang, LIAO Xiaogang, LI Gang.Degradation of methylene blue by catalytic peroxymonosulfate with Ni(OH)2 synthesized through chemical bath deposition method[J].Inorganic Chemicals Industry,2021,53(10):119-124. | |
| 14 | BURROW J N, PENDER J P, GUERRERA J V,et al.CaCl2-activated carbon nitride:Hierarchically nanoporous carbons with ultrahigh nitrogen content for selective CO2 adsorption[J].ACS Applied Nano Materials,2020,3(6):5965-5977. |
| 15 | 张利洁,李德刚,韩文渊,等.磷掺杂碳量子点的制备及活化过一硫酸盐降解亚甲基蓝[J].无机盐工业,2024,56(1):126-133. |
| ZHANG Lijie, LI Degang, HAN Wenyuan,et al.Preparation of phosphorus-doped carbon quantum dots and activation of peroxymonosulfate for degradation of methylene blue[J].Inorganic Che-Industry micals,2024,56(1):126-133. | |
| 16 | ZHANG Hao, TANG Lin, WANG Jiajia,et al.Enhanced surface activation process of persulfate by modified bagasse biochar for degradation of phenol in water and soil:Active sites and electron transfer mechanism[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2020,599:124904. |
| 17 | LI Chunquan, WANG Sidi, ZHANG Xiangwei,et al. In-situ preparation of coal gangue-based catalytic material for efficient peroxymonosulfate activation and phenol degradation[J].Journal of Cleaner Production,2022,374:133926. |
| 18 | 张谌虎,马毅,朱山,等.煤矸石螯合改性后对选矿废水中重金属离子吸附研究[J].无机盐工业,2023,55(4):97-103. |
| ZHANG Chenhu, MA Yi, ZHU Shan,et al.Study on adsorption of heavy metal ions in mineral processing wastewater by chelating modified coal gangue[J].Inorganic Chemicals Industry,2023,55(4):97-103. | |
| 19 | 谢娟,夏润南,杜红霞,等. α-Fe2O3/煤矸石复合光催化剂的制备及其降解五氯酚性能的研究[J].无机盐工业,2019,51(5):74-77. |
| XIE Juan, XIA Runnan, DU Hongxia,et al.Preparation of α-Fe2O3/coal gangue composite photocatalyst and its application in pentachlorophenol degradation[J].Inorganic Chemicals Industry,2019,51(5):74-77. | |
| 20 | FRAISSLER G, JÖLLER M, BRUNNER T,et al.Influence of dry and humid gaseous atmosphere on the thermal decomposition of calcium chloride and its impact on the remove of heavy metals by chlorination[J].Chemical Engineering and Processing:Process Intensification,2009,48(1):380-388. |
| 21 | ZHAO Xinbo, LI Chuanchang, BAI Kaihao,et al.Multiple structure graphite stabilized stearic acid as composite phase change materials for thermal energy storage[J].International Journal of Mining Science and Technology,2022,32(6):1419-1428. |
| 22 | LI Yongxin, ZHANG Ju, YAN Changwang,et al.Effect of activated coal gangue on the hydration and hardening of Portland cement[J].Construction and Building Materials,2024,422:135740. |
| 23 | LI Bei, LV Hongpeng, DENG Jun,et al.Effect of calcium chloride on the self-ignition behaviours of coal using hot-plate test[J].Fuel,2021,304:121451. |
| 24 | ZHONG Guoyu, XIE Haomin, XU Zhihao,et al.Calcium chloride activation of mung bean:A low-cost,green route to N-doped porous carbon for supercapacitors[J].ChemistrySelect,2019,4(12):3432-3439. |
| 25 | ZHOU Jinghong, SUI Zhijun, ZHU Jun,et al.Characterization of surface oxygen complexes on carbon nanofibers by TPD,XPS and FT-IR[J].Carbon,2007,45(4):785-796. |
| 26 | 李春全,王丝蒂,汪欣林,等.风化煤基催化材料的制备及其活化PMS降解苯酚[J].煤炭学报,2022,47(5):2067-2077. |
| LI Chunquan, WANG Sidi, WANG Xinlin,et al.Preparation of weathered coal-based catalytic material and its performance in phenol degradation by activating peroxymonosulfate[J].Journal of China Coal Society,2022,47(5):2067-2077. | |
