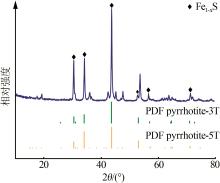
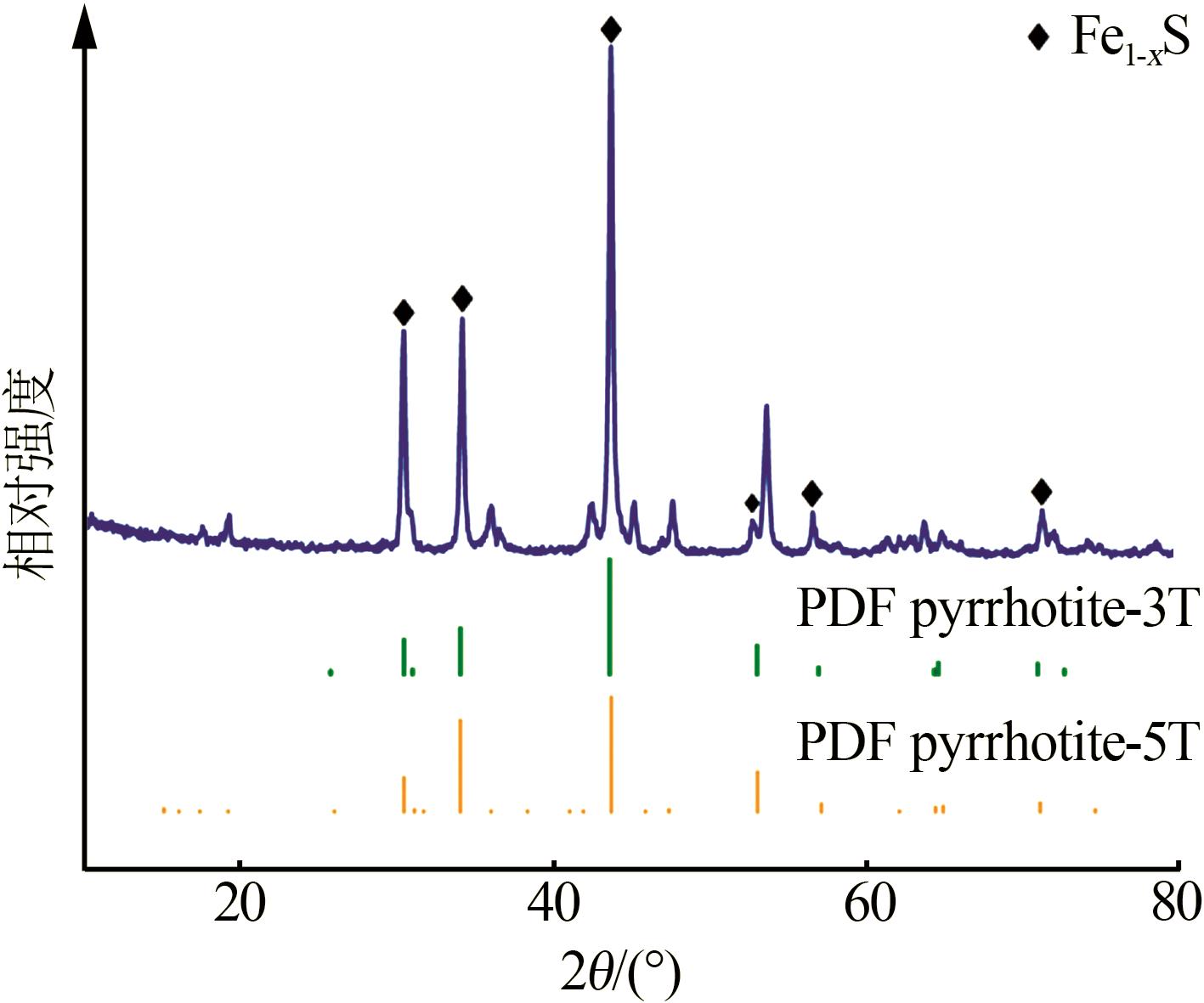
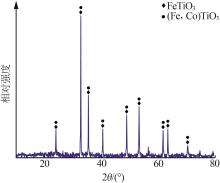
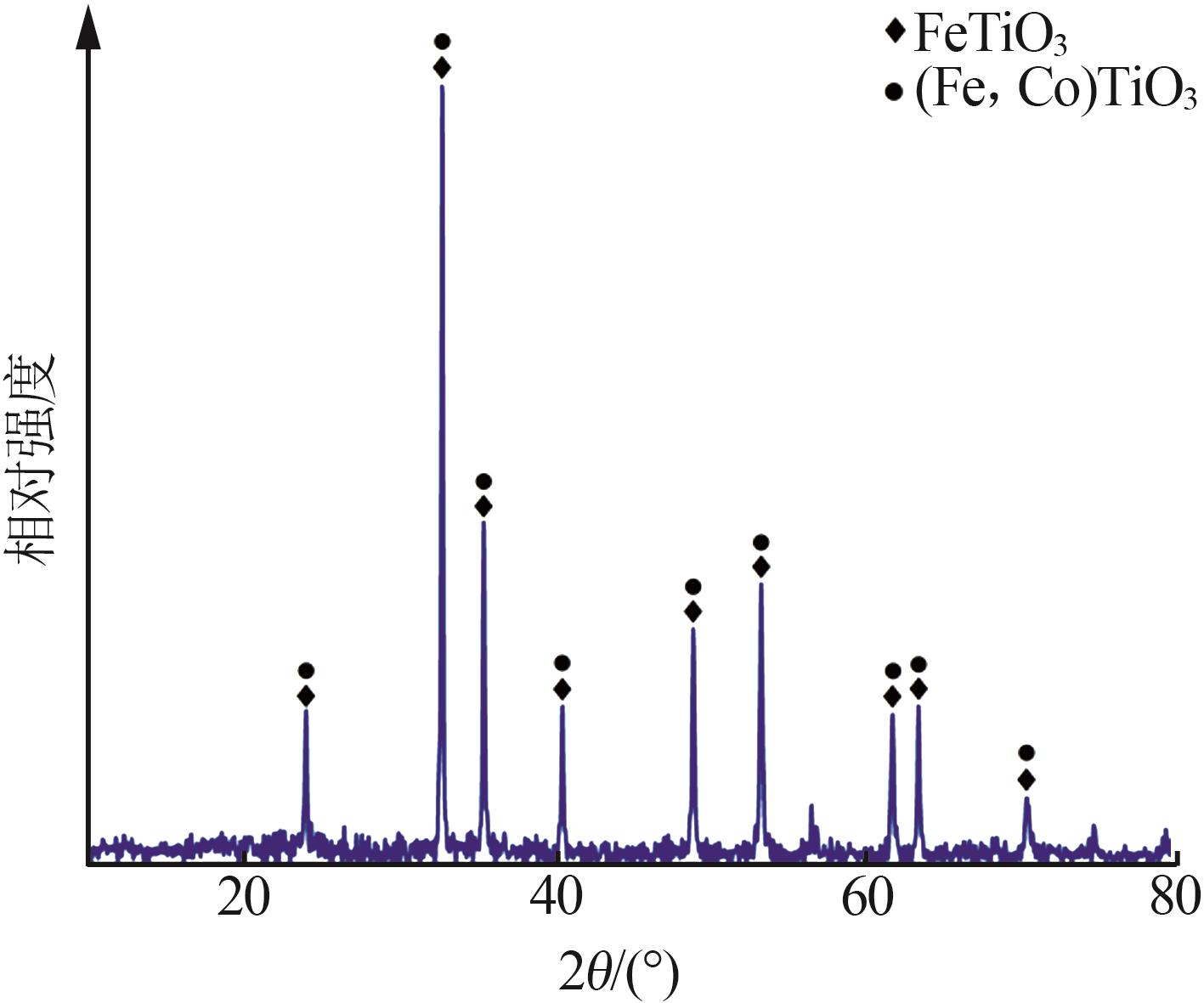
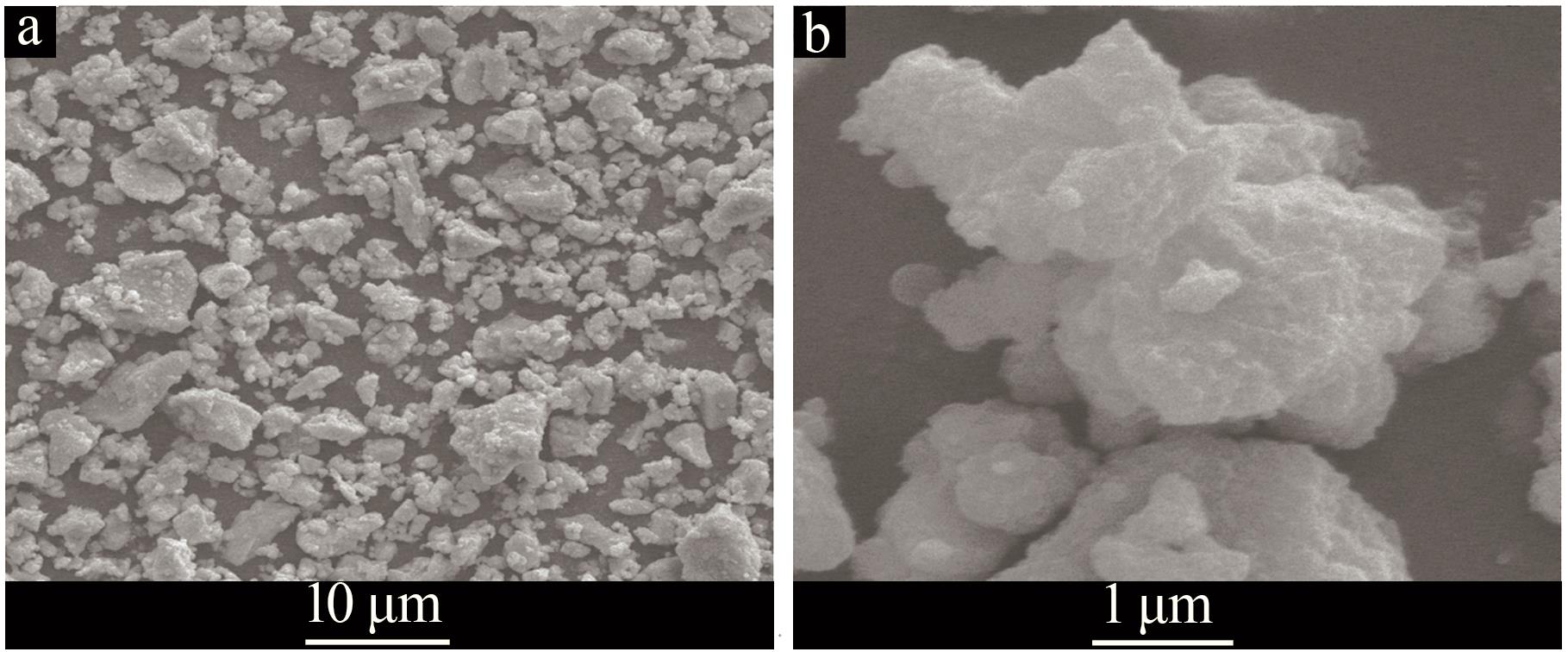
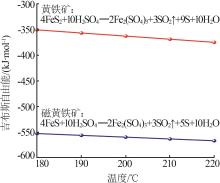
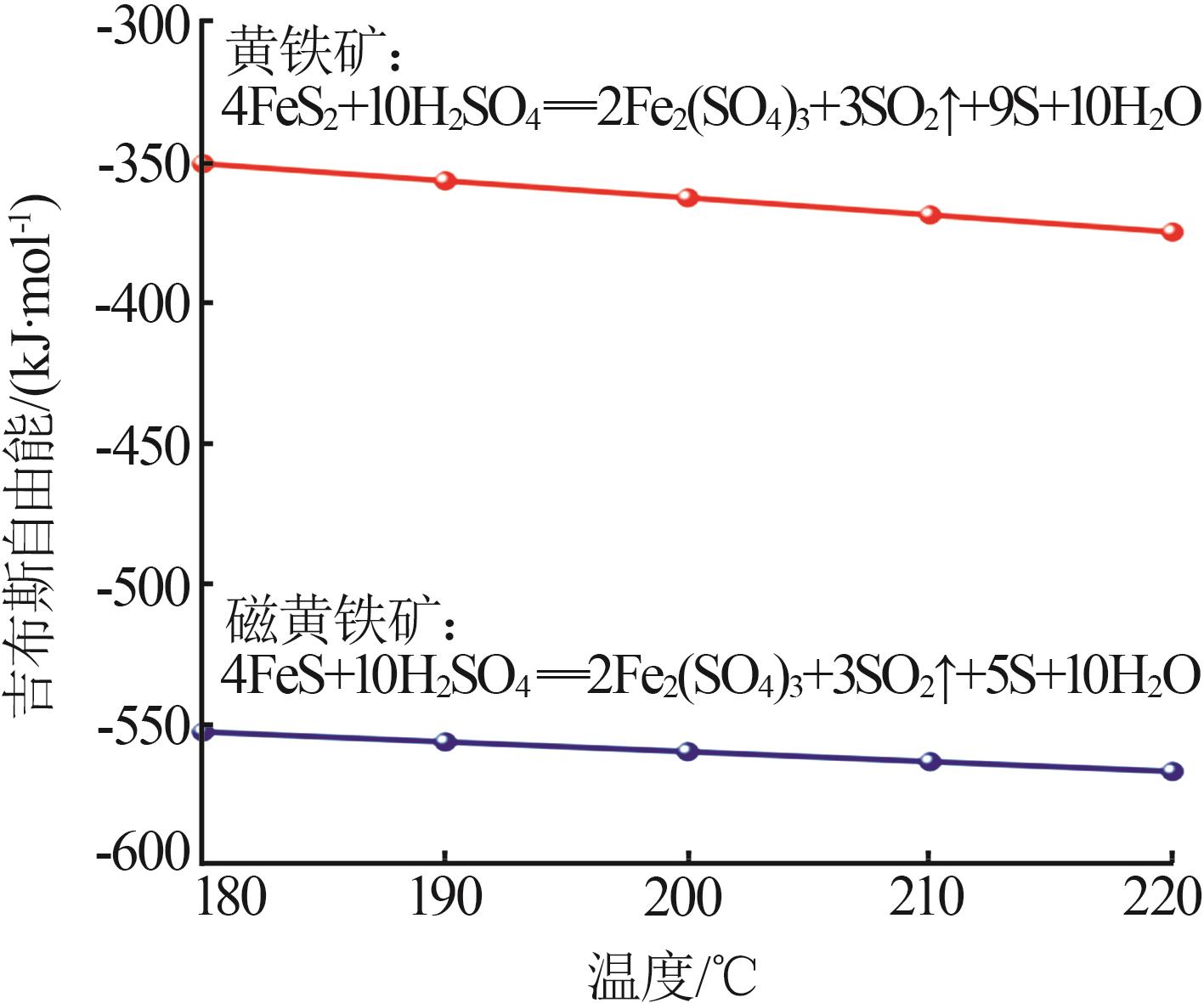
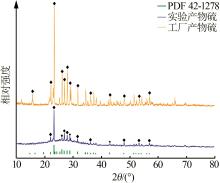
