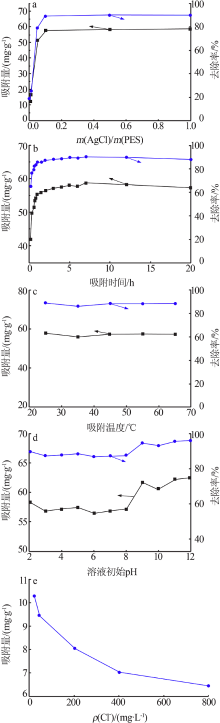
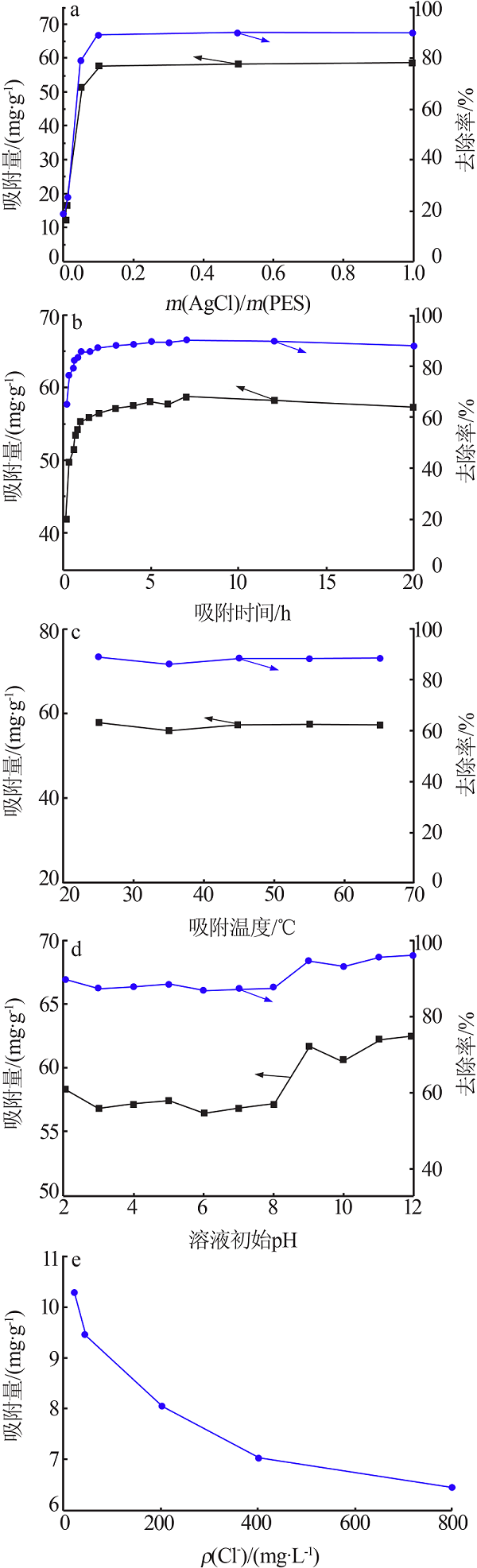
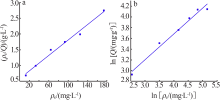
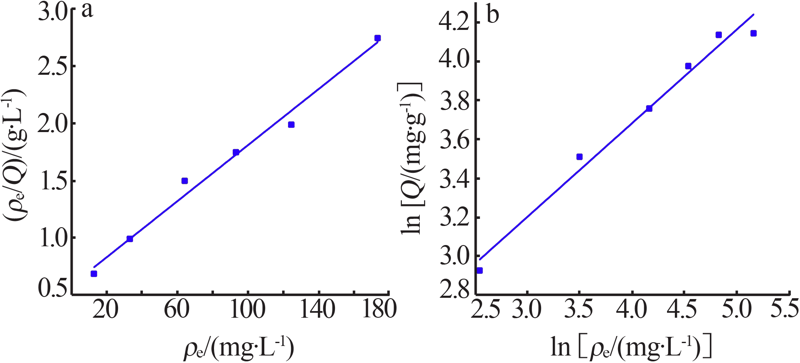
| [1] |
Zhang X, Ji Z Y, Liu F, et al. Investigation of electrochemical oxida-tion technology for selective bromine extraction in comprehensive utilization of concentrated seawater[J]. Separation and Purification Technology, 2020, 248.Doi: 10.1016/j.seppur.2020.117108.
doi: 10.1016/j.seppur.2020.117108
|
| [2] |
管若伶, 王海增. 焙烧层状氢氧化镁铝/聚醚砜复合膜的制备及对溴离子的吸附[J]. 精细化工, 2017, 34(11):1201-1207.
|
| [3] |
Ezechiáš M, Covino S, Cajthaml T. Ecotoxicity and biodegradability of new brominated flame retardants:A review[J]. Ecotoxicology and Environmental Safety, 2014, 110:153-167.
doi: 10.1016/j.ecoenv.2014.08.030
pmid: 25240235
|
| [4] |
俞春雷, 王彦林, 刘颖棋, 等. 含氯溴硅酸酯阻燃剂的合成及表征[J]. 精细化工, 2014, 31(1):90-93.
|
| [5] |
欧育湘, 房晓敏, 沈琦. 阻燃剂对环境和人类健康影响评估的最新进展[J]. 精细化工, 2007, 24(12):1232-1235.
|
| [6] |
Cohen I, Shapira B, Avraham E, et al. Bromide ions specific removal and recovery by electrochemical desalination[J]. Environmental Science & Technology, 2018, 52(11):6275-6281.
doi: 10.1021/acs.est.8b00282
|
| [7] |
管若伶, 王海增. 溴资源与主要化工品[J]. 无机盐工业, 2018, 50(4):6-10.
|
| [8] |
Zhang Q, Liu W, Chai S, et al. The application of structured packing in industrial extraction of bromine from concentrated seawater[J]. IOP Conference Series Earth and Environmental Science, 2019, 252(4):1-6.
|
| [9] |
Theiss F L, Couperthwaite S J, Ayoko G A, et al. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides[J]. Journal of Colloid and Interface Science, 2014, 417:356-368.
doi: 10.1016/j.jcis.2013.11.040
|
| [10] |
朱云华, 丁磊, 钟梅英, 等. 载银沸石吸附去除水中溴离子的特性研究[J]. 硅酸盐通报, 2015, 34(7):1857-1863.
|
| [11] |
Gong C, Zhang Z, Qian Q, et al. Removal of bromide from water by adsorption on silver-loaded porous carbon spheres to prevent bromate formation[J]. Chemical Engineering Journal, 2013, 218:330-340.
|
| [12] |
高灿, 张慧芳, 刘海宁, 等. 溴、碘离子吸附研究进展[J]. 环境化学, 2014, 33(11):1906-1911.
|
| [13] |
林源, 王浩宇, 周亚蓉, 等. 海水提溴技术的发展与研究现状[J]. 无机盐工业, 2012, 44(9):5-7.
|
 ),Sun Chang,Zhuo Xianqin
),Sun Chang,Zhuo Xianqin