| 27 | 王奇洲,李春全,殷帅军,等.CaCl2-热处理煤泥制备复合材料及其活化PMS降解苯酚性能[J].矿业科学学报,2023,8(1):127-136. |
| WANG Qizhou, LI Chunquan, YIN Shuaijun,et al.Preparation of CaCl2-heat treated coal slime based composite catalytic material and its performance toward phenol degradation by activating peroxymonosulfate[J].Journal of Mining Science and Technology,2023,8(1):127-136. | |
| 28 | 龚焱,汤琪,白纯,等.废弃聚氯乙烯塑料衍生的氮掺杂碳纳米片活化过一硫酸盐去除水中四环素[J].环境工程学报,2024,18(4):1161-1171. |
| GONG Yan, TANG Qi, BAI Chun,et al.Nitrogen doped carbon nanosheet derived from waste PVC plastic activated peroxymonosulfate for tetracycline removal[J].Chinese Journal of Environmental Engineering,2024,18(4):1161-1171. | |
| 29 | CAO Zhao, CAO Yongdan, DONG Hongjuan,et al.Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue[J].International Journal of Mineral Processing,2016,146:23-28. |
| 30 | 李敏敏,杨伟光,魏恒,等.活化过硫酸盐修复焦化场地多环芳烃污染土壤效果对比[J].环境工程,2023,41(S1):460-466. |
| LI Minmin, YANG Weiguang, WEI Heng,et al.Comparison of effects of activated persulfate on soil contaminated with polycyclic aromatic hydrocarbons in coking sites[J].Environmental Engineering,2023,41(S1):460-466. | |
| 31 | 姜文超.热脱附耦合过硫酸盐对土壤苯并[a]芘去除效果研究[J].安全与环境学报,2022,22(3):1518-1524. |
| JIANG Wenchao.Study of thermal desorption coupled persulfate on removal of benzo[a]pyrene in soil[J].Journal of Safety and Environment,2022,22(3):1518-1524. | |
| 32 | 魏彤宇,霍宁,商晓甫,等.硫酸亚铁活化过硫酸盐氧化土壤中多环芳烃[J].中国环保产业,2021(12):46-51. |
| WEI Tongyu, HUO Ning, SHANG Xiaofu,et al.Oxidative degradation of polycyclic aromatic hydrocarbons in soil by ferrous sulfate activated persulfate[J].China Environmental Protection Industry,2021(12):46-51. | |
| 33 | 古祺鹏,杨洁,刘亚男.原位热脱附技术修复多环芳烃污染土壤的研究进展[J].中国资源综合利用,2021,39(11):99-101. |
| GU Qipeng, YANG Jie, LIU Yanan.Research progress of in situ thermal desorption technology for remediation of polycyclic aromatic hydrocarbons contaminated soil[J].China Resources Comprehensive Utilization,2021,39(11):99-101. | |
| 34 | 张宏玲,李森,张杨,等.活化过硫酸盐体系原位模拟去除土壤中多环芳烃[J].浙江农业学报,2018,30(6):1044-1049. |
| ZHANG Hongling, LI Sen, ZHANG Yang,et al. In situ simulated remediation of polycyclic aromatic hydrocarbons by activated sodium persulfate system[J].Acta Agriculturae Zhejiangensis, 2018,30(6):1044-1049. | |
| 35 | 黄可.过硫酸钠氧化技术修复PAHs污染土壤的案例分析[J].资源节约与环保,2018(6):76-77,83. |
| HUANG Ke.Case study on remediation of PAHs contaminated soil by sodium persulfate oxidation technology[J].Resources Economization & Environmental Protection,2018(6):76-77,83. | |
| 36 | 邸莎,张超艳,颜增光,等.过硫酸钠对我国典型土壤中多环芳烃氧化降解效果的影响[J].环境科学研究,2018,31(1):95-101. |
| DI Sha, ZHANG Chaoyan, YAN Zengguang,et al.Oxidative degradation effect of sodium persulfate on polycyclic aromatic hydrocarbons in typical Chinese soils[J].Research of Environmental Sciences,2018,31(1):95-101. | |
| 37 | KE Yuxin, ZHANG Xing, REN Yuhang,et al.Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate:Performance,process and mec-hanisms[J].Bioresource Technology,2024,399:130633. |
| 38 | LI Xiaoying, TAN Qiren, ZHOU Ying,et al.Synergic remediation of polycyclic aromatic hydrocarbon-contaminated soil by a combined system of persulfate oxidation activated by biochar and phytoremediation with basil:A compatible,robust,and sustainable approach[J].Chemical Engineering Journal,2023,452:139502. |