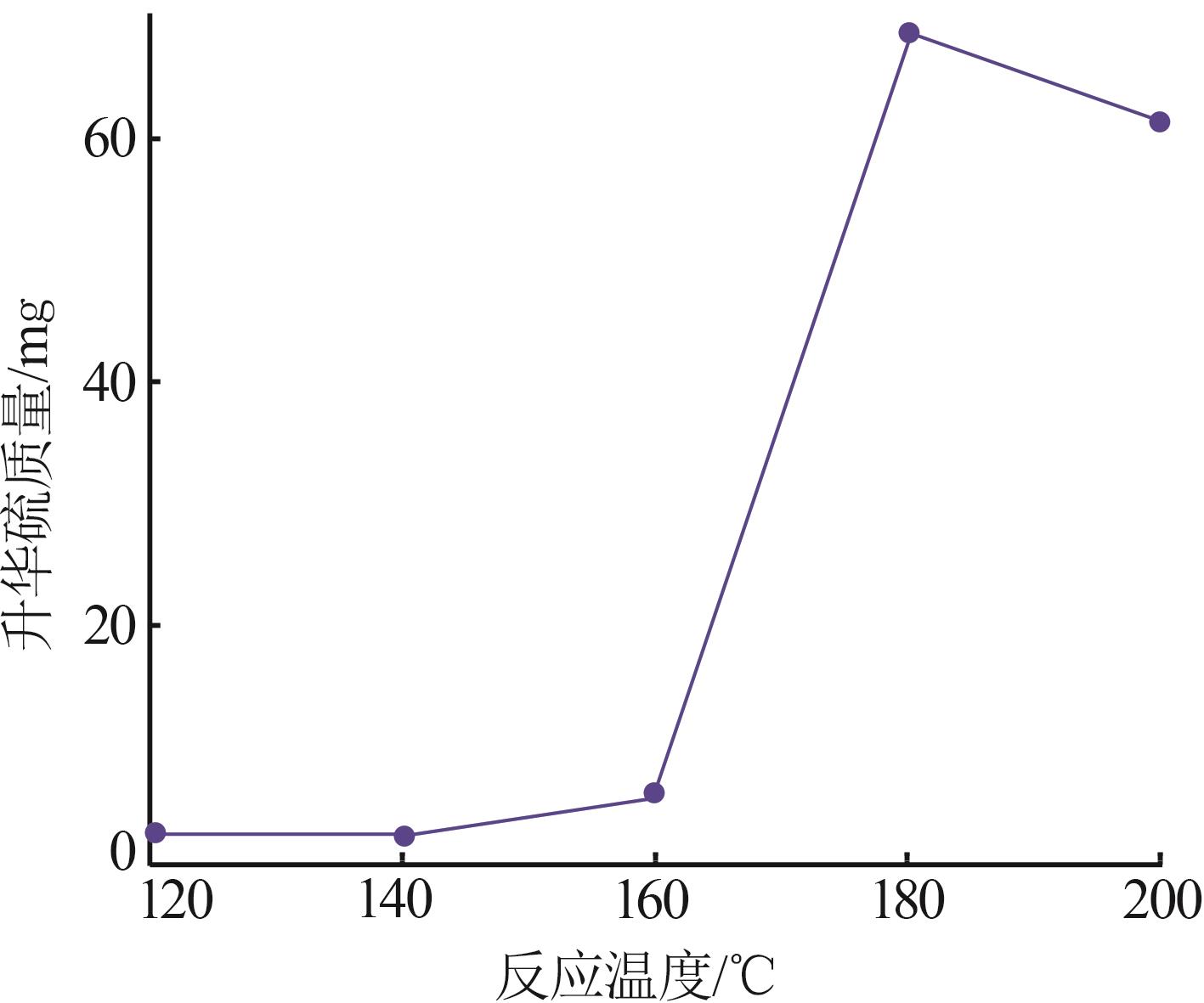
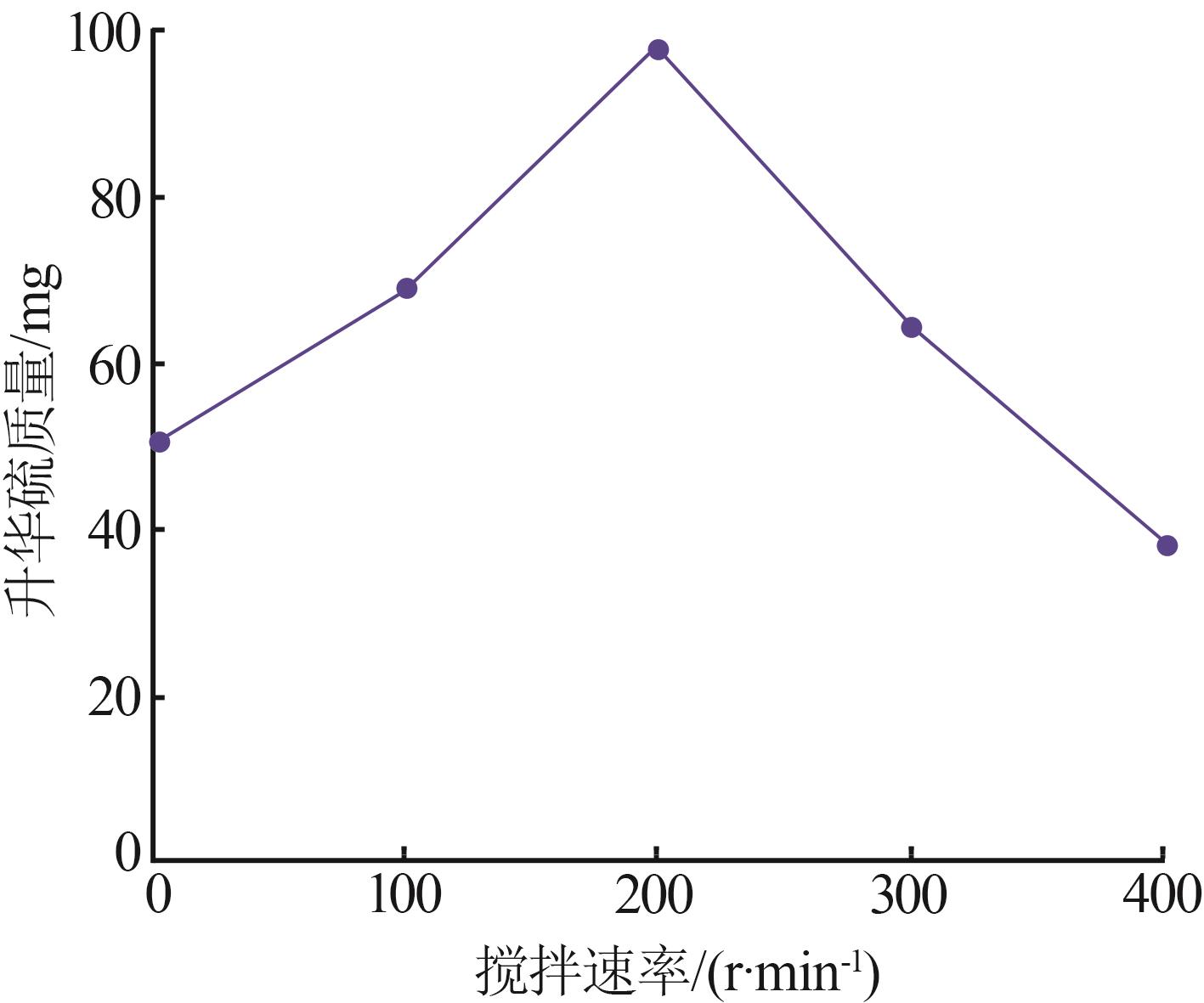
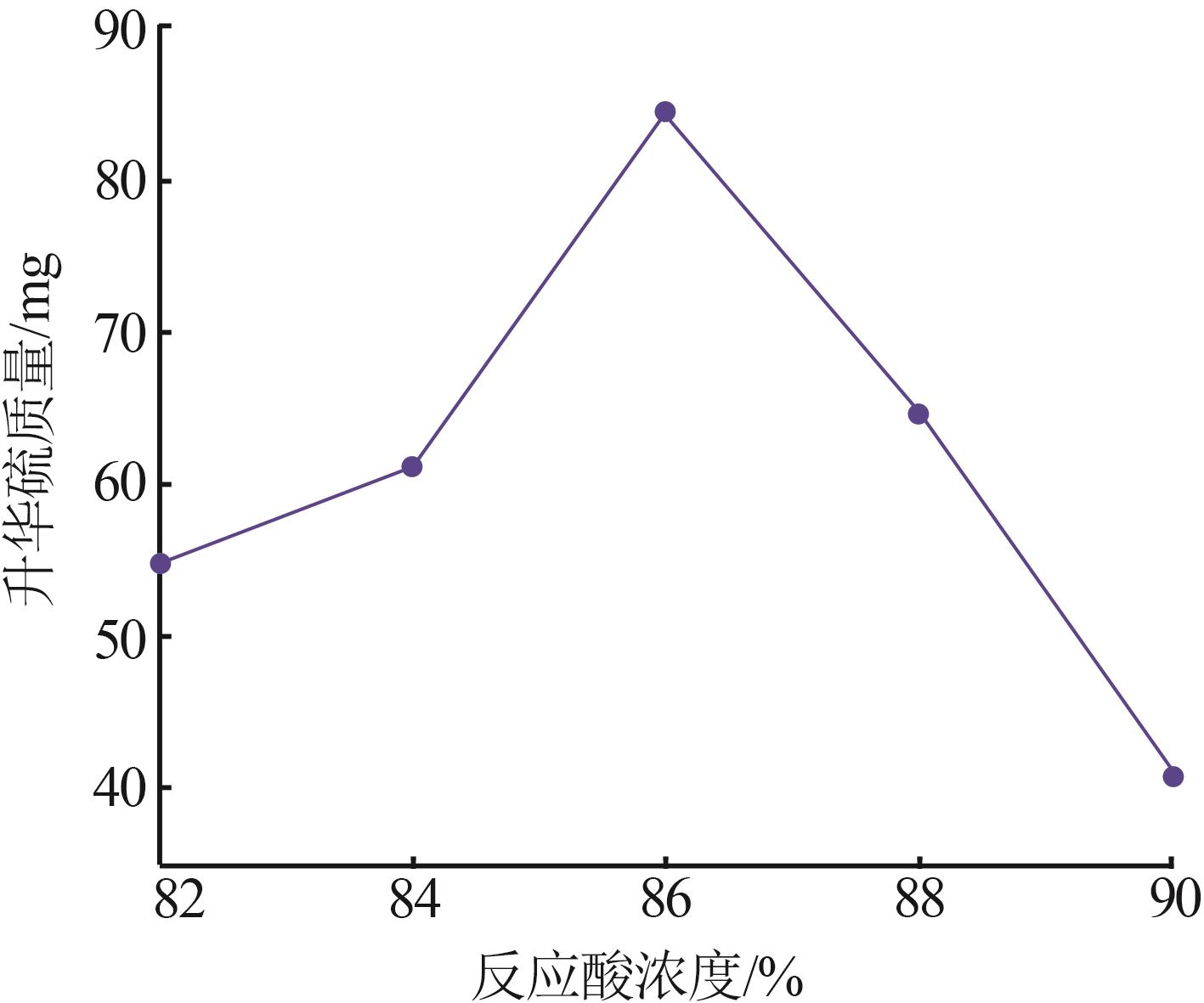
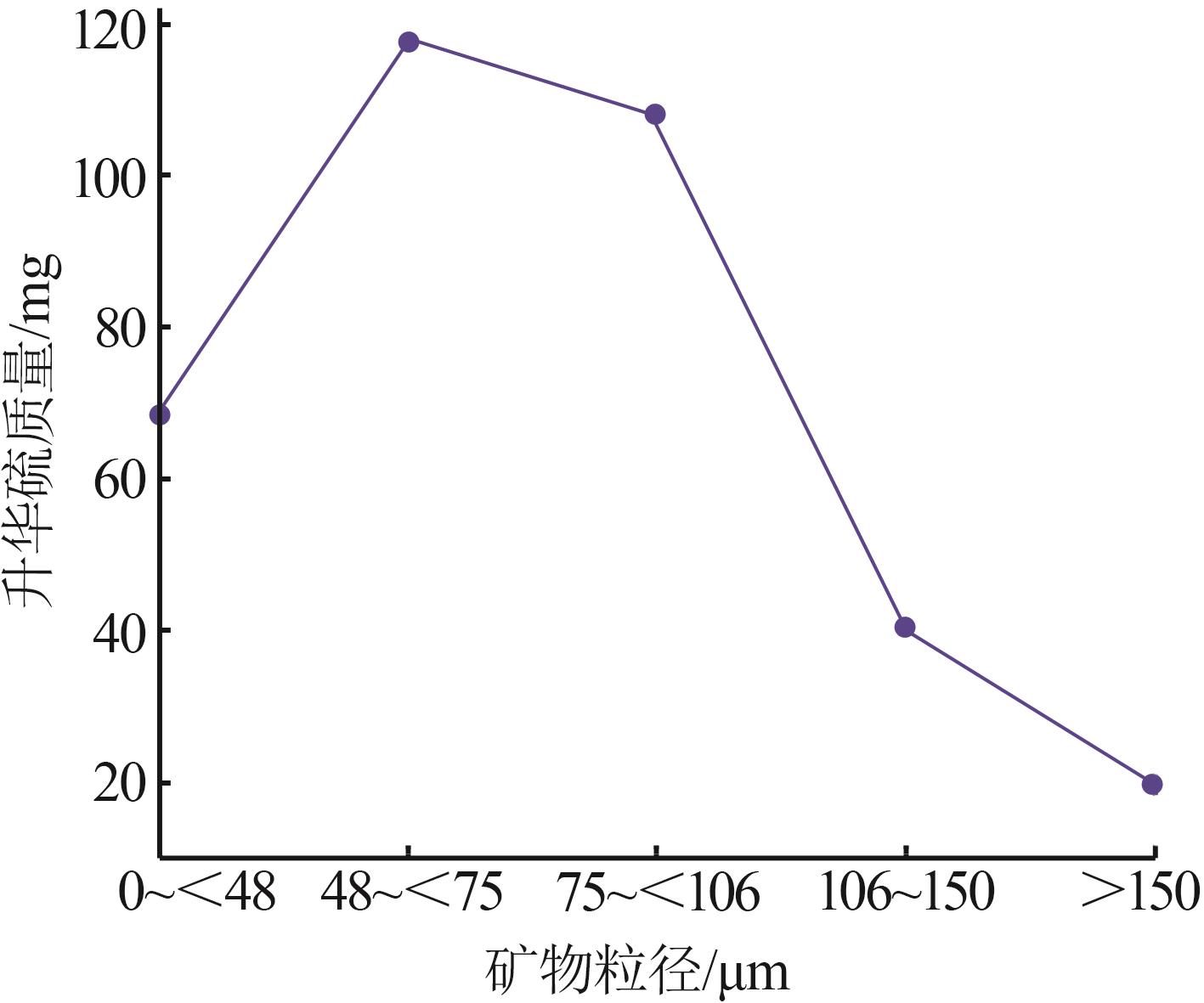
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (7): 96-103.doi: 10.19964/j.issn.1006-4990.2023-0542
• Environment·Health·Safety • Previous Articles Next Articles
Formation rules and emission reduction method of sublimed sulfur in acidolysis exhaust gas of titanium concentrate
XIANG Quanjin1,2( ), QUAN Xuejun2(
), QUAN Xuejun2( ), LI Li2, WANG Haibo1, CHEN Xinhong3, LI Ping2
), LI Li2, WANG Haibo1, CHEN Xinhong3, LI Ping2
- 1.State Key Laboratory of Vanadium and Titanium Resources Comprehensive Utilization,Panzhihua 617000,China
2.School of Chemistry and Chemical Engineering,Chongqing University of Technology,Chongqing 400054,China
3.Panzhihua Steel Group Chongqing Titanium Industry Co. ,Ltd. ,Chongqing 400054,China
-
Received:2023-11-13Online:2024-07-10Published:2024-08-01 -
Contact:QUAN Xuejun E-mail:1904995539@qq.com;hengjunq@cqut.edu.cn
CLC Number:
Cite this article
XIANG Quanjin, QUAN Xuejun, LI Li, WANG Haibo, CHEN Xinhong, LI Ping. Formation rules and emission reduction method of sublimed sulfur in acidolysis exhaust gas of titanium concentrate[J]. Inorganic Chemicals Industry, 2024, 56(7): 96-103.