| [1] | MAO Shize. Study on microwave roasting-activated coal gangue to improve sulfate corrosion resistance of concrete [J]. Inorganic Chemicals Industry, 2025, 57(3): 86-93. |
| [2] | CHAI Chunjing, FENG Zhengjun, WU Haibin, SHI Xiaokai, ZHANG Junjie, SONG Huiping. Effect of coal gangue-based porous matrix on soil solute transport [J]. Inorganic Chemicals Industry, 2024, 56(9): 107-116. |
| [3] | ZHANG Yu, ZHAO Guiyan, TIAN Yongchang, QIU Xiaokui, SUN Jiali, XU Lixin. Reaction kinetics of ethylenediamine hydrochloride with calcium hydroxide [J]. Inorganic Chemicals Industry, 2024, 56(5): 64-69. |
| [4] | DENG Yinxiang, CHEN Chaoyi, WANG Shiyu, GAO Yingxue, PENG Shuang. Effect of cell voltage on electrochemical conversion of CO2 to carbon materials in CaCl2 based molten salt [J]. Inorganic Chemicals Industry, 2024, 56(1): 40-46. |
| [5] | WANG Lijuan, YAN Kezhou, GUO Zhiqiang, ZHAO Zhonghe, GUO Yanxia, CHENG Fangqin. Preparation of poly-aluminum chloride from acid leaching liquor of red mud-coal gangue activated by sodium salt [J]. Inorganic Chemicals Industry, 2023, 55(4): 76-83. |
| [6] | ZHANG Chenhu, MA Yi, ZHU Shan, CHEN Peng, WANG Chengyong, LI Ziwen. Study on adsorption of heavy metal ions in mineral processing wastewater by chelating modified coal gangue [J]. Inorganic Chemicals Industry, 2023, 55(4): 97-103. |
| [7] | Hu Caixia,Hu Chenxin,Peng Jianlong,Zhong Xing. Preparation of porous spherical calcium carbonate with soluble starch as crystallization controller [J]. Inorganic Chemicals Industry, 2021, 53(5): 51-55. |
| [8] | Ye Weihui,Lü Qi,Long Changjiang. Study on rapid preparation process of high-density spherical basic nickel carbonate [J]. Inorganic Chemicals Industry, 2021, 53(5): 69-72. |
| [9] | Yang Quancheng,Zhang Kaiyong,Guo De,Shi Changsheng,Ma Ruixin,Tang Ligang,Du Zhenyu. Research on adsorption properties of methylene blue by mesoporous calcium silicate [J]. Inorganic Chemicals Industry, 2021, 53(10): 86-91. |
| [10] | Wang Xiaoxiong,Zhang Jiangang,Zhang Wenjing,Chen Ye. Removal of calcium chloride from crude phosphoric acid by hydrochloric acid method [J]. Inorganic Chemicals Industry, 2020, 52(8): 17-19. |
| [11] | Liu Xiaoting,Wen Jiuran,Wang Siyu,Liu Kaiping,Gao Ni,Zhong Jiaqiang. Study on strengthening technology of raw coal gangue aggregate [J]. Inorganic Chemicals Industry, 2020, 52(4): 65-71. |
| [12] | Zhang Dongxue,Yang Baojun,Chen Yujia,Wang Bainian,Shao Zongqi. Preparation of calcium sulfate dihydrate whiskers from leachate of phosphate ore by hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 37-42. |
| [13] | Yang Quancheng,Gong Zhiming,Mao Yanyu,Li Xiaodong,Zhang Yancheng,Shi Changsheng,Zeng Ming. Preparation of mesoporous calcium silicate with alumina-extracted coal gangue by hydrothermal method [J]. Inorganic Chemicals Industry, 2020, 52(11): 86-90. |
| [14] | Yan Xin1,Lu Yunfeng2,Ma Yuanyuan3,Xie Long3. Study on composite carbonization mechanism of nano calcium carbonateproduced by calcium chloride-ammonia water system [J]. Inorganic Chemicals Industry, 2019, 51(7): 77-80. |
| [15] | Chen Lifang,Zhang Qinqin,Lin Tian,Yue Peiying. Determination and correlation of solubility of boric acid in calcium chloride solution [J]. Inorganic Chemicals Industry, 2019, 51(5): 70-73. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||