share this article
Table 1
Occurrence of main elements in each mineral of titanium concentrate %"
| 矿物 | w (Al) | w (Ca) | w (Fe) | w (Mg) | w (Mn) | w (S) | w (Ti) |
|---|---|---|---|---|---|---|---|
| 钛铁矿 | 13.78 | 11.84 | 93.16 | 82.30 | 71.61 | 0.00 | 97.30 |
| 磁铁矿 | 6.71 | 0.00 | 3.37 | 1.46 | 0.00 | 0.00 | 0.45 |
| 钛闪石 | 15.19 | 29.11 | 0.52 | 3.24 | 6.65 | 0.00 | 0.40 |
| 绿泥石 | 30.10 | 1.06 | 0.88 | 7.30 | 0.00 | 0.00 | 0.17 |
| 榍石 | 1.13 | 16.46 | 0.05 | 0.02 | 0.00 | 0.00 | 0.34 |
| 镁橄榄石 | 0.77 | 0.01 | 0.16 | 2.61 | 2.17 | 0.00 | 0.01 |
| 透辉石 | 0.38 | 4.28 | 0.04 | 0.38 | 0.00 | 0.00 | 0.01 |
| 钙长石 | 2.27 | 4.50 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 |
| 磁铁矿+绿泥石 | 1.77 | 2.54 | 0.51 | 0.67 | 2.52 | 0.00 | 0.12 |
| 辉石 | 1.19 | 2.03 | 0.07 | 0.20 | 0.80 | 0.00 | 0.01 |
| 白云母 | 3.63 | 0.33 | 0.02 | 0.03 | 0.00 | 0.00 | 0.00 |
| 黑金红石 | 0.18 | 0.12 | 0.02 | 0.02 | 0.00 | 0.00 | 0.18 |
| 钛铁矿+石灰 | 0.23 | 8.12 | 0.25 | 0.42 | 0.00 | 0.00 | 0.30 |
| 微斜长石 | 4.78 | 3.56 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| 钛铁矿+金 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| 刚玉 | 7.54 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| 铁板钛矿 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 |
| 钛铁矿+绿泥石 | 2.26 | 9.22 | 0.35 | 0.00 | 9.95 | 0.00 | 0.52 |
| 钠长石 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| 磁黄铁矿 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 36.83 | 0.00 |
| 镁铝尖晶石 | 2.79 | 0.00 | 0.02 | 0.26 | 0.00 | 0.00 | 0.00 |
| 磷酸钙 | 0.00 | 5.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 钙钛矿 | 0.00 | 1.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| 黑云母 | 0.10 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 |
| 镁铁硫酸盐 | 0.17 | 0.00 | 0.02 | 0.09 | 0.00 | 6.55 | 0.00 |
| 石英 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 黄铁矿 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 56.62 | 0.01 |
| 铝硅酸盐 | 1.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 单质铁 | 0.07 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.01 |
| 绿泥石+钛铁矿 | 2.34 | 0.38 | 0.12 | 0.79 | 0.00 | 0.00 | 0.12 |
| 含铁刚玉 | 1.05 | 0.00 | 0.02 | 0.03 | 0.55 | 0.00 | 0.00 |
| 顽火辉石 | 0.02 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.00 |
| 二氧化钛+锰铁尖晶石 | 0.00 | 0.02 | 0.01 | 0.00 | 5.71 | 0.00 | 0.01 |
| 总计 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | OGUNSANYA O A, AKINWANDE A A, BALOGUN O A,et al.Mechanical and damping behavior of artificially aged Al 6061/TiO2 reinforced composites for aerospace applications[J].Particulate Science and Technology,2023,41(2):196-208. |
| 2 | SOBCZYK-GUZENDA A, SZYMANSKI W, JEDRZEJCZAK A,et al.Bactericidal and photowetting effects of titanium dioxide coatings doped with iron and copper/fluorine deposited on stainless steel substrates[J].Surface and Coatings Technology,2018,347:66-75. |
| 3 | LIU Qiang, JIANG Quan, HUANG Mojia,et al.The fresh and hardened properties of 3D printing cement⁃base materials with self⁃cleaning nano-TiO2:An exploratory study[J].Journal of Cleaner Production,2022,379(2):134804. |
| 4 | EHRENBRING H, CHRIST R, PACHECO F,et al.Analysis of the self⁃cleaning potential of glass fiber reinforced concrete(GRC) with TiO2 nanoparticles[J].Sustainability,2022,14(14):8738. |
| 5 | FU Zhaoyue, HOU Yongli, HAUGEN H J,et al.TiO2 nanostructured implant surface⁃mediated M2c polarization of inflammatory monocyte requiring intact cytoskeleton rearrangement[J].Journal of Nanobiotechnology,2023,21(1):1-16. |
| 6 | ZHANG Wensheng, ZHU Zhaowu, CHENG C.A literature review of titanium metallurgical processes[J].Hydrometallurgy,2011,108:177-188. |
| 7 | MIDDLEMAS S, FANG Z Z, FAN Peng.Life cycle assessment comparison of emerging and traditional titanium dioxide manufacturing processes[J].Journal of Cleaner Production,2015,89:137-147. |
| 8 | 唐舒扬,郭宇峰,郑富强,等.钛白粉的制备方法现状及展望[J].无机盐工业,2022,54(7):27-34. |
| TANG Shuyang, GUO Yufeng, ZHENG Fuqiang,et al.Present situation and prospects of preparation methods of titanium dioxide[J].Inorganic Chemicals Industry,2022,54(7):27-34. | |
| 9 | NGUYEN T H, LEE M S.A review on the recovery of titanium dioxide from ilmenite ores by direct leaching technologies[J].Mineral Processing and Extractive Metallurgy Review,2018,40:231- 247. |
| 10 | JIA Linie, LIANG Bin, Li LÜ,et al.Beneficiation of titania by sulfuric acid pressure leaching of Panzhihua ilmenite[J].Hydrometallurgy,2014,150:92-98. |
| 11 | 王海波,吴小平,马鑫,等.钛精矿酸解固相物中钛的浸出行为及动力学研究[J].中国有色金属学报,2021,31(12):3655-3663. |
| WANG Haibo, WU Xiaoping, MA Xin,et al.Research on leaching behavior and kinetics of titanium from solid phase of acidolysis of titanium concentrate[J].The Chinese Journal of Nonferrous Metals,2021,31(12):3655-3663. | |
| 12 | LUO Yi, CHE Xiaokui, CUI Xinglan,et al.Selective leaching of vanadium from V-Ti magnetite concentrates by pellet calcification roasting-H2SO4 leaching process[J].International Journal of Mining Science and Technology,2021,31(3):507-513. |
| 13 | KORDZADEH-KERMANI V, SCHAFFIE M, HASHEMIPOUR RAFSANJANI H,et al.A modified process for leaching of ilmenite and production of TiO2 nanoparticles[J].Hydrometallurgy,2020,198:105507. |
| 14 | 唐文骞.硫酸法钛白生产中的环保治理措施[J].无机盐工业,2007,39(7):39-41. |
| TANG Wenqian.Environment control measures in titanium white production by H2SO4 process[J].Inorganic Chemicals Industry,2007,39(7):39-41. | |
| 15 | 李有霖,马瑞邦,廖光瑞,等.钛白粉酸解尾气处理技术探析[J].清洗世界,2023,39(5):65-67. |
| LI Youlin, MA Ruibang, LIAO Guangrui,et al.Discussion on treatment technology of acid hydrolysis tail gas of titanium dioxide[J].Cleaning World,2023,39(5):65-67. | |
| 16 | 王海波,罗志强,吴小平,等.钛渣酸解尾气S含量研究[J].钢铁钒钛,2020,41(4):92-96. |
| WANG Haibo, LUO Zhiqiang, WU Xiaoping,et al.Study on the S content of titanium slag acid hydrolysis tail gas[J].Iron Steel Vanadium Titanium,2020,41(4):92-96. | |
| 17 | 徐童章.铜冶炼烟气制酸过程中升华硫的成因及解决措 施[J].化工管理,2021(33):83-85. |
| XU Tongzhang.Causes and solutions of sublimated sulfur in sulfuric acid production from copper smelting flue gas[J].Chemical Enterprise Management,2021(33):83-85. | |
| 18 | NICHOLSON R V, SCHARER J M.Environmental geochemistry of sulfide oxidation[M].Washington DC:American Chemical Society,1994. |
| 19 | 赵青娥.表外钛精矿冶炼钛渣硫的走向分析[J].钢铁钒钛,2018,39(2):97-101. |
| ZHAO Qing′e.Study on trend of sulfur in titanium slag smelting process with the boundary titanium concentrate[J].Iron Steel Vanadium Titanium,2018,39(2):97-101. | |
| 20 | 江日容,熊凤英,李锦燊,等.紫外分光光度法测定复方硫洗剂中硫的含量[J].齐齐哈尔医学院学报,2006,27(3):325-326. |
| JIANG Rirong, XIONG Fengying, LI Jinshen,et al.Determination of sulfur content in compound sulfur lotion by ultraviolet spectrophotometry[J].Journal of Qiqihar Medical College,2006,27(3):325-326. | |
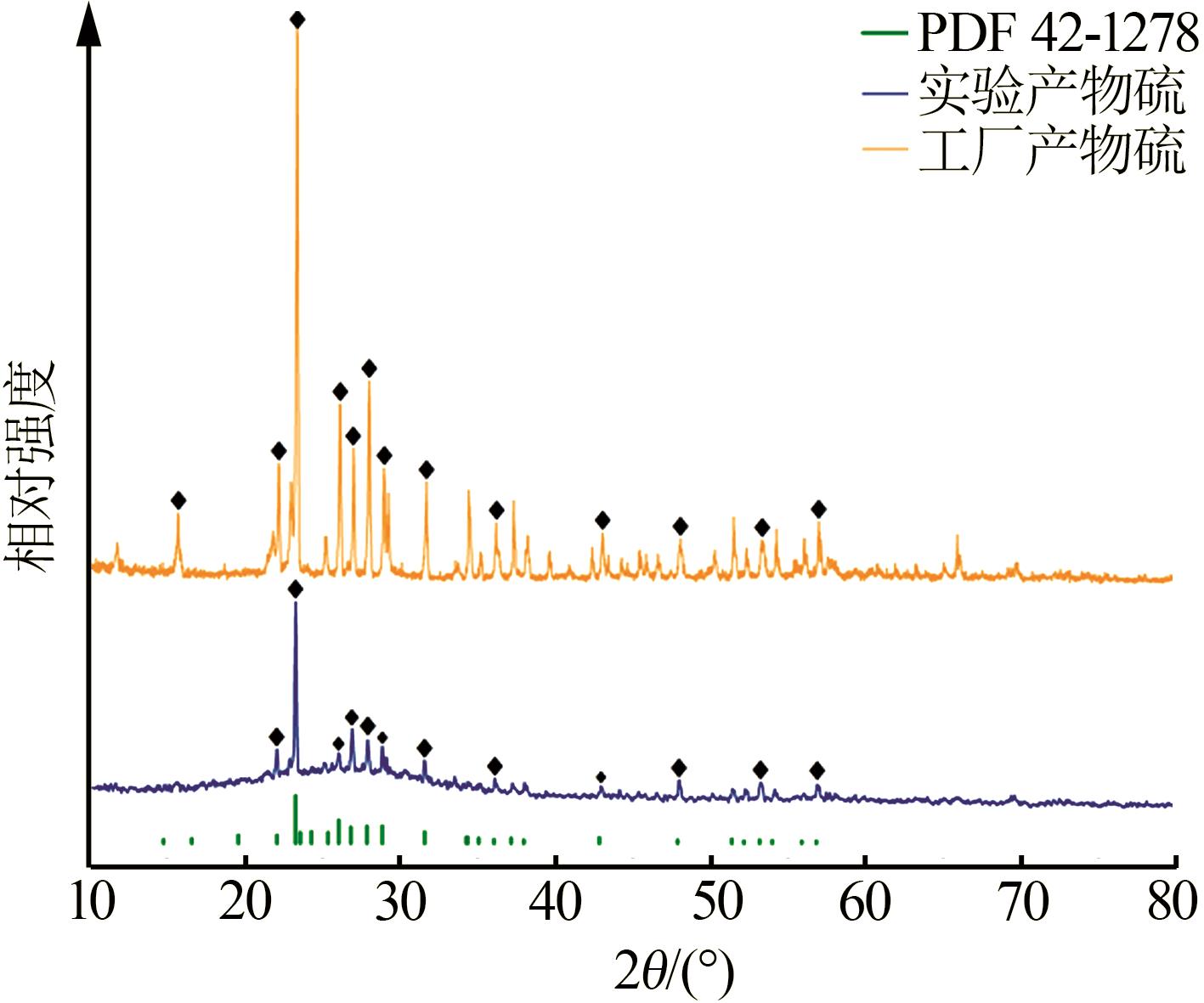
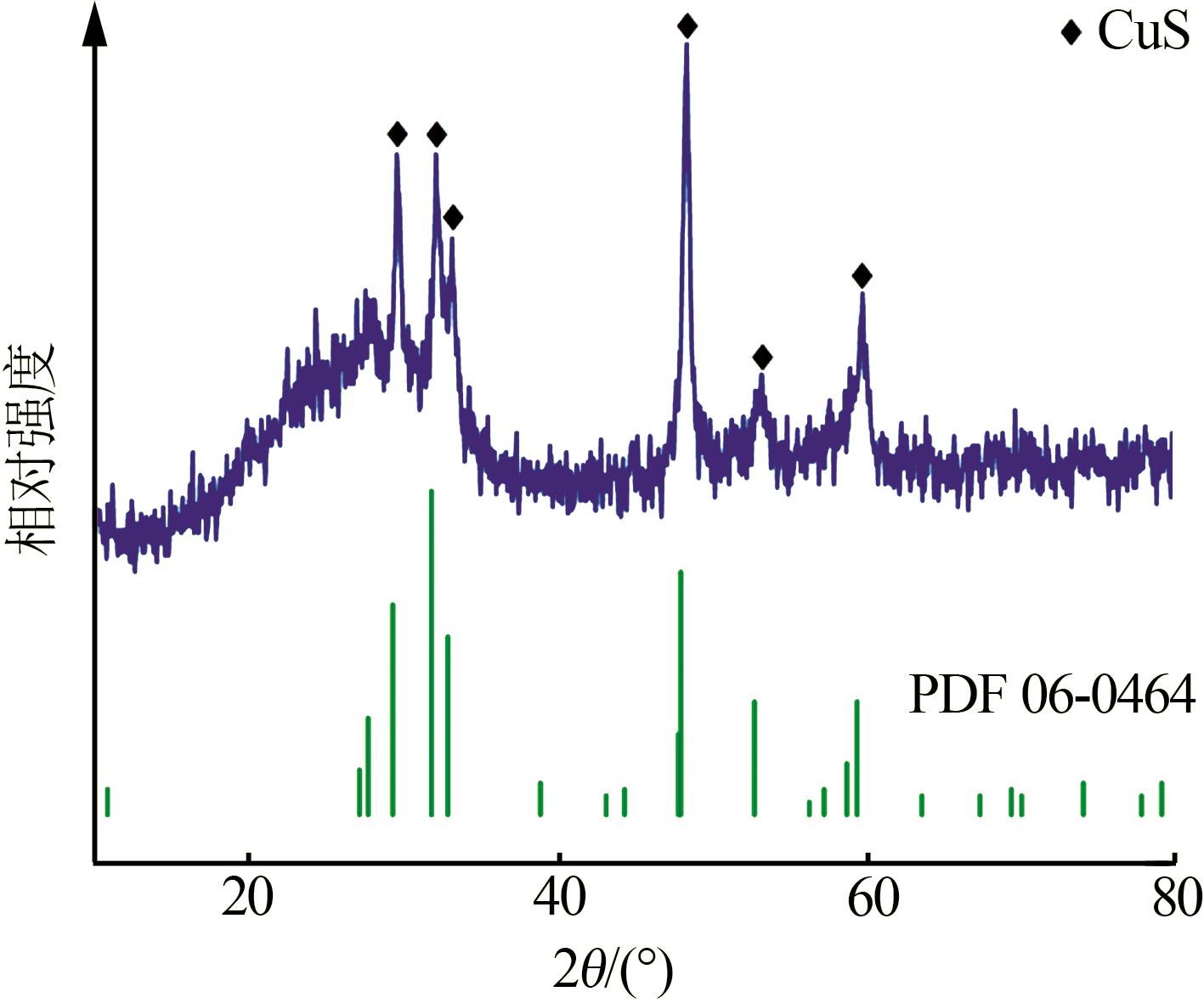
| 21 | 向泉锦,全远霞,全学军,等.钛精矿酸解尾气中升华硫产生的机理分析[J].钢铁钒钛,2023,44(3):100-104. |
| XIANG Quanjin, QUAN Yuanxia, QUAN Xuejun,et al.Mechanism analysis of the production of sublimated sulfur in the tail gas of acidolysis of titanium concentrate[J].Iron Steel Vanadium Titanium,2023,44(3):100-104. | |
| 22 | 张小普,艾光华,严华山.磁黄铁矿选矿研究进展与发展趋 势[J].矿产综合利用,2022(5):102-107. |
| ZHANG Xiaopu, AI Guanghua, YAN Huashan.Research progress and development trend of pyrrhotite beneficiation[J].Multipurpose Utilization of Mineral Resources,2022(5):102-107. | |
| 23 | 张振华,陈宝智,赵杉林,等.硫化亚铁的氧化反应历程[C]∥2012(沈阳)国际安全科学与技术学术研讨会,2012:205- 210. |
| 24 | 李兵,杨义,刘作华,等.湿法磷酸固-液体系混沌混合与浸出强化行为[J].化工学报,2019,70(5):1742-1749. |
| LI Bing, YANG Yi, LIU Zuohua,et al.Solid-liquid chaotic mixing and leaching enhancement performance in phosphoric acid leaching process[J].CIESC Journal,2019,70(5):1742-1749. | |
| 25 | 任南琪,王爱杰,李建政,等.硫化物氧化及新工艺[J].哈尔滨工业大学学报,2003,35(3):265-268,275. |
| REN Nanqi, WANG Aijie, LI Jianzheng,et al.Sulfide oxidationbacteria and innovative sulfide oxidation process[J].Journal of Harbin Institute of Technology,2003,35(3):265-268,275. | |
| 26 | 马维平.硫酸酸解攀西钛精矿技术研究[J].无机盐工业,2013,45(5):24-26. |
| MA Weiping.Research on acidolysis of Panxi titanium concentrate with sulfuric acid[J].Inorganic Chemicals Industry,2013,45(5):24-26. | |
| 27 | SASIKUMAR C, RAO D S, SRIKANTH S,et al.Effect of mechanical activation on the kinetics of sulfuric acid leaching of beach sand ilmenite from Orissa,India[J].Hydrometallurgy,2004,75(1/2/3/4):189-204. |
| 28 | JANZEN M P, NICHOLSON R V, SCHARER J.Pyrrhotite reaction kinetics:Reaction rates for oxidation by oxygen,ferric iron,and for nonoxidative dissolution[J].Geochimica et Cosmochimica Acta,2000,64:1511-1522. |
| 29 | 蔡美芳,党志.磁黄铁矿氧化机理及酸性矿山废水防治的研究进展[J].环境污染与防治,2006,28(1):58-61. |
| CAI Meifang, DANG Zhi.A review on pyrrhotite oxidation mechanism and acid mine drainage prevention[J].Environmental Pollution & Control,2006,28(1):58-61. | |
| 30 | BELZILE N, CHEN Yuwei, CAI Meifang,et al.A review on pyrrhotite oxidation[J].Journal of Geochemical Exploration,2004,84(2):65-76. |
| [1] | Liao Xin1,2,Yang Shaoli1,Ma Lan1,Li Hong1. Comparison and analysis of sulfuric acid process titanium dioxide prepared with titanium concentrate and titanium slag as raw materials respectively [J]. Inorganic Chemicals Industry, 2019, 51(10): 7-11. |
| [2] | MA Guang-Qiang, ZOU Min, XIA Dong. Experimental study on titanium concentrate leaching to prepare Ti-rich material with titanium dioxide waste acid [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 67-. |
| [3] | MA Wei-Ping. Research on acidolysis of Panxi titanium concentrate with sulfuric acid [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 24-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||